1. Introduction
With the exploration of the battery, lithium was discovered as a metal with significant energy density and electrochemical properties. As the research goes on, people use lithium metal in disposable battery production, but with the demand for storing and releasing energy more efficiency, the idea of the first rechargeable battery came out in the USA, 1973, which is. the idea of using MoS2/Li as the electrodes was given by Moli corporation and be the first kind of rechargeable battery in the market, two years later, in 1991, the Sony Corporation creatively provided an ideal of the lithium-ion battery. The first lithium-ion rechargeable battery, known as LiCoO2/C6 rechargeable battery. Due to the special chemical properties of lithium, the reaction in the battery becomes unstable in low and high temperature environments, which are higher than 60℃ or lower than 0℃ [1]. Calculation provided by Idaho National Laboratory shows that the actual capacity and power are weakly inversely proportional to temperature [2]. But with the development of cathode, anode, separator and electrolyte in recent years, the negative effect on battery caused by temperature is reduced. In recent study, people are willing to test batteries in environmental test chamber, in order to discover the effect of different temperature on batteries. Studies of charge-discharge cycle, change of internal resistance, capacity and energy efficient are take place in these chambers, the range can cover around -30 to 60℃ [3].
To address these challenges, recent developments in solid-state electrolytes, like ceramic, polymer, and hybrid types, are explored as promising solutions for enhancing battery stability and performance across a wider temperature range.
2. Research on lithium-ion battery
Oxidation reaction occurs in the cathode, releasing lithium ions and electrons. (Figure 1). But different in factory production (Figure 2), products are different from the structure in theory. A stainless-steel shell is normally used for protection, cathode and anode are doughnut-like, with a separator between them.
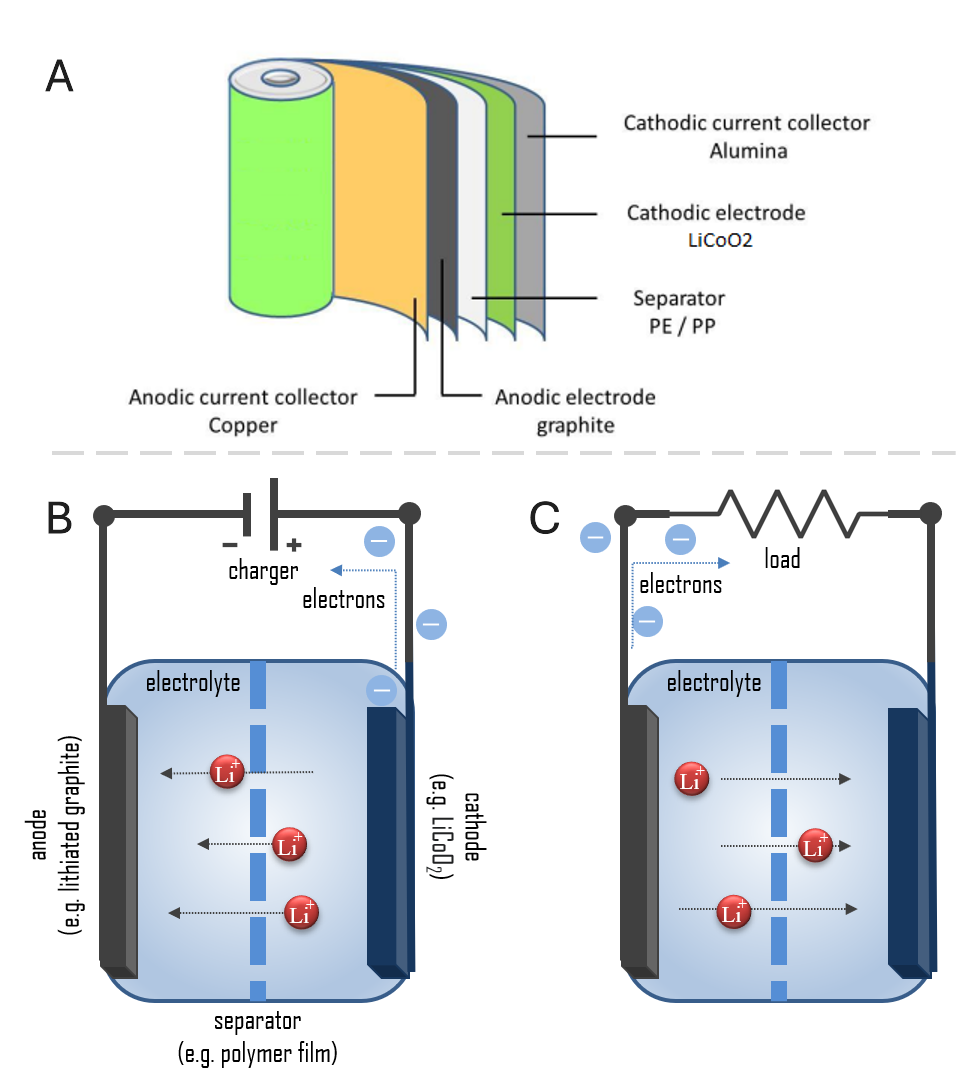

2.1. Common batteries
The following part will talk about several common batteries, they have similar properties, but not all, as they are used in different temperature ranges. More detail mainly focusing on the effect of extreme temperature about these will also be provided.
2.1.1. Lithium cobalt oxide
It used as the main type of battery in mobile devices, thanks to its excellent dense energy and stable output voltage at 3.7 V. But it is not stable enough to withstand the effect of large temperature changes and physical damage. To help this battery perform better in a high temperature range, trifluoroacetic acid is used to treat the surface of LiCoO2, a flat of LiF shell will be formed and cover the cathode, preventing the transfer metal Co into the electrolyte [6].
