1. Introduction
1.1. Background
The term "graphene" was first coined by A. Geim and K. Novoselov, who were awarded the 2010 Nobel Prize for their discovery of a two-dimensional material with a thickness of a single carbon atom of high crystalline quality. They obtained the first monolayer and few-layer metastable graphene nanosheets by mechanically exfoliating graphite samples [1][2].
Graphene oxide (GO) is a two-dimensional (2-D) monolayer graphite that is valued for its accessibility and material compatibility. It can be produced by mechanically agitating or ultrasonically treating graphite oxide to exfoliate it into lamellar flakes. GO and its derivatives are rapidly becoming the most studied carbon-based materials in many fields due to their high stability, two-dimensional planar structure, large surface area, ease of chemical adaptation through their functionality, efficient surface loading of many biomolecules, as well as optical, electrical and mechanical properties [3]. GO has attracted a lot of attention because of its ease of use and compatibility with many materials. GO is relatively inexpensive, physically robust and chemically stable due to the strong influence of C-C bonds in its structure [4]. In terms of material properties, graphene oxide and its reduced state show two different appearances. Graphene oxide is insulating and the oxidized groups (epoxy, C-O) and hydroxyl, C-OH) are removed by reduction [5]. The electronic structure of reduced graphene oxide changes from insulating to semiconducting by removing the oxidized groups, which are carboxy1 (C-O) and hydroxyl (C-OH), and carboxy (COOH) and carbonyl (carbonyl (C=O)) at the boundary [6].
1.2. Atomic structure of graphene oxide
