1. Introduction
Industrialization and urbanization have led to massive discharges of heavy metals and organic pollutants (e.g., PPCPs, antibiotics), with conventional water treatment technologies struggling to efficiently remove low-concentration contaminants. Adsorption techniques have emerged as a core solution due to their operational simplicity, low cost, and absence of secondary pollution. Biochar-based materials have demonstrated particularly significant potential, showing an application growth rate exceeding 40% over the past decade [1, 2].
Sun Xin et al. highlighted that utilizing agricultural waste (e.g., rice husks, straw, sheep manure) for biochar production achieves carbon fixation efficiency surpassing 50% while reducing CO₂ emissions by >60% compared to conventional activated carbon processes, underscoring its low-carbon and eco-friendly advantages [2]. Research of Lu et al. and G Enaime et al. revealed through pyrolysis temperature regulation experiments that biochar pyrolyzed below 500°C retains abundant oxygen-containing functional groups (-COOH, -OH), enhancing its adsorption capacity for heavy metal ions. Conversely, pyrolysis above 600°C promotes dominant aromatic structures that preferentially adsorb organic pollutants [3, 4]. However, Xiao Yang et al. reported critical limitations of unmodified biochar: phosphorus adsorption capacity remains only 1.2–8.7 mg/g, while the maximum adsorption capacity for cadmium ions (Cd²⁺) is typically <20 mg/g [5, 6], clearly indicating the necessity of material modification to enhance adsorption performance.
Existing studies confirm that biochar adsorption mechanisms exhibit significant multi-pathway synergistic characteristics involving physical adsorption, chemical complexation, and ion exchange [7, 8]. Nevertheless, in actual complex water bodies, the coexistence of multiple pollutants triggers competitive effects, making it difficult to identify dominant adsorption pathways. Concurrently, a prominent gap exists in engineering-scale research data. Statistics from references [1, 7] indicate that ~95% of studies are confined to laboratory batch experiments, lacking validation in continuous-flow reactor systems or real water treatment scenarios. Furthermore, regeneration technology faces critical bottlenecks: acid regeneration causes >15% leaching of metal modifiers [9], while thermal regeneration consumes up to 4.2 kWh/m3, severely restricting engineering applications [8].
This review comprehensively analyzes performance optimization strategies for biochar modification technologies and the intrinsic mechanisms governing their adsorption efficacy. By elucidating synergistic removal patterns for heavy metals and organic pollutants, this work establishes a theoretical foundation for overcoming barriers to large-scale engineering implementation.
2. Adsorption mechanism
Biochar is produced by heating biomass feedstock under anaerobic conditions (pyrolysis). Its raw materials are widely available and low-cost, mainly derived from agricultural crop straw, bagasse, seed shells, animal manure, and distiller's grain s from agricultural production, among other plant and animal wastes [10, 11]. The adsorption performance of biochar for pollutants in water is not determined by a single mechanism.And adsorption is not determined solely by a single factor but is a complex process jointly governed by the intrinsic physicochemical structural characteristics of biochar and the properties of pollutants. Core structural parameters, such as specific surface area, pore structure characteristics, and the types and abundance of surface functional groups, are key foundations affecting adsorption capacity [3, 4].
Studies have shown that pyrolysis temperature, as a critical preparation parameter, can precisely regulate the surface properties of biochar: high-temperature pyrolysis (>600℃) tends to form highly aromatic, hydrophobic structures, which favor the adsorption of organic pollutants (such as methyl orange) through hydrophobic interactions and π-π stacking [3,4]; whereas lower pyrolysis temperatures ( < 500℃) help retain abundant oxygen-containing functional groups (such as -COOH, OH), which significantly enhance affinity for heavy metal ions through chemical actions such as electrostatic attraction, complexation (chelation), ion exchange (such as K⁺/Na⁺ replacing Cd²⁺/Pb²⁺) [3, 12, 13].
The selectivity of adsorption mechanisms also shows significant raw material dependence. For example, halophyte biochar (such as European saltwort) achieves efficient removal of ca2t mainly due to carbonate precipitation mechanisms induced by its rich inorganic components [13], while corn stalk biochar relies more on ion exch ange processes [12]. For organic pollutants like antibiotics, hydrogen bonding (-OH···O=C) and pore filling together constitute the main adsorption pathways [14, 15], whereas dissolved organic matter (DOM) can be chemically adsorbed through electron transfer mechanisms on biochar Fe-N-C [8]. Therefore, the adsorption mechanism of biochar is a synergistic result of physical adsorption (pore filling, van der Waals forces) and multiple chemical interactions (complexation, precipitation, ion exchange, electrostatic interactions, hydrogen bonding, T-T interactions), with the dominant path ways jointly regulated by the properties of biochar itself (raw materials, pyrolysis conditions) and the characteristics of tar get pollutants [3, 4, 13].