2.1.2. Lithium iron phosphate
LiFePO4 is used as the cathode which is a kind of stable battery with high theoretical capacity, shows good performance when using, cheap and environmentally friendly. However, it has a disappointing performance under cold conditions, the movement rate of lithium ions has a significant decrease [7]. In order to get close to the theoretical capacity, combine LiFePO4 and nano-carbon to increase the conductivity and prevent the growth of dendrites [8]. A relevant patent performance material, which separates LiFePO4 molecules into the surface or inside of the material, can highly improve conductivity and energy transformation speed [9]. But due to its high voltage at 4.2V.
2.1.3. Lithium manganate
LiMn2O4, a kind of spinel oxidise, is easy to have the Jahn-Teller effect, caused by its octahedral structure. Mn³⁺ ions separate out, which speeds up the decomposition of the electrolyte, capacity drops after using it several times. But it attracts the industrial field’s attention by relying on low cost, and stable output voltage [10].
2.1.4. Ternary battery
Ternary battery has a cathode that is mostly made of Li(NixCo1-x-yMny)O2. It shows a very stable capacity at room temperature, only have slightly accessible decreasing after 1000 cycles at 1C (Figure 3) [11]. However, it is disappointment at higher temperatures, the capacity drops slowly until around the 875th cycle and then drops sharply (Figure 4). In addition, when operating at high temperature, toxic gases from the decomposing electrolyte will release.
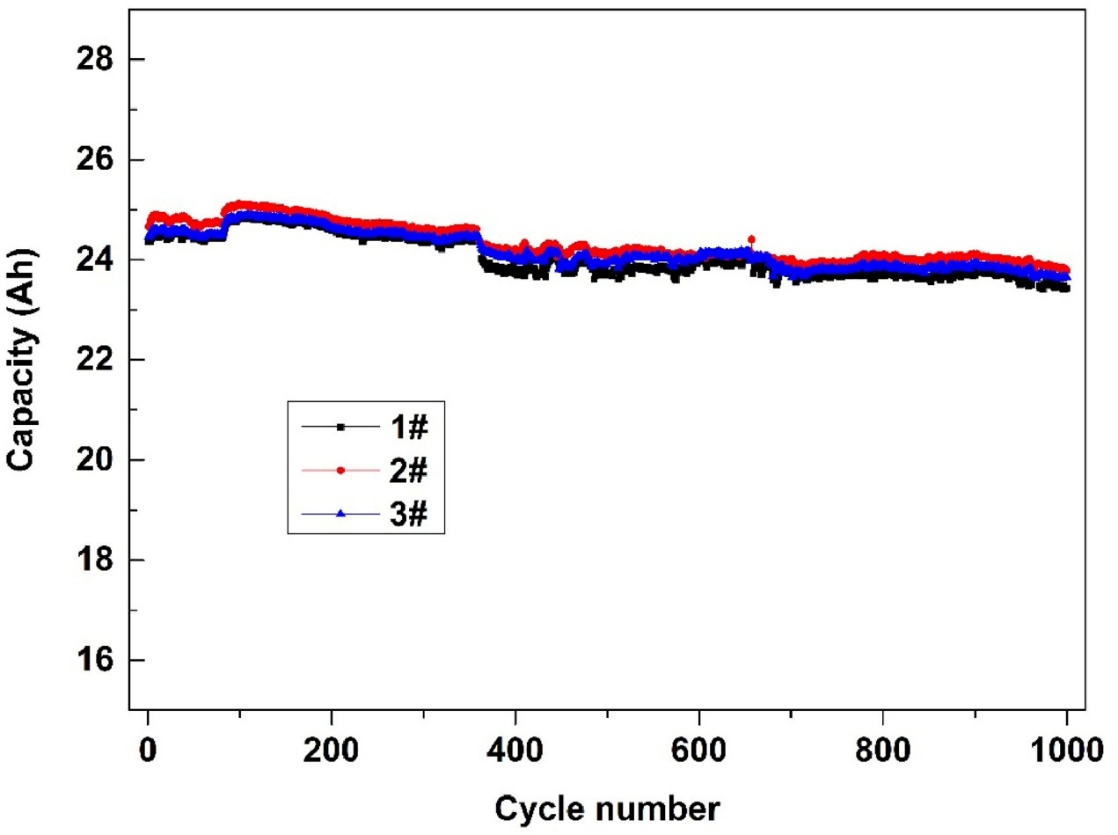

2.2. Anode
2.2.1. Carbon anode
In battery production, industries prefer to use graphite as the anode, which is made from high temperature, because of this progress, the carbon crystallite gets into order, which gives graphite good electrochemical properties, include high irreversible capacity at 0.1C [14,15]. Graphite can be replaced by the new graphene due to its lower cost, porous structure, high conductivity, and large surface area improve the electrochemical properties. With further research, carbon fiber is discovered that has not only great strength but also high conductivity, lithium ions can easily intercalate and deintercalate in the empty space [16].
2.2.2. Lithium metal anode
Lithium is an ideal material for making the anode of batteries. However, due to its extremely high reactivity, will releasing large amount of heat. As the research progressed, Li/Cu composite materials were regarded as more stable materials for manufacturing the anode. The addition of copper ions enhanced the conductivity and the stability of capacity. Figure 4a shows that the lithium metal anode burns continuously when ignited, the temperature can reach above 1000 ℃. In figure 4b, composite material with copper ions added was ignited and then quickly extinguished, the maximum temperature did not exceed 650℃ [17].