Graphene Oxide (GO) is a derivative of graphene produced by the oxidative treatment of graphite. From the picture below, it can be seen that the atomic structure of graphene oxide consists of carbon atoms in sp2 hybridized orbitals to form a hexagonal honeycomb lattice planar film. The graphite layer of GO contains hydroxyl (-OH) and epoxy (-O-) groups on both sides and carboxyl (-COOH) groups on the edges. These oxygen-containing groups make it easy for GO to form modified graphene oxide (MGO) through covalent or non-covalent interactions with organic small molecules and polymers [7].
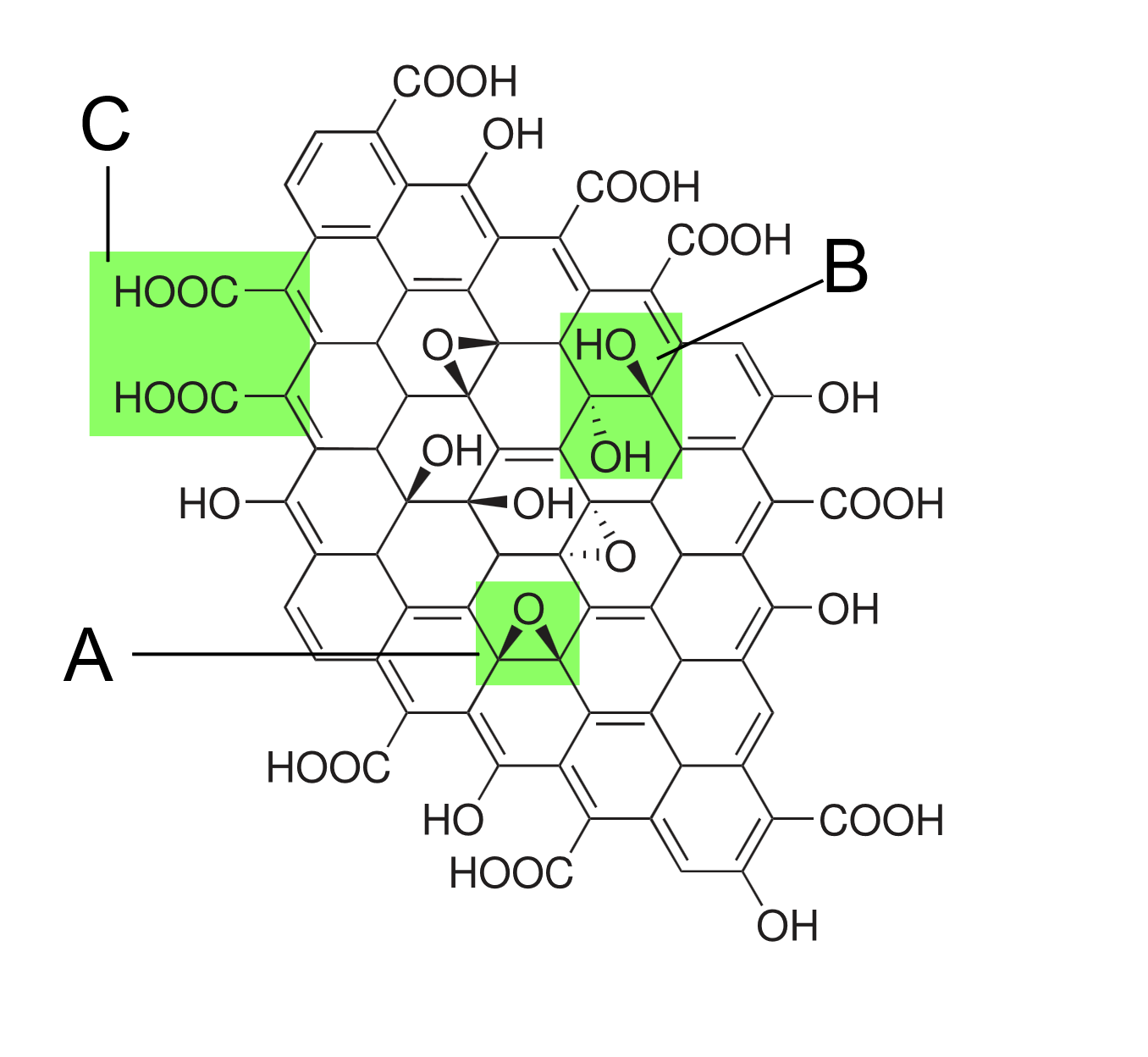
Figure 1: Structure proposed in 1998, contains functional groups: A: epoxy bridge, B: hydroxyl group, C: paired carboxyl group [8]
1.3. Comparison of graphene oxide and reduced graphene oxide
Graphene Oxide and Reduced Graphene Oxide are two different forms of graphene materials that differ significantly in their structure, chemical properties and applications.
Reduced Graphene Oxide (rGO) exhibits improved electrical conductivity compared to GO, although it remains inferior to pristine graphene. The reduction process partially restores the conjugated structure of graphene by removing some oxygen groups, but the perfect lattice structure is not fully recovered.
The hydrophilicity of rGO is reduced, but the chemical stability and mechanical properties are improved. So GO is more used in composites and biomedical fields, while rGO is more suitable for fields such as electronic devices and energy storage devices due to its better conductivity and chemical stability [9].
2. Methodology
2.1. Materials
This experiment used a 0.8-1.2nm nano sized graphene oxide aqueous solution (concentration: 1mg/ml) purchased by Xi'an Qiyue Biotechnology Co., Ltd.
2.2. Experimental procedure
Uniformly coat 4 glass substrates with graphene oxide (GO) solution, and divide them into two groups after drying:
Exposure group (2 pieces): Siemens Multix Select DR (40 kV, 100 mA, 1.0 mAs/time, SID=100 cm) was used for 4 X-ray exposures, with a 5-minute interval between each exposure (in a room temperature and ventilated environment).
Control group (2 pieces): placed in the same environment but not irradiated.