3. Biochar modification technology
Biochar modification technologies aim to enhance its adsorption capacity and selectivity for pollutants through physical, chemical, biological, and composite methods. Physical modification, such as pyrolysis regulation, can optimize pore structure, and steam activation can expand mesopore volume [3, 12, 16]. Chemical modification includes various methods, such as acid/base treatment, metal loading to impart magnetic separation functionality, heteroatom doping to enhance DOM adsorption via electron transfer, and surface oxidation to introduce carboxyl groups for reinforcement [8, 9, 16, 17]. Biological modification involves microbial colonization to form biofilms, producing coupled adsorption and degradation effects [3,14]. Composite modification integrates physical pore expansion and chemical activation to enhance material stability [9].
3.1. Physical modification
Two physical modification methods are generally pyrolysis regulation and steam activation. Pyrolysis in pyrolysis regulation refers to the thermochemical process of converting biomass at high temperatures under oxygen-limited conditions. Its core feature is the directional regulation of the physicochemical properties of biochar by precisely controlling temperature parameters [3]. For example, after pyrolyzing corn stalks at roo-c for 2 hours, their specific surface area significantly in creased from 80 m2/g to 310 m2/g, and their adsorption capacity for Cd2+also increased by 2.3 times [12]. However, high-temperature pyrolysis has limitations, as it may cause degradation of surface functional groups on biochar, thereby weakening its adsorption capacity for polar pollutants.
Steam activation is a process of physical etching of pre-carbonized biochar by introducing steam in an inert atmosphere at high temperature [3,16]. Xiao Guangli et al. used bamboo sawdust biochar as the subject, introducing steam for continuous etching for 1 hour, after pore expansion the mesopore volume increased by 47%, significantly optimizing the mass transfer pathway; The adsorption capacity of the modified carbon for Cd2+ reached 45.6 mg/g after adsorption verification, and since no chemical reagents were introduced throughout the process, the risk of secondary pollution was avoided, while achieving precise control of the mesoporous structure [16].
3.2. Chemical modification
As shown in Table 1, chemical modification significantly enhances the affinity of biochar for specific pollutants by directionally modifying the surface chemical properties. Acid/base treatment can greatly increase the surface negative charge density, thereby strengthening electrostatic attraction and ion exchange capacity; however, strong acid etching may damage the microporous structure, leading to the loss of physical adsorption sites [2, 17]. The metal loading strategy not only introduces magnetism for easy separation and recovery but also efficiently captures heavy metals through hydroxyl coordination on the surface of metal ox ides. Nevertheless, excessive metal leaching in acidic environments poses a risk of secondary pollution [9]. Heteroatom doping enhances electron transfer ability by forming active sites such as pyridinic nitrogen, increasing the adsorption capacity of the material for dissolved organic matter (DOM), but the increased cost of reagents limits large-scale application [8]. Surface oxidation modification selectively introduces oxygen-containing functional groups such as carboxyl groups to promote complexation adsorption, but high-temperature oxidation simultaneously leads to a decrease in specific surface area, requiring a balance between chemical activity and physical structural stability [16]. In summary, the core value of chemical modification lies in the precise regulation of surface chemical sites, but its enhancement effects are often accompanied by structural sacrifice or environmental risks, necessitating the optimization of process parameters based on raw material characteristics and target pollutants.
|
Types of modification |
Specific operations |
Adsorption enhancement |
Limitations |
|
Acid/Base Treatment |
Soaked in1M H2SO4 or NaOH for 24 hours, then neutralized and washed [17] |
Surface negative charge density increased by 2 times, Pb2+ adsorption capacity +180% [17] |
Acid treatment destroys the microporous structure [2] |
|
Metal Loading [9] |
Co-precipitation method:FeCl3/FeCl2(molar ratio 2:1) loading, ammonia precipitation to formFe3O4 |
The adsorption capacity of magnetic rice husk charcoal for Cd2+ reaches 89.3 mg/g |
Fe leaching rate >10 (pH <3) |
|
Hetero atom doping [8] |
Nitrogen doping: Urea mixed with biochar (mass ratio 1:2), pyrolyzed for 1 hour |
Fe - N - increased the adsorption capacity for DOM to 210 mg/g |
Reagent costs increased by 30% |
|
Surface oxidation modification [16] |
Hot air oxidation: 300℃ air atmosphere treatment for 2 hours, introducing carboxyl groups |
The adsorption capacity increased to 38.7 mg/g |
Specific surface area decreased by 20% |
3.3. Biological modification
The surface of biochar can create a suitable environment for microorganisms to colonize, allowing them to form stable bio film structures, thereby enhancing the synergistic effects in the biodegradation process [3]. In addition, Duan Xuejun et al. confirmed through white rot fungus treatment of biochar that biochar materials modified by white rot fungus showed a significant improvement in the removal efficiency of tetracycline antibiotics, reaching a removal rate of 95%. Among this, the contribution of degradation to the overall removal effect exceeded 40% [14]. However, G Enaime et al. further reported that when the chemical oxygen demand (COD) of the water body exceeds 500 mg/L , toxic effects cause a significant decline or even inactivation of microbial activity, and this limitation severely restricts the application of this technology in complex wastewater [3].
