1. Introduction
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder associated with aging, marked by permanent cognitive decline and distinct pathological brain lesions that severely affect the quality of life for those patients. Around 44 million people around the globe have AD, with more than 90% of these cases being late-onset and occurring sporadically.[1] According to statistical data, Alzheimer’s disease accounts for approximately 60% to 80% of all dementia cases.[2]
The etiology of sporadic Alzheimer’s disease (AD) remains elusive. To date, despite various therapeutic interventions that can mitigate the symptoms of AD, no definitive cure exists for the disease.[3] Most current research on AD primarily focuses on late-onset AD (LOAD). The majority of AD patients develop LOAD, which emerges later in life and is typically sporadic. While these cases are not hereditary and lack a single genetic cause, current research indicates several genetic risk factors are involved, with the E4 allele of the ApoE (apolipoprotein E) gene—present in about 16% of the population—being the most significant.[4]
Research has identified that degus exhibits spontaneous AD-like characteristics in both cognitive performance and neuropathology.[5] This observation suggests that degus could serve as an effective and practical model for studying the natural progression of sporadic Alzheimer’s disease.
An experiment is proposed to investigate the early-stage changes in the degus’ brain as Alzheimer’s disease (AD) begins to develop. This research aims to identify the primary causative factors of AD, by observing the early change in the brain, that potentially contribute to developing more effective therapeutic strategies.
2. Project Description
The main goal of our research is to identify the initial change in degus’ brain as AD begins to develop. Then, compare the results with non-AD degus’s brain, in order to find out the primary causative factors of AD. It will potentially contribute to the development of more effective therapeutic strategies by stopping the changes in the brain.
Hypothesis: There is an initial change or sign of change at the start of AD development. The initial change will trigger other lesions that indicate AD, such as Amyloid-β plaques, NFTs, and neuroinflammation.