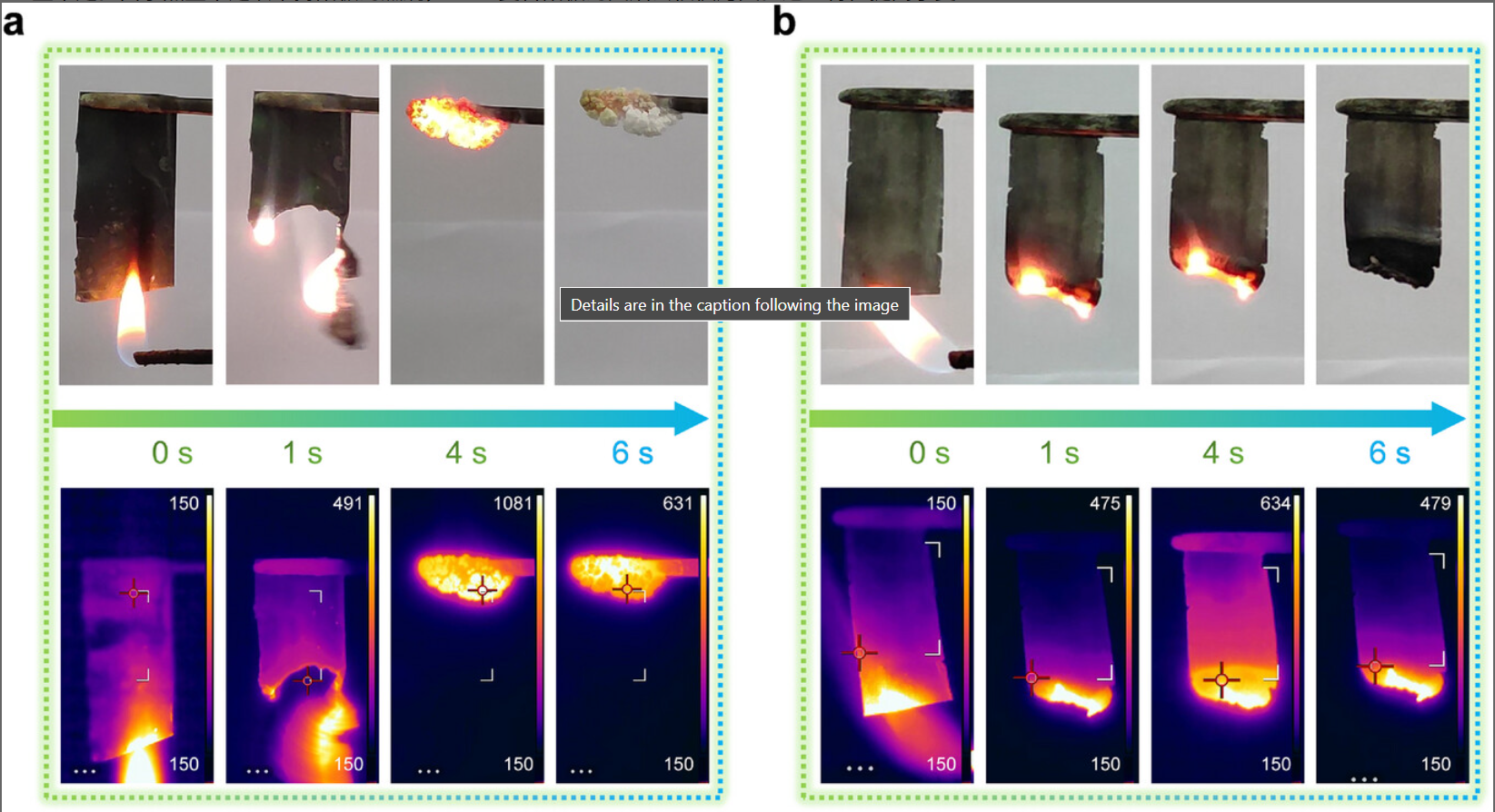
3. Effect of temperature on lithium-ion batteries
3.1. Temperature effect
3.1.1. In high temperature range
Most cathodes are made of lithium oxidise, gas may release due to the hot working temperature, while liquid electrolytes are mostly flammable, which are easily cause safety hazard. Anode will out of protection in high temperature at around 120℃, lithium ion in anode reacts with electrolyte, while the cathode may start to decompose at this temperature and release oxygen gas, in this case, more heat is produced [19]. Seriously high temperatures can cause irreversible damage to cells’ structure, even break the shell and cause fire. As the equation shows, when the temperature increases to much, while the activation energy is kept constant, the reaction rate, k, will increase and make the ratio of the reaction out of control.
K is the reaction rate constant, while A is preexponential constant, R is gas constant, Ea is activation energy of the chemical, and T is temperature in kelvin.
Then, according to Dongxu Ouyang’s test [20], the capacity of LiFePO4/graphite batteries reduces significantly after several charge-discharge cycles in elevated temperature space (Figure 6b), and with number of cycle increase, battery shows unstable in capacity [21]. As we can know from Figure 6, the current in capacity is decreasing while the voltage is increasing, and internal resistance can be calculated based on the equation, the rate of internal resistance will increase to varying degrees after each cycle.
![Figure 6. LiFePO4/graphite batteries (a)carry out 400 charge-discharge cycles at room temperature at 25 ℃, (b)carry out 400 charge-discharge cycles at 55℃ [22].](https://file.ewadirect.com/press/media/markdown/document-image6_zxXzyga.jpeg)
Separators are always polyporous and thin, those in common lithium-ion battery mostly made of organic materials (polyethylene or polypropylene) which has low melting point, under elevated temperature, these materials are prone not only to melt but also to thermal shrinkage. This shrink reduces the effective surface area of the separator, compromising its ability to maintain a safe physical barrier between the electrodes. As a result, the probability of short circuit increase [23]. By the way, capacity is to be also affected. With the thermal degradation of separator, pores are collapse or blocked. The ionic transport failure disrupts the charge-discharge process and may ultimately result in a complete loss of capacity or premature battery failure [24].
In addition to safety hazards, with charge-discharge cycles going under high current and voltage, this thermal buildup not only stress the electrochemical system but also accelerates the onset of deleterious side effects, one of the most critical consequences is the enhanced deposition of lithium metal particularly on the surface of anode and increase the risk of thermal runaway [25]. Anode especially graphite is easy to be broken, SEI membranes on both electrodes are going to decompose and become thicker, increase internal resistance and cause irreversible damage on lithium oxides, capacity drops as a result [26]. These thermal and electrochemical degradations underscore the critical importance of thermal management in battery integrity and performance.