One sample per group was used for UV-Vis (monitoring π→π * transitions) and FTIR (analyzing functional groups) detection. All samples were subjected to spectral measurements before and after irradiation to track dynamic changes. "
3. RESULT
3.1. Results and analysis of UV-Vis
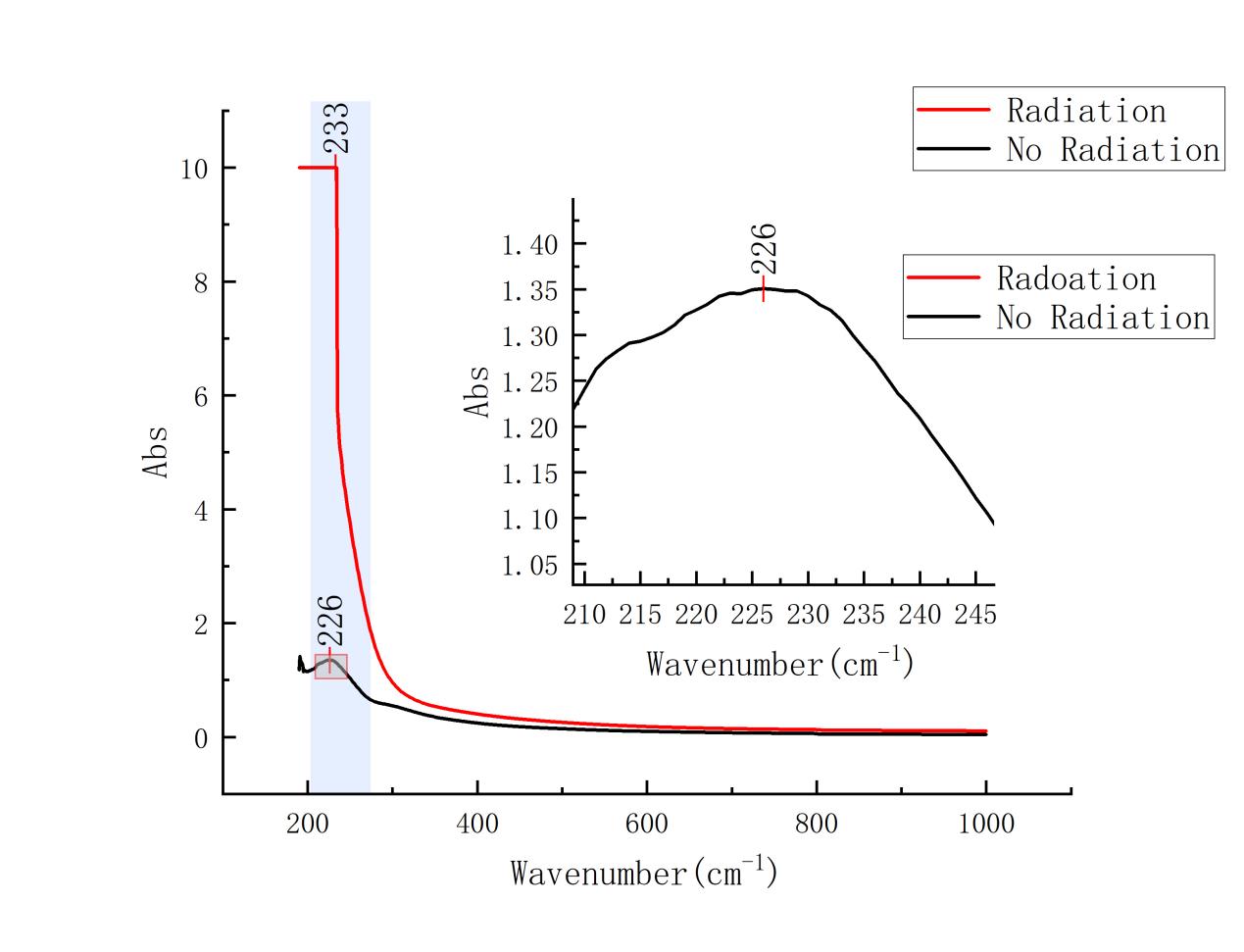
Figure 2: Comparative UV-Vis images of post-irradiation and unirradiated samples at a concentration of 1.0 mg/ml
The absorption peak after radiation treatment appeared at 234 nm. the absorption peak without radiation treatment appeared at 228 nm.
A slight change in the position of the absorption peaks after radiation treatment suggests a change in the GO structure, possibly due to a radiation-induced reduction reaction.
In Figure it is shown that the radiation-free sample has a high absorption at about 228 nm, while the radiation-treated sample has a diminished absorption around this wavelength. This change verifies that the radiation treatment led to the reduction of GO, reducing the oxygen functional groups (e.g., hydroxyl and carboxyl groups) and making the carbon backbone structure tend to be characterized by rGO.