Objective 1: Identify the degus who is in the initial stage of Alzheimer’s disease development.
Methods: Prepare a group of degus from the same parents. They will grow in the same conditions. Each degu will undergo periodic assessments to evaluate the potential onset of Alzheimer's disease.
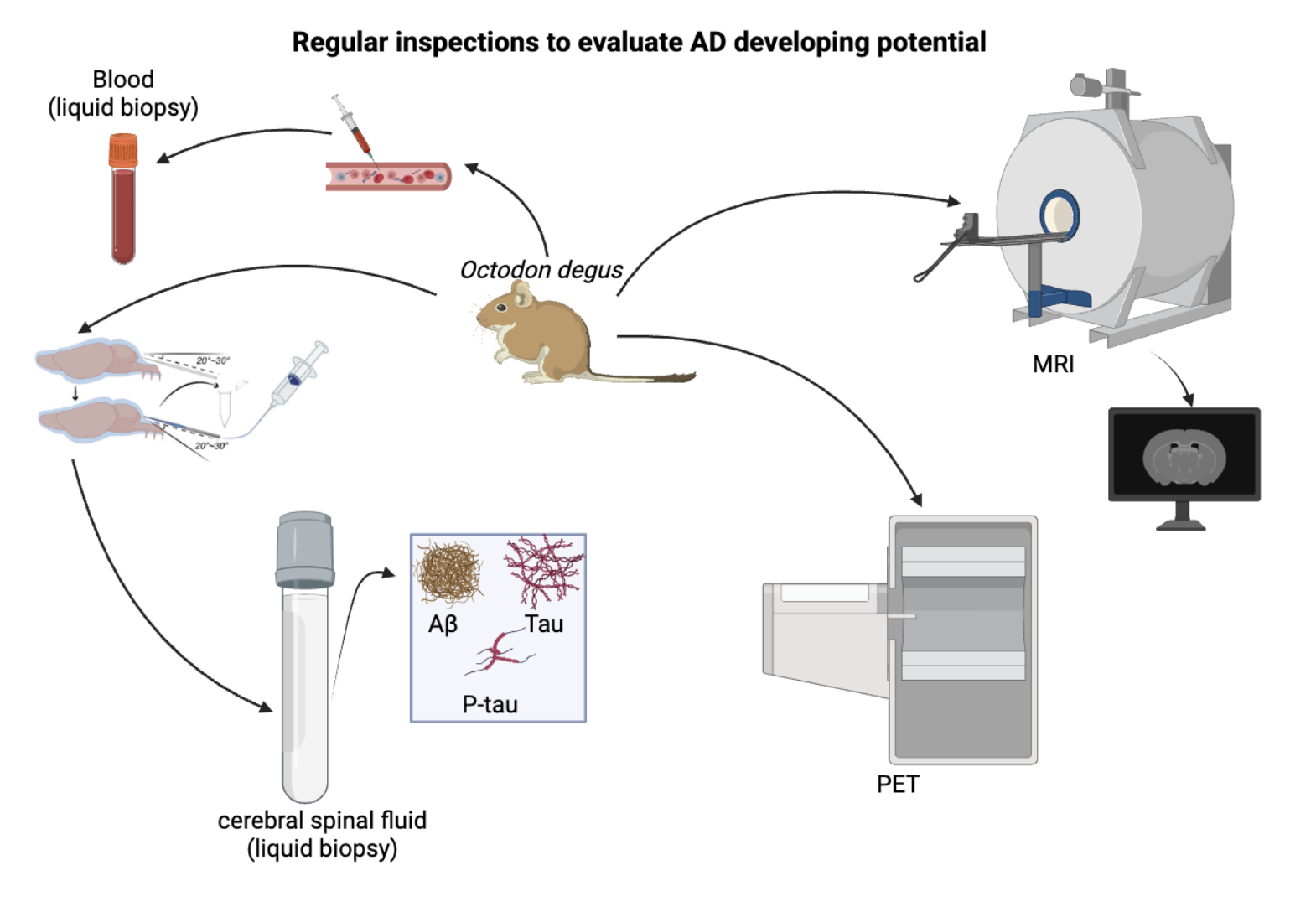
Figure 1. Degus are accessed through those inspections regularly, using blood & CSF (liquid biopsy) and MRI & PET (imaging)
Created with BioRender.com.
Magnetic resonance imaging (MRI) can be used to diagnose AD because it provides detailed images of the brain to identify key changes such as hippocampal atrophy, cortical thinning, and ventriculomegaly.[6] To diagnose AD, degus undergo high-resolution structural MRI, diffusion tensor imaging (DTI), and possibly functional MRI (fMRI) to assess brain anatomy, white matter integrity, and brain activity, respectively. Analyzing brain volume and cortical thickness, as well as comparing scans, can help detect early signs and monitor disease progression.
Positron Emission Tomography (PET) imaging is used to diagnose AD by detecting metabolic activity, NTF-like structure, and amyloid-β plaques in the brain.[6] PET scans involve injecting a radioactive tracer, such as fluorodeoxyglucose (FDG) to measure glucose metabolism or amyloid-specific tracers to visualize amyloid deposits. Reduced glucose metabolism in regions like the posterior cingulate, precuneus, and parietal lobes indicates AD. Amyloid PET imaging identifies amyloid plaque accumulation, a hallmark of AD. PET imaging aids in early diagnosis, distinguishing AD from other dementias, and monitoring disease progression by providing detailed functional and biochemical information about brain activity.
Liquid biopsy of cerebrospinal fluid (CSF) is used to diagnose AD by analyzing specific biomarkers.[7][8] CSF is collected via arteria dorsalis spinalis, and the levels of amyloid-β, total tau, and phosphorylated tau proteins are measured. [9][10][11] increased amyloid-β and raised tau levels indicate AD. [12] This approach helps in the early identification of AD from other dementias together with the tracking of the progression of the disease due to its demonstration of biochemical realities in the brains of patients.