In order to control the heat, which come from the internal and external environment during charge-discharge cycle, active heat dissipation of cell is now considered as a possible solvent at present.
3.1.2. In low temperature range
Low temperature can expose lots of problems of lithium-ion batteries, performance of batteries decreases sharply. In this condition, the kinetics of lithium-ion intercalation into the anode become significantly hindered, leading to uneven lithium deposition on the anode surface, instead of forming a uniform and dense lithium layer, metallic lithium tends to plate irregularly and create dendrites. Dendrites have the potential to grow and lengthen over several cycles, eventually penetrating the separator. An internal short-circuit could take place because of the direct contact between the two electrolytes, caused by the growth of dendritic structures following this breach [27].
An additional problem occurring during the charging process is lithium plating, which is more noticeable when the anode is made up of carbon-based materials such as graphite [28]. During charging, lithium ions transport from the cathode through the electrolyte and intercalate into the layered structure of the graphite anode. However, under low temperatures, high charging rates or overcharging lead the intercalation kinetics slow down dramatically. When the oxidation number of anode getting close to lithium ion, metallic lithium begins to deposit directly on the surface of the carbon instead of intercalating into its structure, then lithium plate formed. The reducing performance may speed up plated lithium reacting with the electrolyte to produce an unstable SEI due to its potential for chemical instability.
Finally, there are multiple limitation on the power output and capacity retention of lithium-ion batteries at low temperatures. At cooler temperatures, the viscosity of the electrolyte increases dramatically, reducing the mobility of the solvated lithium ions in the solution. The kinetics of the electrochemical reaction suffers by the slower charge transport between the electrodes due to this reduced ionic conductivity [29]. The capacity release is constrained by the significant reduction in the lithium-ion diffusion coefficients of positive and negative electrode materials (such as graphite).
3.2. Electrolyte
3.2.1. Organic electrolyte
Due to 4-fluorobenzenethiolate has special chemical reactivity with the lithium-sulfur cathode,it has been decided as an ideal electrolyte especially for lithium-sulfur batteries, upon introduction into the electrolyte system, it can engage in interfacial reactions that facilitate the formation of a more conductive and stable cathode–electrolyte interface, thereby accelerating the redox conversion of polysulfide species. This developed electrochemical kinetics not only contributes to mitigating the shuttle effect but also enables the generation of additional active sites, which leads to an increase in the battery capacity as a result. In addition, 4-fluorobenzenethiolate demonstrates exceptional low-temperature performance, a critical parameter for the practical deployment of Li-S batteries in cold climate applications. Under conditions as -20 °C, the electrolytes enable the battery to maintain a high reversible capacity at 1300mAh/g under 0.1 C [30].
3.2.2. Quasi-solid electrolyte
A hybrid electrolyte system called quasi-solid electrolytes, it combines most of the benefits in both liquid and solid electrolytes in the past. The incorporation of alkali metal salts into the polymer mixture not only improves the electrochemical stability of the electrolyte within the battery environment but also improves the migration of metal ions through the polymer framework. Due to the plasticizer's solvating effect and the polymer chains coordinated motion, a significantly improvement can be found on internal ionic conductivity. Given that the polymer backbone maintains structural integrity, the conductivity advantages are accompanied by mechanical robustness, which strengthens the battery both chemically and physically [31]. Gel-polymer is kind of prominent subclass of quasi-solid electrolytes. Their special viscoelastic and resilient physical properties minimize direct contact between electrodes, reducing the risk of short circuits, and enable them to function simultaneously as electrolytes and separators. Furthermore, their performance at elevated temperatures remains excellent, as the quasi-solid structure reduce the evaporation and leakage issues typically associated with liquid electrolytes [32].
3.3. Thermal management of lithium batteries
Temperature management system is an important part of the battery industry, especially in electric vehicle production. Vehicles need to charge and discharge under different temperatures, sometimes the difference is huge, in order to keeping the battery temperature at a suitable range will make driving safer. When interface material is used in a battery pack, in a test of a 4*5 battery group discharge for 1500s, the rising rate of temperature will be much slower than that without the interface [33]. For some large battery groups in electric cars, an external thermal management system is necessary. A water-cooling system and a heating system are used to manage temperature, water-cooling system used in hot days while heating system used in winter, in order to keep the battery within an ideal working temperature range in most conditions. As YU Hs’ invention shows, management systems can be used in most kinds of cars [34].
4. Possible solution
Temperature change has a great influence on the performance of lithium-ion batteries. It is roughly to make improvements on the basement, which it already has, but there are still some ways to treat this problem. Solid electrolyte is decided as one of the most possible solutions for improving the efficiency and stability of batteries in fatal conditions [36]. Inorganic ceramic electrolyte shows a great electric conductivity in a high temperature range, and the increasing rate is highly proportional to temperature. While another type is polymer electrolyte, which is a kind of gel-like material, this property gives it two main advantages, this polymer can also be used as a separator and can be used to make soft-battery. In order to maximize the benefit, the ideal hybrid electrolyte was decided upon during research, and it equally shared the properties of both inorganic ceramic and polymer electrolyte [37].