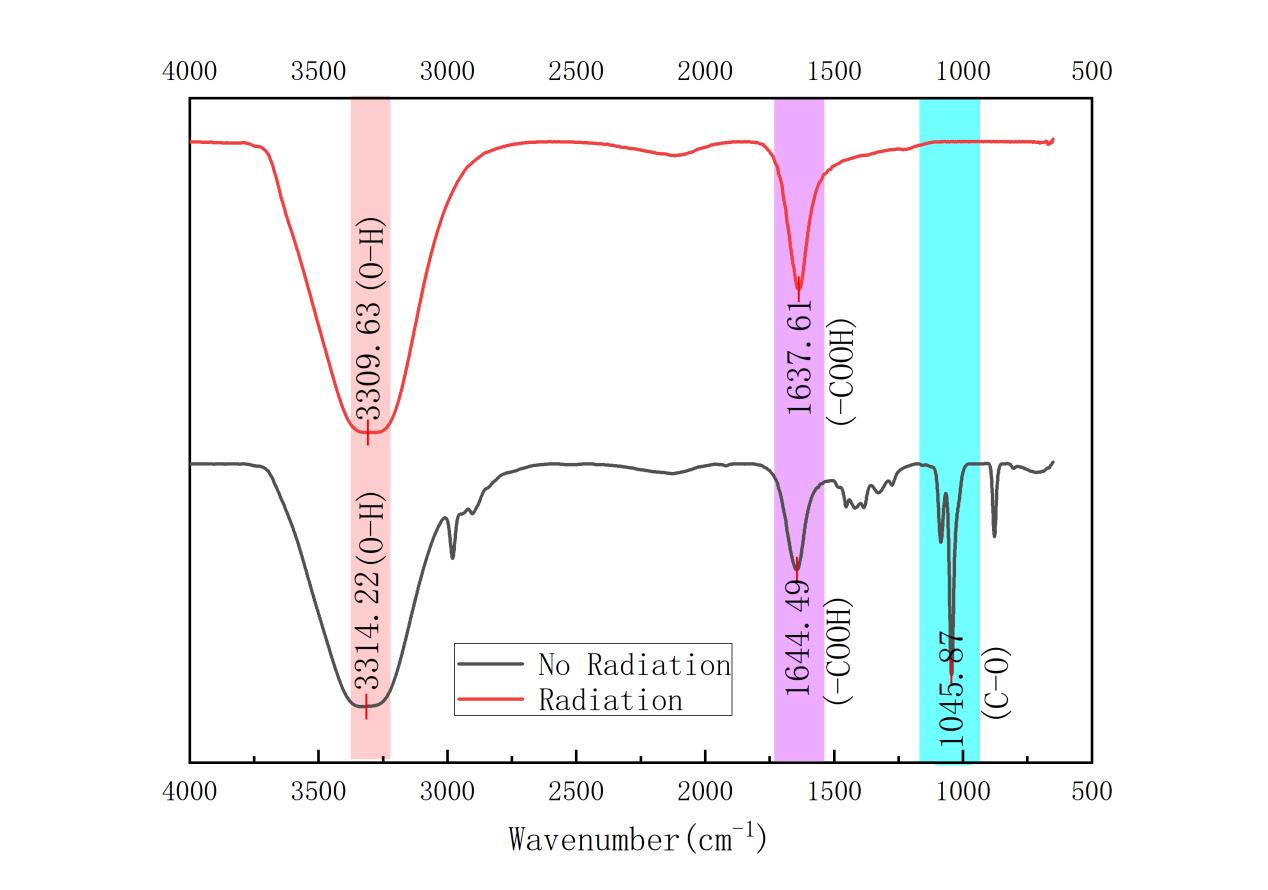
Figure 3: FTIR comparison images of post-irradiation and unirradiated samples at a concentration of 1.0 mg/ml
3.2. Results and analysis of FTIR
The absorption peaks at 3302 cm-1, 1641 cm-1, and 1044 cm-1 were weakened in the radiation-treated samples as compared to those without radiation. This may indicate that the radiation resulted in GO reduction, reducing oxygen functional groups (e.g., hydroxyl and carboxyl groups).
4. Discussion
The characteristic absorption peaks of GO usually appeared in the 230-240 nm wavelength region, corresponding to the π-π* leap.
The characteristic peaks of rGO usually appear in the wavelength region of 260-270 nm.
the samples with concentrations of 1.0 mg/ml were red-shifted. During the reduction process of GO, the oxygen-containing functional groups on the surface were removed, and the conjugated structure of the sp2 carbon atoms was restored, and the increase in this conjugated structure led to a decrease in the energy required for the electron leaps, resulting in a red-shift of the absorption peaks.
In the case of the samples, the absorption peaks appear to increase substantially, and when GO is reduced to rGO, the removal of the oxidation groups leads to an increase in the sp2 hybridization between the carbon atoms, which enhances the absorption of the π-π* leaps. This usually leads to an increase in absorption in a specific wavelength range.
Whereas rGO has a more complete conjugate structure than GO, the degree of electron delocalization is increased, leading to enhanced absorption in the visible and near-infrared regions. This enhanced absorptivity is usually expressed as an increase in absorbance.