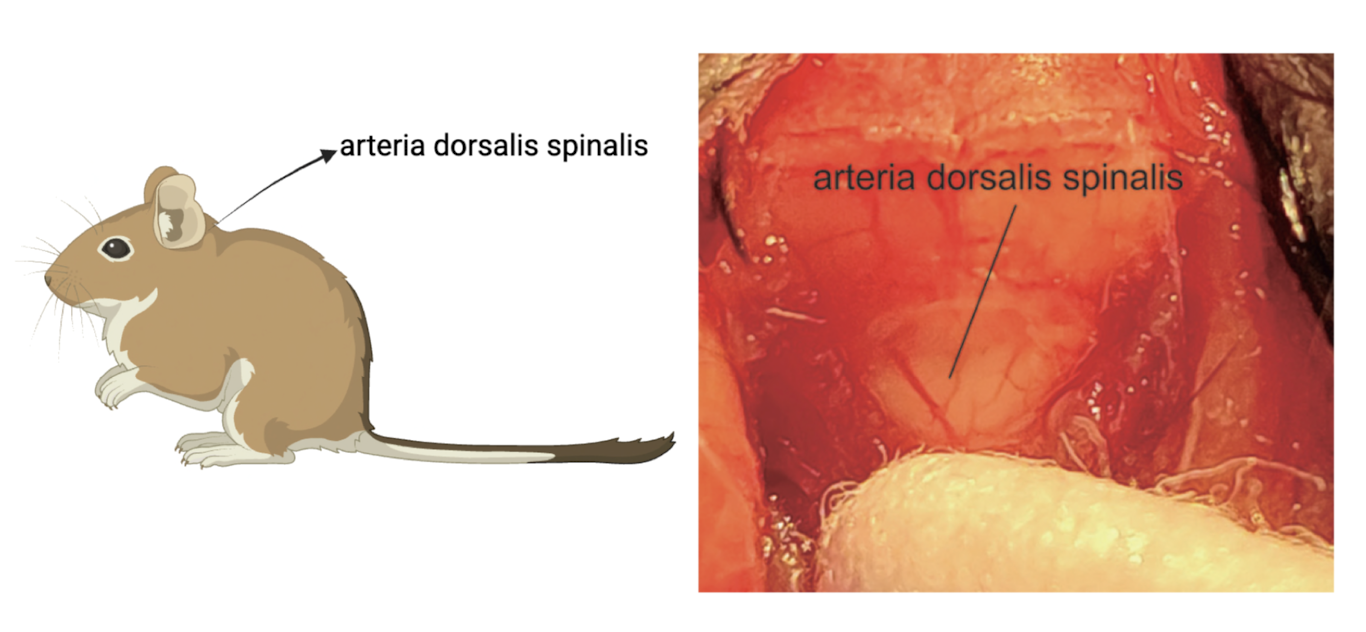
Figure 2. CSF is collected via arteria dorsalis spinalis Created with BioRender.com.
Liquid biopsy of blood for diagnosing AD involves measuring biomarkers such as amyloid-β, total tau, and phosphorylated tau proteins.[13][14] Blood samples are collected and analyzed for these markers, with increased amyloid-β and raised tau levels indicating AD.[12][15] This non-invasive method allows for early detection, differentiation from other dementias, and monitoring of disease progression by providing valuable biochemical information about brain pathology.
Degus undergo these inspections monthly. By observing subtle changes and trends in the results, it is possible to identify degus that are developing AD.
Expected Results:
Aged Outbred degus will spontaneously develop AD in the patient’s brain. It is expected that the regular inspections on degus will show changes in degus’ body that indicate AD is starting to develop in their brain. Those degus will be grouped as AD-developing degus.
Liquid biopsy of blood: It is expected to show the presence of amyloid-β, total tau, and phosphorylated tau proteins.
Liquid biopsy of CSF: It is expected to show the presence of amyloid-β, total tau, and phosphorylated tau proteins.
MRI: It is expected to show hippocampal atrophy, cortical thinning, and enlarged ventricles, which signify brain tissue loss. DTI shows white matter degradation and loss of integrity.
PET: It is expected to show changes in brain metabolism (like a decrease in glucose) and the presence of amyloid plaques.
In contrast, I expect at least one of those four tests to show a sign of AD developing in the degus brain.
Objective 2: Identify the initial changes in degus’ brain at the start of Alzheimer’s disease development.