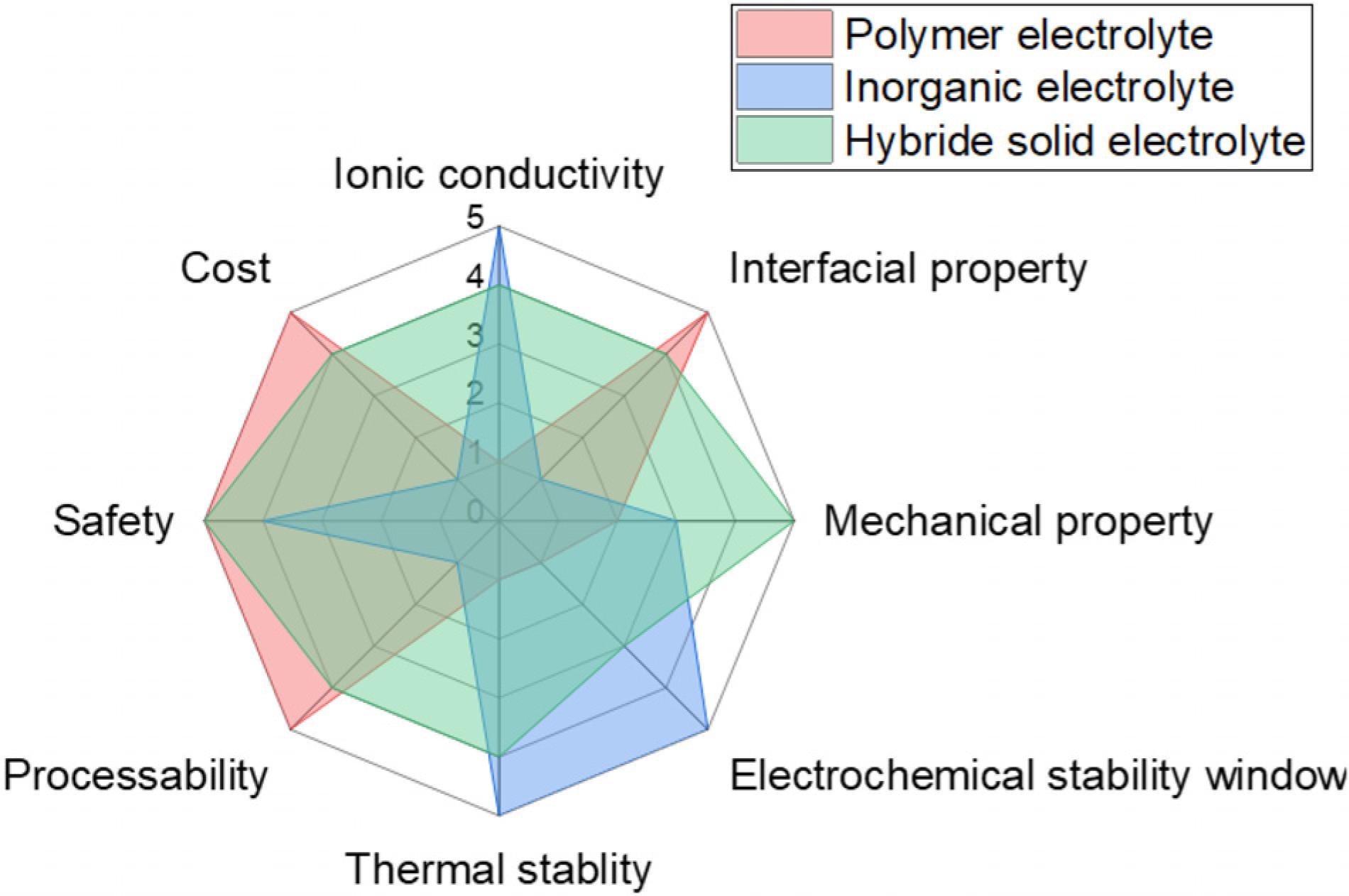
5. Conclusion
In this review, we highlight two kinds of problems lithium-ion batteries may meet in fatal temperatures, discuss details of how these problems happen and provide some possible solutions. However, to a certain extent, it indicates that this kind of battery still has many issues that need to be resolved in terms of usage. Fortunately, to increase safety, efficiency, stability and lower the cost, hybrid electrolyte catches people’s attention, its improvement is very likely to help the lithium-ion battery industry to against the effect of fatal temperature and break through the bottleneck period. In future studies, developed lithium-ion batteries can replace a great part of the usage of fossil fuels in daily life.
References
[1]. Limin Zhou, Hua Ma, Zhonghan Wu, Qing Zhao, Haixia Li, Kai Zhang and Jun Chen, (2022), Challenges and advances in wide-temperature rechargeable lithium batteries Yang Feng, Energy Environ. Sci., 15, 1711.
[2]. Jeffrey R. Belt, Chinh D. Ho, Ted J. Miller, M. Ahsan Habib, Tien Q. Duong, (2005), The effect of temperature on capacity and power in cycled lithium-ion batteries, Journal of Power Sources, 142 (1–2), 354-360.
[3]. Zhigang Qi, (2006), Steve Buelte, Effect of open circuit voltage on performance and degradation of high temperature PBI–H3PO4 fuel cells, Journal of Power Sources, 161, 2, 1126-1132.
[4]. https: //www.electricity-magnetism.org/electric-battery/electric-car-battery/
[5]. https: //batteryswapcabinet.com/lithium-ion-battery-structure/
[6]. Xiao, Z.; Zhu, X.; Wang, S.; Shi, Y.; Zhang, H.; Xu, B.; Zhao, C.; Zhao, Y., (2024), Construction of Uniform LiF Coating Layers for Stable High-Voltage LiCoO2Cathodes in Lithium-Ion Batteries. Molecules, 29, 1414.
[7]. Nannan Zhao, Xiaoke Zhi, Li Wang, Yanhui Liu, Guangchuan Liang, (2015), Effect of microstructure on low temperature electrochemical properties of LiFePO4/C cathode material, Journal of Alloys and Compounds, 645, 301-308.
[8]. Ali Eftekhari, (2017), LiFePO4/C nanocomposites for lithium-ion batteries, Journal of Power Sources, 343, 395-411.
[9]. GONG X; ZHOU M; WANG J; CHEN L; CHEN K; WEI H; LIU J; XIE L; WU C; CHEN B; LI B; YING D, (2024), Lithium iron phosphate composite material useful in battery anode material for lithium iron phosphate battery, comprises carbon nanofiber microsphere carrier and lithium iron phosphate.