3.4. Composite modification
Composite modification technology combines physical and chemical modification methods (such as steam activation combined with iron loading [16,9]) to synergistically enhance the adsorption performance and material stability of biochar. This combined strategy effectively overcomes the limitations of single modification methods. A typical successful case is the treatment process of rice husk biochar: first, pore expansion through steam activation, followed by loading with magnetite (Fe3O4), ultimately achieving an adsorption capacity for cadmium ions (Cd2+) of 112.4 mg/g [9].
Notably, this adsorption capacity is increased by approximately 40% compared to a single modification method (whether it is steam activation alone or Fe3O4 loading alone) [9]. This significant synergistic effect arises from the complementary effects of different modification techniques—physical modification (such as steam activation) primarily optimizes the pore structure of the material to provide greater adsorption space and mass transfer channels, while chemical modification (such as metal loading) introduces abundant active sites to enhance the chemical affinity for target pollutants, thereby achieving a dual improvement in adsorption capacity and material stability.
4. Feedstock influence
The intrinsic properties of biochar feedstock have a decisive impact on its modification effectiveness, making the targeted selection of modification strategies crucial. For feedstocks with relatively low specific surface area (such as rice husks), their initial adsorption capacity is limited; in this case, modification methods that expand pores and increase capacity are most effective, such as steam activation or metal loading. For example, rice husk biochar modified by loading with ferric oxide (Fe3O4) significantly enhanced its adsorption capacity for cadmium ions (Cd2+), with the adsorption amount increasing by as much as 400% times [9].
For high-ash raw materials, their abundant inorganic mineral components may hinder the effective binding of modifiers or the coverage of active sites, making the pretreatment step very important. In particular, "acid washing to remove ash" has become a key prerequisite for enhancing subsequent modification effects. Research has confirmed that after effectively removing ash impurities, further heteroatom doping such as nitrogen doping can be carried out.
Nitrogen-doped modification can further enhance the adsorption performance by an additional 25% [8]. Notably, halophyte raw materials (such as European glasswort) exhibit unique advantages. These plants are naturally rich in specific minerals or possess special cellular structures, enabling their biochar to demonstrate excellent adsorption performance through direct pyrolysis without any additional chemical or physical modification. Experimental data show that the adsorption capacity of European glasswort biochar for Cd2+ can reach as high as 108.54 mg/g [13]. Therefore, a thorough understanding of the inherent properties of the raw materials (specific surface area, ash content, special components) is fundamental to designing and optimizing biochar modification processes for efficient pollutant removal.
5. Analysis of pollutant adsorption mechanisms and regulation patterns
Heavy metal adsorption relies on the synergy of precipitation, ion exchange, and complexation (e.g., precipitation dominates in halophytes, ion exchange dominates in corn stalks) [12, 13], while pH is a key switch for heavy metal adsorption [2, 17]; organic pollutant adsorption depends on molecular interaction matching (hydrophobic/ π-π interactions for dyes, hydrogen bonding for antibiotics) [3, 4, 18], and pore size and surface chemistry determine the efficiency of organic pollutant capture [8, 14, 15].
5.1. Dominant mechanisms for heavy metal removal
The adsorption pathways of Cd²⁺ show significant raw material dependence: halophyte biochar (such as European saltgrass) mainly relies on carbonate precipitation to achieve the removal rate of >60% [13], while corn stalk biochar primarily depends on K⁺/Na⁺ ion exchange (accounting for 45%) [12]; when the pH value is in the range of 5-7, precipitation and complexation synergistically enhance adsorption, whereas at PH<4, H+competition leads to adsorption inhibition [19]; modification strategies can significantly improve performance, such as phosphate-modified apricot kernel biochar achieving an adsorption capacity of Cd²⁺ for 105.9 mg/g, approximately 12 times that of unmodified materials [18].For Pb²+ , biochar loaded with magnetite(Fe3O4@BC) combines the advantages of magnetic separation and surface hydroxyl complexation, with an adsorption capacity as high as 345 mg/g [18]; nano metal oxides such as MnO2 and MgO modified biochar remove more than 90% of Pb2+ within 3 minutes in actual wastewater through inner-sphere complexation [18].