Methods: 3D microscopy analysis: Degus that showed AD-developing features will be euthanized. Brain tissue is sampled at autopsy in coronal sections and fresh tissue is flash-frozen in cassettes. Fresh frozen tissue samples are then sectioned on a cryostat at 5 μm thick. The samples will be prepared on glass slides and subjected to immunofluorescent staining to highlight amyloid-β and tau protein. Neuron images are captured using a Leica SP8 confocal microscope equipped with LAS X LIGHTNING for deconvolution. Imaris software is then utilized for 3D image reconstruction and quantitative analysis of neuronal endosomes.[16] It is applied to achieve highly stereoscopic imaging of the brain tissues of degus with emerging AD and healthy ones. Thanks to the comparison of neuronal cells, amyloid-β accumulation, tau protein deposition among other relevant features, a comprehensive insight into the progression of the disease can be provided.
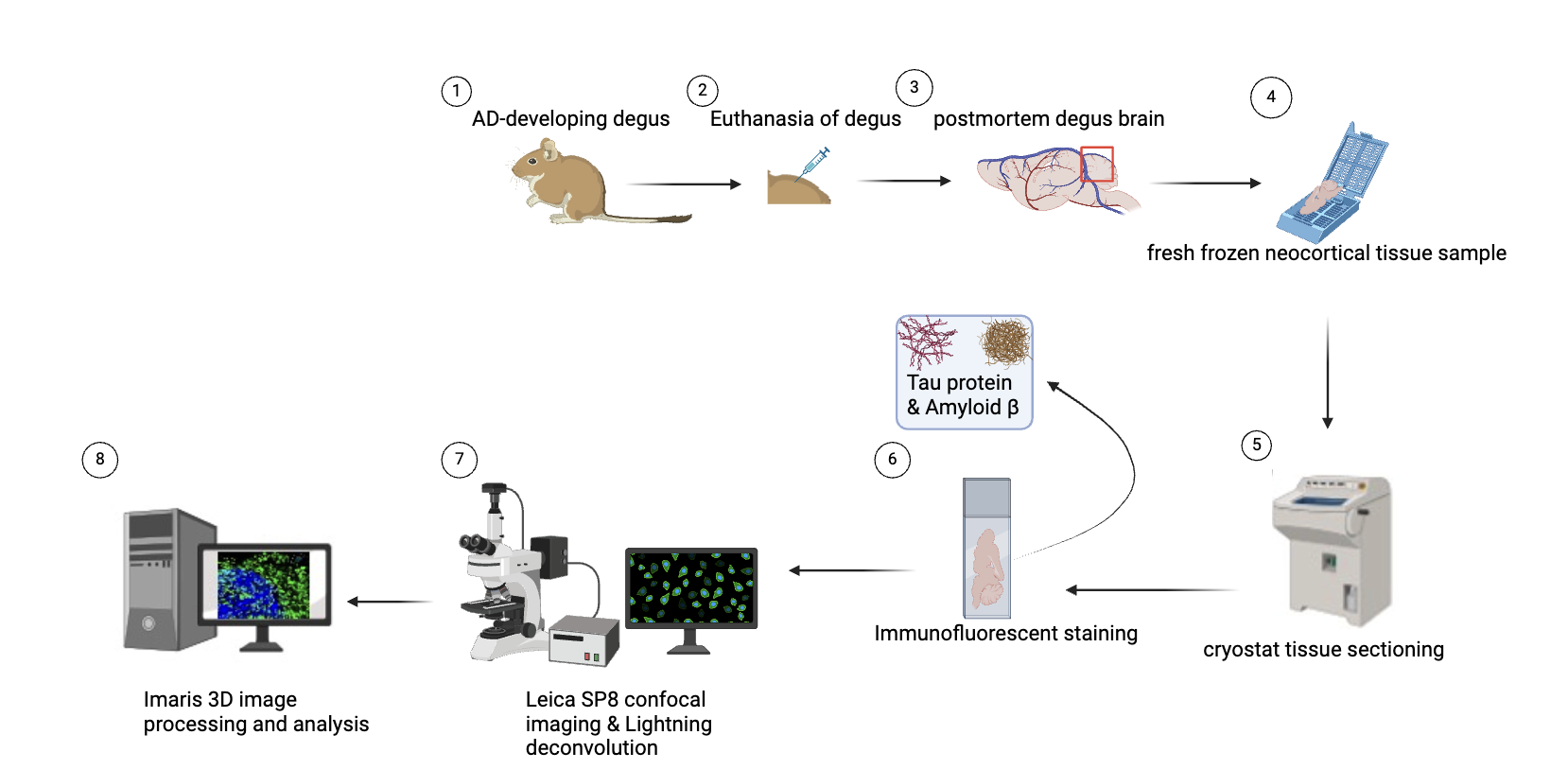
Figure 3. Steps of 3D imaging
Created with BioRender.com.