The reduction process may lead to a decrease in the optical band gap of the material, allowing more photons to be absorbed by the material, resulting in an increase in absorbance. rGO's improved electrical conductivity also leads to an increase in absorbance, which also implies better absorptive properties. All of these reasons can lead to a large increase in the absorption peaks.
In the FTIR detection of GO, there are several major characteristic peaks of GO:
Hydroxyl group (-OH): in the range of 3200-3600 cm-1, there is usually a broad absorption peak indicating the presence of hydroxyl group.
Epoxy group (C-O): characteristic peaks in the range 1000-1300 cm-1 indicate the presence of an epoxy group.
Carboxy group (-COOH): a strong absorption peak occurs in the range of 1700-1750 cm-1 indicating the presence of carboxy group.
Carbon-carbon double bond(C=C): characteristic peaks in the range 1000-1300 cm-1 indicate the presence of a carbon-carbon double bond.
The main feature of rGO's on FTIR is the significant weakening or disappearance of the absorption peaks in the range of 3200-3600 cm-1 and 1700-1750 cm-1 in the FTIR spectrum of rGO as compared to GO, indicating the removal of hydroxyl and carboxyl groups. The absorption peaks in the range of 1000-1300 cm-1 are also attenuated, reflecting the removal of epoxy groups.
And on Figure 3, it can also be found that the absorption peaks in the range of 3200-3600 cm-1 and 1000-1300 cm-1 are significantly weakened or disappeared, which verifies the formation of reduced graphene oxide, as rGO is characterized by the reduction of oxygen-containing functional groups and the increase of carbon-carbon double bonds However, we did not find carbonyl group (C=O) at 1700-1750 cm-1.
Finally, the combined results from UV-Vis and FTIR spectroscopy confirm the hypothesis that X-ray irradiation induces the reduction of graphene oxide. The observed shifts in absorption peaks and the weakening of characteristic FTIR bands indicate that X-ray treatment reduces oxygen functional groups, transforming GO into rGO. This reduction is crucial for modifying the electronic properties of GO, potentially enhancing its conductivity and making it more suitable for applications requiring graphene-like materials.
In this experiment, the improvement needed is to increase the exposure time and intensity of the sample, but due to the use of medical X-ray machines, this may lead to a series of problems such as insufficient response. Instruments capable of long-term stable exposure should be used to ensure more thorough reaction of the sample.
Since only UV-Vis and FTIR were used to test the samples in this experiment, there may be some errors, for this problem we can use Ramon spectroscopy, Scanning Probe Microscopy (SPM), X-ray Photoelectron Spectroscopy (XPS), Scanning Photoelectron Microscopy (SPEM), etc., which can help us to determine whether or not the samples are generating rGO. this can help us to be more sure of the results of the experiment. This way, we can be more sure of the accuracy of the experimental results.
5. Conculsion
This study aims to investigate the effect of X-ray irradiation on graphene oxide (GO). From the data obtained by UV Vis, it was found that the sample with a concentration of 1.0mg/ml after irradiation showed a red shift and a significant increase in the absorption peak. In addition, the reduction of oxygen functional groups can confirm that the reduction reaction of GO has occurred, but it is uncertain whether rGO has been produced. Therefore, we need to perform Fourier transform infrared spectroscopy analysis on the irradiated samples.
After detecting the reduction of oxygen functional groups and the increase of C=C in the irradiated samples, it also indicates that the reduction reaction of the irradiated samples has taken place and rGO has been produced.
Finally, it is concluded that after a certain amount of X-ray irradiation, GO undergoes a reduction reaction and rGO is produced.
The significance of these findings lies in the potential applications of irradiated GO in various fields such as electronics, biomedicine, and environmental remediation.
References
[1]. Geim, A. K., & Novoselov, K. S. (2007). The rise of graphene. Nature Materials, 6(3), 183–191. https://doi.org/10.1038/nmat1849
[2]. Novoselov, K. S., Geim, A. K., Morozov, S. V., Jiang, D. E., Zhang, Y., Dubonos, S. V., ... & Firsov, A. A. (2004). Electric field effect in atomically thin carbon films. science, 306(5696), 666-669.