[10]. Junda Huang, Yuhui Zhu, Yu Feng, Yehu Han, Zhenyi Gu, Rixin Liu, Dongyue Yang, Kai Chen, (2022), Research Progress on Key Materials and Technologies for Secondary Batteries, Acta Phys. -Chim. Sin., 38, 2208008 (1 of 146)
[11]. Suijun Wang, Jialiang Liu, Jerry Y.S. Lin, (2024), Failure analysis of ternary lithium-ion batteries throughout the entire life cycling at high temperature, Electrochimica Acta, 508, 145238
[12]. Suijun Wang, Jialiang Liu, Jerry Y.S. Lin, (2024), Failure analysis of ternary lithium-ion batteries throughout the entire life cycling at high temperature, Electrochimica Acta, 508, 145238
[13]. Suijun Wang, Jialiang Liu, Jerry Y.S. Lin, (2024), Failure analysis of ternary lithium-ion batteries throughout the entire life cycling at high temperature, Electrochimica Acta, 508, 145238
[14]. Liang Han, Xiao Zhu, Fei Yang, Qian Liu, Xilai Jia, (2021), Eco-conversion of coal into a nonporous graphite for high-performance anodes of lithium-ion batteries, Powder Technology, 382, 40-47.
[15]. Shuai Xu, Johannes van der Watt, Daniel Laudal, Ruiqing Zhang, Rahate Ahmed, Xiaodong Hou, (2025), Coal-derived carbon anodes for lithium-ion batteries: Development, challenges, and prospects, Journal of Power Sources, 628, 235858.
[16]. Norio Takami, Asako Satoh, Michikazu Hara and Takahisa Ohsaki, (1995), Rechargeable Lithium‐Ion Cells Using Graphitized Mesophase‐Pitch‐Based Carbon Fiber Anodes, ECS, The Electrochemical SocietyJournal of The Electrochemical Society, 142-8, 2564
[17]. Longfei Han, Mengdan Zhang, Yukun Cao, Xinru Zhang, Can Liao, Liying Cheng, Qiang Gu, Yongchun Kan, Jixin Zhu, Yuan Hu, (2025), Advanced Functional Material, 2504427.
[18]. Longfei Han, Mengdan Zhang, Yukun Cao, Xinru Zhang, Can Liao, Liying Cheng, Qiang Gu, Yongchun Kan, Jixin Zhu, Yuan Hu, (2025), Advanced Functional Material, 2504427.
[19]. Luyao Zhao, Wei Li, Weiyi Luo, Minxue Zheng, Mingyi Chen, (2024), Numerical study of critical conditions for thermal runaway of lithium-ion battery pack during storage, Journal of Energy Storage, 84, Part B, 110901.
[20]. Dongxu Ouyang, Jingwen Weng, Mingyi Chen, Jian Wang, (2020), Impact of high-temperature environment on the optimal cycle rate of lithium-ion battery, Journal of Energy Storage, 28, 101242.
[21]. Caisheng Li, Xianqing Liu, Changhong Wang, Lisheng Ye, Tingting Wu, Zhixuan Liang, Zejie Zhang, Ying Zeng, Kaizhe Li, (2024), Electrochemical-thermal behaviors of retired power lithium-ion batteries during high-temperature and overcharge/over-discharge cycles, Case Studies in Thermal Engineering, 61, 104898.
[22]. Dongxu Ouyang, Jingwen Weng, Mingyi Chen, Jian Wang, (2020), Impact of high-temperature environment on the optimal cycle rate of lithium-ion battery, Journal of Energy Storage, 28, 101242
[23]. Chen X, Duan T, Li L. (2024), Recent Progress of High Safety Separator for Lithium-Ion Battery. Green Chemical Technology, 1(1): 10006.
[24]. Xiankai Yu, Yixiang Shi, Hongjian Wang, Ningsheng Cai, Chen Li, Rumen I. Tomov, Jeffrey Hanna, Bartek A. Glowacki, Ahmed F. Ghoniem, (2013), Experimental characterization and elementary reaction modeling of solid oxide electrolyte direct carbon fuel cell, Journal of Power Sources, 243, 159-171.
[25]. Liu, S., Zhang, G., and Wang, C., (2023), Challenges and Innovations of Lithium-Ion Battery Thermal Management Under Extreme Conditions: A Review, ASME. J. Heat Mass Transfer, 145(8): 080801.
[26]. Alipour M, Ziebert C, Conte FV, Kizilel R. (2020), A Review on Temperature-Dependent Electrochemical Properties, Aging, and Performance of Lithium-Ion Cells. Batteries. 6(3): 35.
[27]. Yujie Wang, Xingchen Zhang, Zonghai Chen, (2022), Low temperature preheating techniques for Lithium-ion batteries: Recent advances and future challenges, Applied Energy, 313, 118832.
[28]. J. Jaguemont, L. Boulon, Y. Dubé, (2016), A comprehensive review of lithium-ion batteries used in hybrid and electric vehicles at cold temperatures, Applied Energy, 164, 99-114.
[29]. Dr. Qian Li, Gang Liu, Haoran Cheng, Dr. Qujiang Sun, Prof. Junli Zhang, Prof. Jun Ming, (2021), Low-Temperature Electrolyte Design for Lithium-Ion Batteries: Prospect and Challenges, Chemistry A European Journal, 27-64, 15842-15865.
[30]. Pengfei Sang, Shuai Tang, Fengli Li, Yubing Si, Yongzhu Fu, (2024), Organic Thiolate as Multifunctional Salt for Rechargeable Lithium–Sulfur Batteries, Nano-Micro SMALL, 20-48, 2406972.
[31]. Weizhong Liang, Kun Zhao, Liuzhang Ouyang, Min Zhu, Jun Liu, (2025), A review of functional group selection and design strategies for gel polymer electrolytes for metal batteries, Materials Science and Engineering: R: Reports, 164, 100973.
[32]. Yubing Dong, Xinming Qi, Manabu Tanaka, Hiroyoshi Kawakami, (2025), Gel polymer electrolyte membranes consisted of solvate ionic liquid and crosslinked network polymers bearing different main chains: Fabrication and lithium battery application, Electrochimica Acta, 514, 145661.