Cyclic multiplex fluorescent immunohistochemistry combined with machine learning models [17]: cyclic MFI is considered as a very advanced imaging method employed in analysis of tissue samples of great architecture. This method implies the use of staining, imaging and washing steps in a sequential manner on the correspondent tissue section. In each round, certain antibodies conjugated to fluorescent probes are employed for marking proteins or any other molecule of interest present in the tissue. After the imaging, the signals are quenched and the process continues with a new set of fluorophore-labelled antibodies. This enables the analysis of several biomarkers in a given tissue section at the same time. It is especially useful for mapping protein distribution and their association within tissues with high complexity such as the tissue of the Alzheimer’s disease brains. The integration of Ml with cyclic MFI improves the efficiency of handling the large datasets of high-order dimensions that are produced. This data can easily be fed to an algorithm with capabilities of analyzing and drawing correlations and patterns which would otherwise be hard for one to notice. By employing the aforementioned approach, the development, and progression of Alzheimer’s disease (AD) shall be expounded in addition to analyzing the impact of tau protein and amyloid-β on astrocytes, microglia, and other neuronal cells in the degus’ brain.[18]
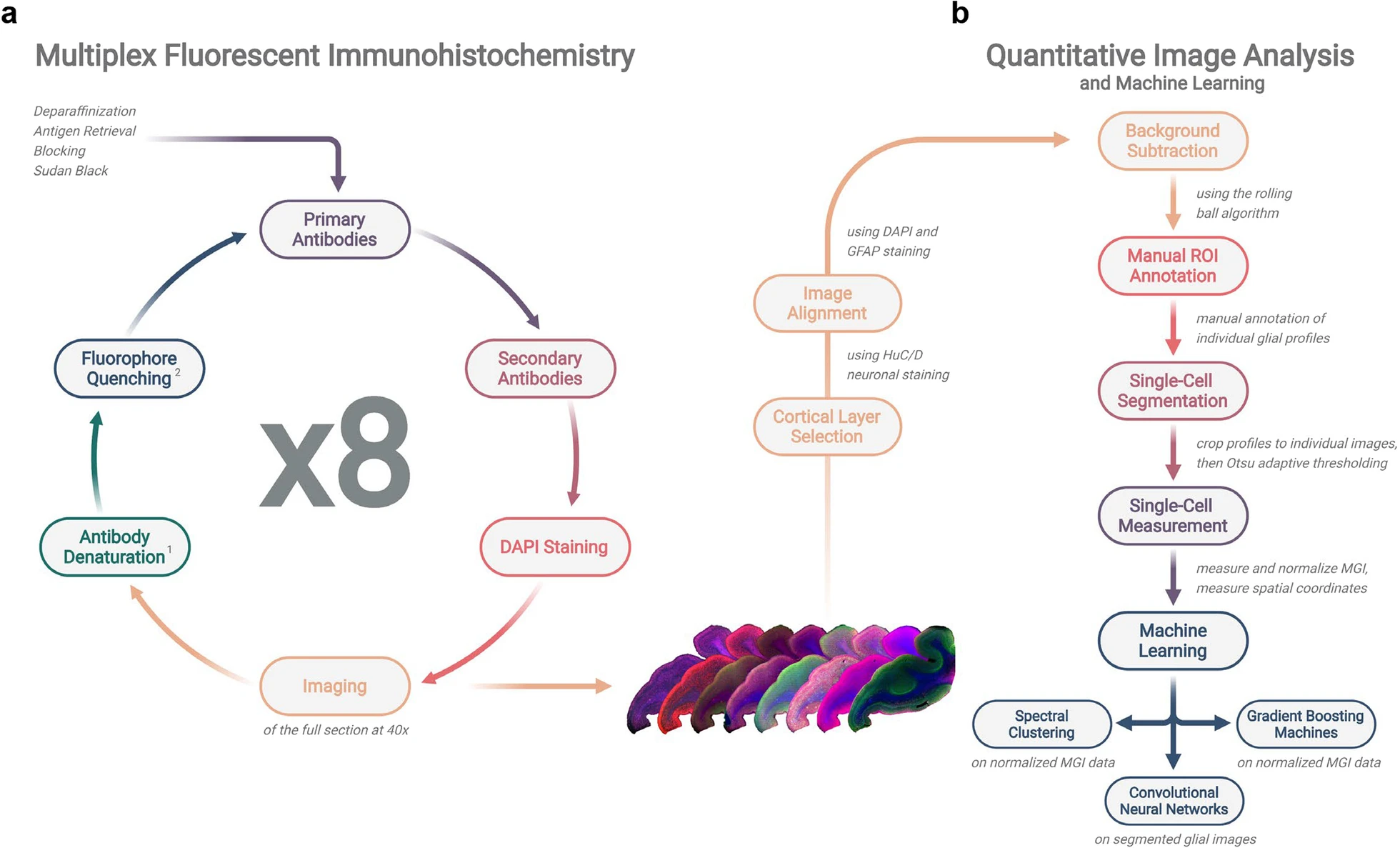
Figure 4. Operation timeline and workflow of cyclic multiplex fluorescent immunohistochemistry and machine learning-based quantitative image analysis.
Adapted from the previous paper. [17]
Expected Results:
When comparing the 3D immunofluorescent images and multiplex fluorescent immunohistochemistry results of AD-developing degus with those of healthy non-AD degus, several subtle but significant differences are expected to be observed.