[3]. Mushahary, N., Sarkar, A., Basumatary, F., Brahma, S., Das, B., & Basumatary, S. (2024). Recent developments on graphene oxide and its composite materials: From fundamentals to applications in biodiesel synthesis, adsorption, photocatalysis, supercapacitors, sensors and antimicrobial activity. Results in Surfaces and Interfaces, 15, 100225. https://doi.org/https://doi.org/10.1016/j.rsurfi.2024.100225
[4]. Bai, L., Tajikfar, A., Tamjidi, S., Foroutan, R., & Esmaeili, H. (2021). Synthesis of MnFe2O4@graphene oxide catalyst for biodiesel production from waste edible oil [Article]. Renewable Energy, 170, 426-437. https://doi.org/10.1016/j.renene.2021.01.139
[5]. Wang, S. (2017). Reduction of graphene oxide by synchrotron radiation soft X-ray beam (Master’s thesis). Donghai University, Taichung City, Taiwan
[6]. Ito, J., Nakamura, J., & Natori, A. (2008). Journal of Applied Physics, 103, 113712
[7]. Zhu, H., Duan, Z., Zhang, L., & Yin, K. (2017). Review on preparation and structure of graphene oxide. *Materials Science and Technology, 25*(6), 82-88. https://doi.org/10.11951/j.issn.1005-0299.20160400
[8]. He, H., Klinowski, J., Forster, M., & Lerf, A. (1998). A model for graphite oxide [Image]. In Chemical Physics Letters, 287(1–2). Public Domain, Wikimedia Commons. https://commons.wikimedia.org/w/index.php?curid=18699429
[9]. Wang, X., Zhou, Y., Li, X., Huang, Y., Tan, Y., Liu, L., et al. (2024). Preparation of NiCo-LDHs@rGO composites with improved electrochemical performance by ammonia diffusion method under closed conditions. Chemical Physics Letters, 852, 141495. https://doi.org/10.1016/j.cplett.2024.141495
Cite this article
Wu,P.;Li,H. (2025). Structural and Optical Evolution of Graphene Oxide under X-Ray Irradiation: Insights from UV-Vis and FTIR Spectroscopy. Applied and Computational Engineering,143,94-99.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Functional Materials and Civil Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Geim, A. K., & Novoselov, K. S. (2007). The rise of graphene. Nature Materials, 6(3), 183–191. https://doi.org/10.1038/nmat1849
[2]. Novoselov, K. S., Geim, A. K., Morozov, S. V., Jiang, D. E., Zhang, Y., Dubonos, S. V., ... & Firsov, A. A. (2004). Electric field effect in atomically thin carbon films. science, 306(5696), 666-669.
[3]. Mushahary, N., Sarkar, A., Basumatary, F., Brahma, S., Das, B., & Basumatary, S. (2024). Recent developments on graphene oxide and its composite materials: From fundamentals to applications in biodiesel synthesis, adsorption, photocatalysis, supercapacitors, sensors and antimicrobial activity. Results in Surfaces and Interfaces, 15, 100225. https://doi.org/https://doi.org/10.1016/j.rsurfi.2024.100225
[4]. Bai, L., Tajikfar, A., Tamjidi, S., Foroutan, R., & Esmaeili, H. (2021). Synthesis of MnFe2O4@graphene oxide catalyst for biodiesel production from waste edible oil [Article]. Renewable Energy, 170, 426-437. https://doi.org/10.1016/j.renene.2021.01.139
[5]. Wang, S. (2017). Reduction of graphene oxide by synchrotron radiation soft X-ray beam (Master’s thesis). Donghai University, Taichung City, Taiwan
[6]. Ito, J., Nakamura, J., & Natori, A. (2008). Journal of Applied Physics, 103, 113712
[7]. Zhu, H., Duan, Z., Zhang, L., & Yin, K. (2017). Review on preparation and structure of graphene oxide. *Materials Science and Technology, 25*(6), 82-88. https://doi.org/10.11951/j.issn.1005-0299.20160400
[8]. He, H., Klinowski, J., Forster, M., & Lerf, A. (1998). A model for graphite oxide [Image]. In Chemical Physics Letters, 287(1–2). Public Domain, Wikimedia Commons. https://commons.wikimedia.org/w/index.php?curid=18699429
[9]. Wang, X., Zhou, Y., Li, X., Huang, Y., Tan, Y., Liu, L., et al. (2024). Preparation of NiCo-LDHs@rGO composites with improved electrochemical performance by ammonia diffusion method under closed conditions. Chemical Physics Letters, 852, 141495. https://doi.org/10.1016/j.cplett.2024.141495