[33]. Sharon Samuel, Antony Poulose, K. Arul Prakash, (2024), Thermal Management of Lithium Titanium Oxide Batteries: A Numerical Study, Advances in Computational Heat and Mass Transfer, 489–498, 31.
[34]. YU H; GUO Z, (2024), Thermal management system for vehicle e.g. car, has second heating circuit that is provided to exchange heat with temperature regulating circuit, where temperature regulating circuit is provided to regulate temperature of battery in vehicle.
[35]. YU H; GUO Z, (2024), Thermal management system for vehicle e.g. car, has second heating circuit that is provided to exchange heat with temperature regulating circuit, where temperature regulating circuit is provided to regulate temperature of battery in vehicle.
[36]. Jeffrey W. Fergus, (2010), Ceramic and polymeric solid electrolytes for lithium-ion batteries, Journal of Power Sources, 195-15, 4554-4569.
[37]. Lu Han, Michelle L. Lehmann, Jiadeng Zhu, Tianyi Liu, Zhengping Zhou Xiaomin Tang, Chien-Te Heish, Alexei P. Sokolov, Pengfei Cao, Xi Chelsea Chen, Tomonori Saito, (2020), Front. Energy Res, Electrochemical Energy Storage, 8 – 2020.
[38]. Lu Han, Michelle L. Lehmann, Jiadeng Zhu, Tianyi Liu, Zhengping Zhou Xiaomin Tang, Chien-Te Heish, Alexei P. Sokolov, Pengfei Cao, Xi Chelsea Chen, Tomonori Saito, (2020), Front. Energy Res, Electrochemical Energy Storage, 8 – 2020.
Cite this article
Wu,X. (2025). Troubles Meet in Extreme Temperatures of Lithium-ion Battery. Applied and Computational Engineering,206,9-19.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of CONF-FMCE 2025 Symposium: Semantic Communication for Media Compression and Transmission
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Limin Zhou, Hua Ma, Zhonghan Wu, Qing Zhao, Haixia Li, Kai Zhang and Jun Chen, (2022), Challenges and advances in wide-temperature rechargeable lithium batteries Yang Feng, Energy Environ. Sci., 15, 1711.
[2]. Jeffrey R. Belt, Chinh D. Ho, Ted J. Miller, M. Ahsan Habib, Tien Q. Duong, (2005), The effect of temperature on capacity and power in cycled lithium-ion batteries, Journal of Power Sources, 142 (1–2), 354-360.
[3]. Zhigang Qi, (2006), Steve Buelte, Effect of open circuit voltage on performance and degradation of high temperature PBI–H3PO4 fuel cells, Journal of Power Sources, 161, 2, 1126-1132.
[4]. https: //www.electricity-magnetism.org/electric-battery/electric-car-battery/
[5]. https: //batteryswapcabinet.com/lithium-ion-battery-structure/
[6]. Xiao, Z.; Zhu, X.; Wang, S.; Shi, Y.; Zhang, H.; Xu, B.; Zhao, C.; Zhao, Y., (2024), Construction of Uniform LiF Coating Layers for Stable High-Voltage LiCoO2Cathodes in Lithium-Ion Batteries. Molecules, 29, 1414.
[7]. Nannan Zhao, Xiaoke Zhi, Li Wang, Yanhui Liu, Guangchuan Liang, (2015), Effect of microstructure on low temperature electrochemical properties of LiFePO4/C cathode material, Journal of Alloys and Compounds, 645, 301-308.
[8]. Ali Eftekhari, (2017), LiFePO4/C nanocomposites for lithium-ion batteries, Journal of Power Sources, 343, 395-411.
[9]. GONG X; ZHOU M; WANG J; CHEN L; CHEN K; WEI H; LIU J; XIE L; WU C; CHEN B; LI B; YING D, (2024), Lithium iron phosphate composite material useful in battery anode material for lithium iron phosphate battery, comprises carbon nanofiber microsphere carrier and lithium iron phosphate.
[10]. Junda Huang, Yuhui Zhu, Yu Feng, Yehu Han, Zhenyi Gu, Rixin Liu, Dongyue Yang, Kai Chen, (2022), Research Progress on Key Materials and Technologies for Secondary Batteries, Acta Phys. -Chim. Sin., 38, 2208008 (1 of 146)
[11]. Suijun Wang, Jialiang Liu, Jerry Y.S. Lin, (2024), Failure analysis of ternary lithium-ion batteries throughout the entire life cycling at high temperature, Electrochimica Acta, 508, 145238
[12]. Suijun Wang, Jialiang Liu, Jerry Y.S. Lin, (2024), Failure analysis of ternary lithium-ion batteries throughout the entire life cycling at high temperature, Electrochimica Acta, 508, 145238
[13]. Suijun Wang, Jialiang Liu, Jerry Y.S. Lin, (2024), Failure analysis of ternary lithium-ion batteries throughout the entire life cycling at high temperature, Electrochimica Acta, 508, 145238
[14]. Liang Han, Xiao Zhu, Fei Yang, Qian Liu, Xilai Jia, (2021), Eco-conversion of coal into a nonporous graphite for high-performance anodes of lithium-ion batteries, Powder Technology, 382, 40-47.
[15]. Shuai Xu, Johannes van der Watt, Daniel Laudal, Ruiqing Zhang, Rahate Ahmed, Xiaodong Hou, (2025), Coal-derived carbon anodes for lithium-ion batteries: Development, challenges, and prospects, Journal of Power Sources, 628, 235858.
[16]. Norio Takami, Asako Satoh, Michikazu Hara and Takahisa Ohsaki, (1995), Rechargeable Lithium‐Ion Cells Using Graphitized Mesophase‐Pitch‐Based Carbon Fiber Anodes, ECS, The Electrochemical SocietyJournal of The Electrochemical Society, 142-8, 2564
[17]. Longfei Han, Mengdan Zhang, Yukun Cao, Xinru Zhang, Can Liao, Liying Cheng, Qiang Gu, Yongchun Kan, Jixin Zhu, Yuan Hu, (2025), Advanced Functional Material, 2504427.