3D immunofluorescent microscopy analysis: It is expected to see the distribution of AD biomarkers (tau protein & amyloid-β) in the brains of degus. This analysis is intended to provide insights into the progression and development of early-onset AD. Understanding the spatial distribution of these biomarkers can also help identify specific brain regions where AD pathology may initially manifest.
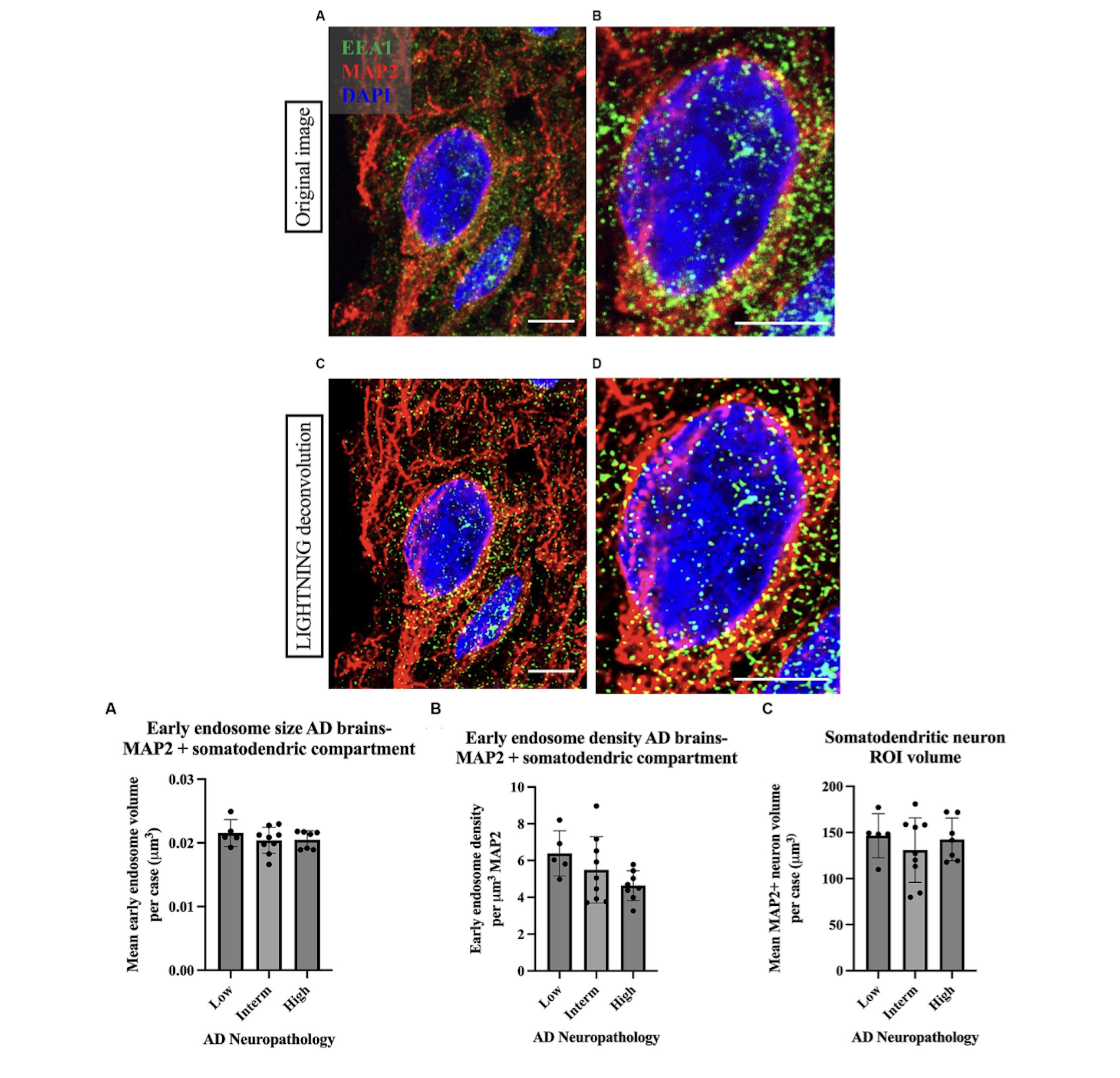
Figure 5. Example of high magnification images of a neuron imaged from Case 9 parietal cortex, stained with cortical neuron marker MAP2 in red, early endosome marker EEA1 in green, and DAPI nuclear marker. In the case of Imaris early endosome analysis, the following is an example of data output that may be obtained. Adapted from the previous paper. [16]
Cyclic multiplex fluorescent immunohistochemistry: It is expected to observe alterations in astrocytes, microglia, and other neuronal cells. Such changes may provide valuable insights into the progression of AD and the heterogeneity of glial responses associated with the condition.