5.2. Structural adaptation mechanism of organic pollutants
Dye pollutants (such as methyl orange) are mainly adsorbed through hydrophobic interactions and π-π stacking. The graphitized structure formed by high-temperature pyrolysis (around 700°C) (such as sheep manure biochar) can enhance this mechanism [4]. The adsorption of antibiotics (such as tetracycline) relies on the synergistic effect of hydrogen bonding (H-bonds) and pore filling. Optimizing the pore structure can significantly improve mass transfer efficiency, as white rot fungus-modified biochar utilizes this mechanism to achieve a tetracycline removal rate of over 90% [14,15]. The removal of pharmaceuticals and personal care products (PPCPs) is mainly dominated by electrostatic interactions and van der Waals forces. The negative charges introduced by surface oxidation modification can enhance the electrostatic attraction to positively charged PPCP molecules under neutral pH conditions [15].
The deep removal of dissolved organic matter (DOM) depends on chemisorption via electron transfer. Nitrogen and sulfur co-doped biochar increases the electron density by raising the pyridinic nitrogen content, thereby enhancing the adsorption capacity for DOM [8]. A notable breakthrough is that nitrogen-doped biochar shows unconventional selective adsorption for cadmium (Cd2+) (although a heavy metal, the mechanism involves electron transfer). After modification with ethylene diamine tetraacetic acid (EDTA), the Cd adsorption capacity increased by about three times, attributed to the ultra-high stability constant of the Cd(II)-EDTA complex [18]. In summary, targeted modification based on the structure-activity characteristics of pollutant molecules is the core approach to overcoming the adsorption bottlenecks of biochar.
5.3. Environmental interference factors
In actual water treatment environments, various interfering factors significantly affect the adsorption efficiency of biochar for target pollutants. Firstly, competitive adsorption of coexisting ions is a common challenge. For example, when the cont ent of Cd2+ in the water exceeds 200 mg/L, the intense competition for adsorption sites leads to a significant decrease in the adsorption capacity of biochar for Cd2+, with the adsorption amount dropping by as much as 35% [19]. This inhibitory effect is particularly pronounced in high-hardness water. Secondly, temperature effects are also important regulatory variables. The thermodynamic characteristics of the adsorption process determine the impact of temperature changes. For instance, the adsorption enthalpy change (ΔH) of methyl orange was measured to be +45.2 KJ/mol, indicating an endothermic process [4]. Therefore, in practical applications, when the temperature rises from the common 25℃ to 45℃, the adsorption amount of methyl orange by biochar increases accordingly, with an increase of up to 22% [4]. This suggests that for endothermic adsorption processes, appropriately raising the water temperature may be beneficial for improving efficiency.
However, the complex composition of actual water bodies often presents the most severe challenges. For example, dissolved organic matter (DOM), commonly present in secondary effluent from wastewater treatment plants, due to its diverse structure, high concentration, and strong interactions with the biochar surface, competes significantly with target pollutants (such as Cd2+) for adsorption. Experimental data clearly reveal this dilemma: in actual secondary effluent containing DOM, even high-performance modified biochar experiences a "halving" of its adsorption capacity for Ca2+ , with adsorption amounts reduced by about 50% compared to ideal laboratory conditions [8].
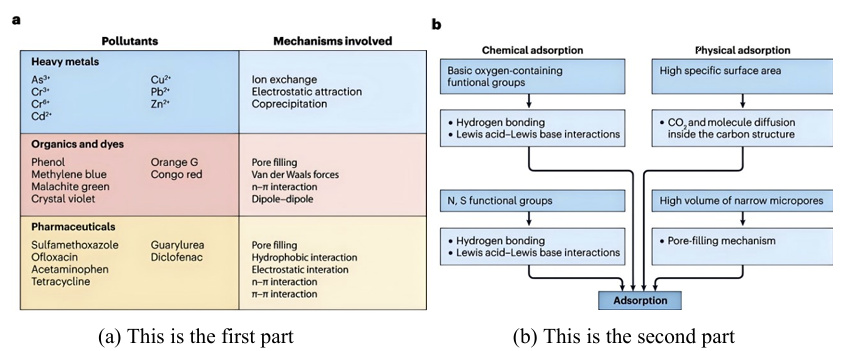
As shown in figure 1, the adsorption mechanisms of heavy metals, organic matter, dyes, and pharmaceuticals onto biochar involve various pathways such as ion exchange, electrostatic attraction, pore filling, van der Waals forces, n-π interactions, and hydrogen bonding. In complex water bodies, competition among these mechanisms may occur, leading to decreased adsorption efficiency [20].