[18]. Longfei Han, Mengdan Zhang, Yukun Cao, Xinru Zhang, Can Liao, Liying Cheng, Qiang Gu, Yongchun Kan, Jixin Zhu, Yuan Hu, (2025), Advanced Functional Material, 2504427.
[19]. Luyao Zhao, Wei Li, Weiyi Luo, Minxue Zheng, Mingyi Chen, (2024), Numerical study of critical conditions for thermal runaway of lithium-ion battery pack during storage, Journal of Energy Storage, 84, Part B, 110901.
[20]. Dongxu Ouyang, Jingwen Weng, Mingyi Chen, Jian Wang, (2020), Impact of high-temperature environment on the optimal cycle rate of lithium-ion battery, Journal of Energy Storage, 28, 101242.
[21]. Caisheng Li, Xianqing Liu, Changhong Wang, Lisheng Ye, Tingting Wu, Zhixuan Liang, Zejie Zhang, Ying Zeng, Kaizhe Li, (2024), Electrochemical-thermal behaviors of retired power lithium-ion batteries during high-temperature and overcharge/over-discharge cycles, Case Studies in Thermal Engineering, 61, 104898.
[22]. Dongxu Ouyang, Jingwen Weng, Mingyi Chen, Jian Wang, (2020), Impact of high-temperature environment on the optimal cycle rate of lithium-ion battery, Journal of Energy Storage, 28, 101242
[23]. Chen X, Duan T, Li L. (2024), Recent Progress of High Safety Separator for Lithium-Ion Battery. Green Chemical Technology, 1(1): 10006.
[24]. Xiankai Yu, Yixiang Shi, Hongjian Wang, Ningsheng Cai, Chen Li, Rumen I. Tomov, Jeffrey Hanna, Bartek A. Glowacki, Ahmed F. Ghoniem, (2013), Experimental characterization and elementary reaction modeling of solid oxide electrolyte direct carbon fuel cell, Journal of Power Sources, 243, 159-171.
[25]. Liu, S., Zhang, G., and Wang, C., (2023), Challenges and Innovations of Lithium-Ion Battery Thermal Management Under Extreme Conditions: A Review, ASME. J. Heat Mass Transfer, 145(8): 080801.
[26]. Alipour M, Ziebert C, Conte FV, Kizilel R. (2020), A Review on Temperature-Dependent Electrochemical Properties, Aging, and Performance of Lithium-Ion Cells. Batteries. 6(3): 35.
[27]. Yujie Wang, Xingchen Zhang, Zonghai Chen, (2022), Low temperature preheating techniques for Lithium-ion batteries: Recent advances and future challenges, Applied Energy, 313, 118832.
[28]. J. Jaguemont, L. Boulon, Y. Dubé, (2016), A comprehensive review of lithium-ion batteries used in hybrid and electric vehicles at cold temperatures, Applied Energy, 164, 99-114.
[29]. Dr. Qian Li, Gang Liu, Haoran Cheng, Dr. Qujiang Sun, Prof. Junli Zhang, Prof. Jun Ming, (2021), Low-Temperature Electrolyte Design for Lithium-Ion Batteries: Prospect and Challenges, Chemistry A European Journal, 27-64, 15842-15865.
[30]. Pengfei Sang, Shuai Tang, Fengli Li, Yubing Si, Yongzhu Fu, (2024), Organic Thiolate as Multifunctional Salt for Rechargeable Lithium–Sulfur Batteries, Nano-Micro SMALL, 20-48, 2406972.
[31]. Weizhong Liang, Kun Zhao, Liuzhang Ouyang, Min Zhu, Jun Liu, (2025), A review of functional group selection and design strategies for gel polymer electrolytes for metal batteries, Materials Science and Engineering: R: Reports, 164, 100973.
[32]. Yubing Dong, Xinming Qi, Manabu Tanaka, Hiroyoshi Kawakami, (2025), Gel polymer electrolyte membranes consisted of solvate ionic liquid and crosslinked network polymers bearing different main chains: Fabrication and lithium battery application, Electrochimica Acta, 514, 145661.
[33]. Sharon Samuel, Antony Poulose, K. Arul Prakash, (2024), Thermal Management of Lithium Titanium Oxide Batteries: A Numerical Study, Advances in Computational Heat and Mass Transfer, 489–498, 31.
[34]. YU H; GUO Z, (2024), Thermal management system for vehicle e.g. car, has second heating circuit that is provided to exchange heat with temperature regulating circuit, where temperature regulating circuit is provided to regulate temperature of battery in vehicle.
[35]. YU H; GUO Z, (2024), Thermal management system for vehicle e.g. car, has second heating circuit that is provided to exchange heat with temperature regulating circuit, where temperature regulating circuit is provided to regulate temperature of battery in vehicle.
[36]. Jeffrey W. Fergus, (2010), Ceramic and polymeric solid electrolytes for lithium-ion batteries, Journal of Power Sources, 195-15, 4554-4569.
[37]. Lu Han, Michelle L. Lehmann, Jiadeng Zhu, Tianyi Liu, Zhengping Zhou Xiaomin Tang, Chien-Te Heish, Alexei P. Sokolov, Pengfei Cao, Xi Chelsea Chen, Tomonori Saito, (2020), Front. Energy Res, Electrochemical Energy Storage, 8 – 2020.
[38]. Lu Han, Michelle L. Lehmann, Jiadeng Zhu, Tianyi Liu, Zhengping Zhou Xiaomin Tang, Chien-Te Heish, Alexei P. Sokolov, Pengfei Cao, Xi Chelsea Chen, Tomonori Saito, (2020), Front. Energy Res, Electrochemical Energy Storage, 8 – 2020.