This fully illustrates the enormous challenges posed by complex water quality compositions in practical application scenarios, and the limitations of relying solely on data from pure laboratory water systems to evaluate adsorbent performance.
6. Engineering application
Scaling up biochar from laboratory research to large-scale engineering applications faces multiple challenges beyond addressing the aforementioned interfering factors in complex water bodies, including material stability, competitive adsorption behavior, and cost-effectiveness. Cheng et al. pointed out that unmodified biochar generally suffers from low adsorption capacity and poor selectivity [21]. Preparation parameters such as pyrolysis temperature significantly regulate its stability: biochar obtained from low-temperature pyrolysis exhibits underdeveloped pore structures and limited functional groups, resulting in poor adsorption performance [22]; Ma et al. further emphasized that biochar is prone to aging in complex aquatic environments, affecting its long-term adsorption efficiency and carbon sequestration potential [23]. To enhance stability, Meng et al. developed polyurethane cross-linked modified biochar (PCB), verifying its ability to enhance hydrological performance and nutrient removal capacity [24]. In practical wastewater systems with coexisting multiple pollutants, competitive adsorption behavior significantly constrains the efficacy of biochar. Wu et al. reported that cations such as Cu²⁺, Pb²⁺, and Zn²⁺ compete for limited adsorption sites, markedly inhibiting the removal rate of individual metal ions [25]. The complexity of this competition mechanism makes predicting and optimizing actual performance extremely difficult. Currently, some studies attempt to enhance the adsorption selectivity of biochar in multicomponent systems through surface modification.
Cost-effectiveness is another critical bottleneck affecting the large-scale application of biochar. Cheng et al. and Wu et al. indicated that although biochar is considered a low-cost adsorbent, the economic feasibility of its modification and application processes still faces challenges. Ma et al. found that complex chemical modifications can significantly increase production costs; An et al. suggested that although certain modifications can enhance performance, their industrial economics still require systematic evaluation [26]. Furthermore, issues related to the separation, recovery, and disposal of post-adsorption materials also constrain the overall techno-economic feasibility. It is necessary to incorporate full life cycle analysis to promote alignment with the circular economy concept, considering the costs and environmental impacts throughout the product's entire life cycle [27,28].
7. Conclusion
This review comprehensively examines the adsorption mechanisms and modification strategies of biochar for removing heavy metals and organic pollutants from wastewater. As a sustainable adsorbent derived from agricultural waste, biochar demonstrates tunable surface properties through pyrolysis temperature control: low-temperature biochar ( <500°C) favors heavy metal adsorption via oxygen-containing functional groups, while high-temperature biochar ( >600°C) enhances organic pollutant removal through aromatic structures. Modification techniques—including physical, chemical, biological, and hybrid methods—significantly improve adsorption capacity and selectivity, yet face challenges such as structural trade-offs and economic feasibility. Competitive adsorption in complex water matrices, limited engineering-scale validation (95% of studies remain lab-based), and inefficient regeneration methods currently hinder large-scale application. Future efforts should focus on developing composite materials, optimizing operation parameters via computational modeling, and establishing standardized evaluation frameworks to bridge the gap between laboratory research and real-world implementation.
Contribution
All the authors contributed equally and their names were listed in alphabetical order.
References
[1]. Wang, X., Guo, Z., Hu, Z. and Zhang, J. (2020). Recent advances in biochar application for water and wastewater treatment: a review. PeerJ, 8, e9164.
[2]. Sun, X., Chen, L., Liu, Q., Chen, B., Cheng, D., Liu, Y. and Lin, Y. (2025). Research Progress on the Application of Modified Biochar in Sewage Treatment. In Henan Chemical Industry (CNKI; Vol. 42, Issue 06, pp. 1-4+8).
[3]. Enaime, G., Baçaoui, A., Yaacoubi, A. and Lübken, M. (2020). Biochar for Wastewater Treatment—Conversion Technologies and Applications. Applied Sciences, 10, 3492.
[4]. Lu, Y., Chen, J., Bai, Y., Gao, J. and Peng, M. (2019). Adsorption Properties of Methyl Orange in Waterby Sheep Manure Biochar. Polish Journal of Environmental Studies, 28, 3791–3797.
[5]. Geng, Q. and Zhang, S. (2022). Application Status and Development Trends of Biochar for Cadmium Removal from Water. In Resources Economization & Environmental Protection (CNKI; Issue 12, pp. 87–90).
[6]. Xiao, Y., Qi, J., Lei, X., Wang, Q. and Ma, Z. (2025). Advances in the mechanism and regeneration application of biochar adsorption for phosphorus removal. Journal of Functional Materials, 56, 2040–2049.
[7]. Han, M., Liu, Z., Huang, S., Zhang, H., Yang, H., Liu, Y., Zhang, K. and Zeng, Y. (2024). Application of Biochar-Based Materials for Effective Pollutant Removal in Wastewater Treatment. Nanomaterials, 14, 1933.
[8]. Wu, C., Xu, L., Jin, X., Shi, X. and Jin, P. (2022). Adsorption Characteristics and Long-term Effectiveness Evaluation of Iron-nitrogen Co-doped Biochar for Secondary Water-Soluble Organic Matter. In Environmental Science (CNKI; Vol. 43, Issue 01, pp. 398–408).
[9]. Hu, S., Yang, J., Yang, B., Wang, J., Zhou, S., Lei, Z. and Luo, Y. (2022). Research Progress of Rice Husk Based Materials in the Field of Water Pollution Control. Materials Reports, 36, 49–59.
[10]. Hoslett, J., Ghazal, H., Ahmad, D. and Jouhara, H. (2019). Removal of copper ions from aqueous solution using low temperature biochar derived from the pyrolysis of municipal solid waste. Science of The Total Environment, 673, 777–789.
[11]. Tan, X., Liu, Y., Zeng, G., Wang, X., Hu, X., Gu, Y. and Yang, Z. (2015). Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere, 125, 70–85.
[12]. Ding, J., Tian, Y., Lu, T. and Kang, J. (2021). Study on the adsorption of heavy metals by biochar modified with different modifiers under room temperature. E3S Web of Conferences, 293, 03014.
[13]. Ge, S., Zhao, S., Wang, L., Zhao, Z., Wang, S. and Tian, C. (2024). Exploring adsorption capacity and mechanisms involved in cadmium removal from aqueous solutions by biochar derived from euhalophyte. Scientific Reports, 14, 450.
[14]. Duan, X., Mao, Y., Du, Y., Dou, Y., Ao, T., Liang, X. and Zeng, H. (2025). Research progress on the application of biochar for antibiotic removal from water. Journal of Zhongyuan University of Technology, 36, 63-71+80.
[15]. Wang, X., Gong, C., Lai, L. and Cheng, J. (2022). Research progress of four new modified adsorbents for removal of PPCPs in water. In Industrial Water Treatment (CNKI; Vol. 42, Issue 09, pp. 23–37).
[16]. Xiao, G., Guo, X., Han, X., Zhang, K., Zhu, S., Gou, Q., Xu, Q. and Huang, H. (2021). Study on Adsorption Characteristics of Cadmium by Thermal Air Oxidation Modified Biochar. In Southwest China Journal of Agricultural Sciences (CNKI; Vol. 34, Issue 12, pp. 2765–2774).
[17]. Ren, Z., Yin, H., Wang, Q., Hou, X., Lu, L., Ren, T. and Lyu, L. (2025). Research progress of chemically modified biochar and its application in water treatment field. In Industrial Water Treatment (CNKI; Vol. 45, Issue 06, pp. 98–106).
[18]. Zhang, A., Li, X., Xing, J. and Xu, G. (2020). Adsorption of potentially toxic elements in water by modified biochar: A review. Journal of Environmental Chemical Engineering, 8, 104196.
[19]. Li, Y., Peng, L. and Li, W. (2020). Adsorption behaviors on trace Pb2+ from water of biochar adsorbents from konjac starch. Adsorption Science & Technology, 38, 344–356.
[20]. Mohanty, A. K., Vivekanandhan, S., Das, O., Millán, L. M. R., Klinghoffer, N. B., Nzihou, A. and Misra, M. Biocarbon materials.
[21]. Cheng, N., Wang, B., Wu, P., Lee, X., Xing, Y., Chen, M. and Gao, B. (2021). Adsorption of emerging contaminants from water and wastewater by modified biochar: A review. Environmental Pollution, 273, 116448.
[22]. Ranjbar, A., Heidarpour, M., Eslamian, S. and Shirvani, M. (2022). Investigating the performance of adsorbents made from the canola stalk for the removal of lead from aqueous solutions. Arabian Journal of Geosciences, 15, 1565.
[23]. Ma, Z., Zheng, D., Liang, B. and Li, H. (2025). Effect of vermiculite-modified biochar on carbon sequestration potential, mercury adsorption stability, and economics. Biomass Conversion and Biorefinery, 15, 9513–9529.
[24]. Meng, Y., Wang, Y., Li, M., Feng, D. and Ren, J. (2025). Enhanced hydrologic performance and nutrient removal of bioretention systems modified with polyurethane-biochar crosslinked material (PCB). Journal of Environmental Management, 393, 127070.
[25]. Wu, Q., Dong, S., Wang, L. and Li, X. (2021). Single and Competitive Adsorption Behaviors of Cu2+, Pb2+ and Zn2+ on the Biochar and Magnetic Biochar of Pomelo Peel in Aqueous Solution. Water, 13, 868.
[26]. An, Y., Lu, J., Niu, R., Li, M., Zhao, X., Huang, X., Huang, H., Garg, A. and Zhussupbekov, A. (2023). Exploring effects of novel chemical modification of biochar on soil water retention and crack suppression: towards commercialization of production of biochar for soil remediation. Biomass Conversion and Biorefinery, 13, 13897–13910.
[27]. Feldman, J., Seligmann, H., King, S., Flynn, M., Shelley, T., Helwig, A. and Burey, P. (Polly). (2024). Circular economy barriers in Australia: How to translate theory into practice? Sustainable Production and Consumption, 45, 582–597.
[28]. Kulakovskaya, A., Wiprächtiger, M., Knoeri, C. and Bening, C. R. (2023). Integrated environmental-economic circular economy assessment: Application to the case of expanded polystyrene. Resources, Conservation and Recycling, 197, 107069.
Cite this article
Deng,B.;Su,Y. (2025). Adsorption Mechanism in Water Treatment by Biochar and Its Modified Materials: A Review. Applied and Computational Engineering,180,191-200.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of CONF-MCEE 2026 Symposium: Advances in Sustainable Aviation and Aerospace Vehicle Automation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Wang, X., Guo, Z., Hu, Z. and Zhang, J. (2020). Recent advances in biochar application for water and wastewater treatment: a review. PeerJ, 8, e9164.
[2]. Sun, X., Chen, L., Liu, Q., Chen, B., Cheng, D., Liu, Y. and Lin, Y. (2025). Research Progress on the Application of Modified Biochar in Sewage Treatment. In Henan Chemical Industry (CNKI; Vol. 42, Issue 06, pp. 1-4+8).
[3]. Enaime, G., Baçaoui, A., Yaacoubi, A. and Lübken, M. (2020). Biochar for Wastewater Treatment—Conversion Technologies and Applications. Applied Sciences, 10, 3492.
[4]. Lu, Y., Chen, J., Bai, Y., Gao, J. and Peng, M. (2019). Adsorption Properties of Methyl Orange in Waterby Sheep Manure Biochar. Polish Journal of Environmental Studies, 28, 3791–3797.
[5]. Geng, Q. and Zhang, S. (2022). Application Status and Development Trends of Biochar for Cadmium Removal from Water. In Resources Economization & Environmental Protection (CNKI; Issue 12, pp. 87–90).
[6]. Xiao, Y., Qi, J., Lei, X., Wang, Q. and Ma, Z. (2025). Advances in the mechanism and regeneration application of biochar adsorption for phosphorus removal. Journal of Functional Materials, 56, 2040–2049.
[7]. Han, M., Liu, Z., Huang, S., Zhang, H., Yang, H., Liu, Y., Zhang, K. and Zeng, Y. (2024). Application of Biochar-Based Materials for Effective Pollutant Removal in Wastewater Treatment. Nanomaterials, 14, 1933.
[8]. Wu, C., Xu, L., Jin, X., Shi, X. and Jin, P. (2022). Adsorption Characteristics and Long-term Effectiveness Evaluation of Iron-nitrogen Co-doped Biochar for Secondary Water-Soluble Organic Matter. In Environmental Science (CNKI; Vol. 43, Issue 01, pp. 398–408).
[9]. Hu, S., Yang, J., Yang, B., Wang, J., Zhou, S., Lei, Z. and Luo, Y. (2022). Research Progress of Rice Husk Based Materials in the Field of Water Pollution Control. Materials Reports, 36, 49–59.
[10]. Hoslett, J., Ghazal, H., Ahmad, D. and Jouhara, H. (2019). Removal of copper ions from aqueous solution using low temperature biochar derived from the pyrolysis of municipal solid waste. Science of The Total Environment, 673, 777–789.
[11]. Tan, X., Liu, Y., Zeng, G., Wang, X., Hu, X., Gu, Y. and Yang, Z. (2015). Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere, 125, 70–85.
[12]. Ding, J., Tian, Y., Lu, T. and Kang, J. (2021). Study on the adsorption of heavy metals by biochar modified with different modifiers under room temperature. E3S Web of Conferences, 293, 03014.
[13]. Ge, S., Zhao, S., Wang, L., Zhao, Z., Wang, S. and Tian, C. (2024). Exploring adsorption capacity and mechanisms involved in cadmium removal from aqueous solutions by biochar derived from euhalophyte. Scientific Reports, 14, 450.
[14]. Duan, X., Mao, Y., Du, Y., Dou, Y., Ao, T., Liang, X. and Zeng, H. (2025). Research progress on the application of biochar for antibiotic removal from water. Journal of Zhongyuan University of Technology, 36, 63-71+80.
[15]. Wang, X., Gong, C., Lai, L. and Cheng, J. (2022). Research progress of four new modified adsorbents for removal of PPCPs in water. In Industrial Water Treatment (CNKI; Vol. 42, Issue 09, pp. 23–37).
[16]. Xiao, G., Guo, X., Han, X., Zhang, K., Zhu, S., Gou, Q., Xu, Q. and Huang, H. (2021). Study on Adsorption Characteristics of Cadmium by Thermal Air Oxidation Modified Biochar. In Southwest China Journal of Agricultural Sciences (CNKI; Vol. 34, Issue 12, pp. 2765–2774).
[17]. Ren, Z., Yin, H., Wang, Q., Hou, X., Lu, L., Ren, T. and Lyu, L. (2025). Research progress of chemically modified biochar and its application in water treatment field. In Industrial Water Treatment (CNKI; Vol. 45, Issue 06, pp. 98–106).
[18]. Zhang, A., Li, X., Xing, J. and Xu, G. (2020). Adsorption of potentially toxic elements in water by modified biochar: A review. Journal of Environmental Chemical Engineering, 8, 104196.
[19]. Li, Y., Peng, L. and Li, W. (2020). Adsorption behaviors on trace Pb2+ from water of biochar adsorbents from konjac starch. Adsorption Science & Technology, 38, 344–356.
[20]. Mohanty, A. K., Vivekanandhan, S., Das, O., Millán, L. M. R., Klinghoffer, N. B., Nzihou, A. and Misra, M. Biocarbon materials.
[21]. Cheng, N., Wang, B., Wu, P., Lee, X., Xing, Y., Chen, M. and Gao, B. (2021). Adsorption of emerging contaminants from water and wastewater by modified biochar: A review. Environmental Pollution, 273, 116448.
[22]. Ranjbar, A., Heidarpour, M., Eslamian, S. and Shirvani, M. (2022). Investigating the performance of adsorbents made from the canola stalk for the removal of lead from aqueous solutions. Arabian Journal of Geosciences, 15, 1565.
[23]. Ma, Z., Zheng, D., Liang, B. and Li, H. (2025). Effect of vermiculite-modified biochar on carbon sequestration potential, mercury adsorption stability, and economics. Biomass Conversion and Biorefinery, 15, 9513–9529.
[24]. Meng, Y., Wang, Y., Li, M., Feng, D. and Ren, J. (2025). Enhanced hydrologic performance and nutrient removal of bioretention systems modified with polyurethane-biochar crosslinked material (PCB). Journal of Environmental Management, 393, 127070.
[25]. Wu, Q., Dong, S., Wang, L. and Li, X. (2021). Single and Competitive Adsorption Behaviors of Cu2+, Pb2+ and Zn2+ on the Biochar and Magnetic Biochar of Pomelo Peel in Aqueous Solution. Water, 13, 868.
[26]. An, Y., Lu, J., Niu, R., Li, M., Zhao, X., Huang, X., Huang, H., Garg, A. and Zhussupbekov, A. (2023). Exploring effects of novel chemical modification of biochar on soil water retention and crack suppression: towards commercialization of production of biochar for soil remediation. Biomass Conversion and Biorefinery, 13, 13897–13910.
[27]. Feldman, J., Seligmann, H., King, S., Flynn, M., Shelley, T., Helwig, A. and Burey, P. (Polly). (2024). Circular economy barriers in Australia: How to translate theory into practice? Sustainable Production and Consumption, 45, 582–597.
[28]. Kulakovskaya, A., Wiprächtiger, M., Knoeri, C. and Bening, C. R. (2023). Integrated environmental-economic circular economy assessment: Application to the case of expanded polystyrene. Resources, Conservation and Recycling, 197, 107069.