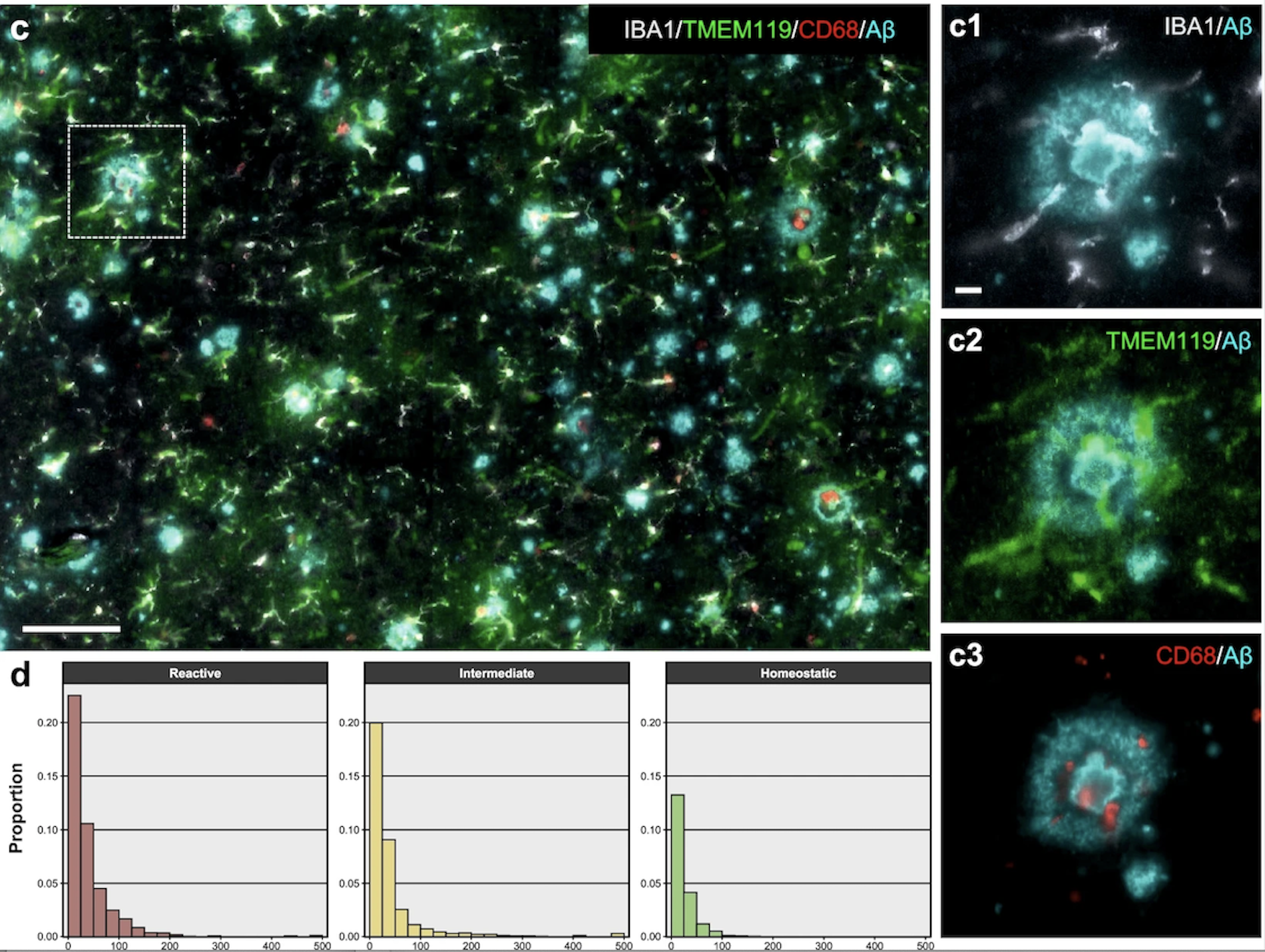
Figure 6. Example of a high-plex image of microglia from an AD subject. For clarity, only IBA1, TMEM119, and CD68 markers are shown together with Aβ. Scale bar: 100 µm, insets c1–c3: 10 µm. Histograms indicate the proportion of each microglial phenotype with respect to all AD microglial profiles as a function of the distance (µm, x axis) to the nearest Aβ plaque or PHF1+ NFT. Reactive microglia were relatively more closely associated with AD neuropathological changes than intermediate microglia, and these more than homeostatic microglia. Adapted from the previous paper. [17]
3. Future trajectories
Objective 3: Find a method to inhibit the early development of AD in the brain as a therapeutic strategy.
Further Directions (Planning): Conduct further research that focuses on inhibiting early pathological changes in the brain that may initiate or exacerbate subsequent neurodegenerative processes. The additional objective is to inhibit the production of molecules that contribute to the formation of brain lesions and to remove these pathogenic molecules from the system.
By focusing on these early intervention strategies, I aim to not only improve our understanding of the underlying mechanisms driving neurodegeneration but also develop novel therapeutic approaches that can be applied at earlier stages of the disease.
4. Conclusion
The study provides critical insights into the initial neuropathological changes occurring in the brains of degus during the onset of Alzheimer’s disease (AD). By characterizing these early changes and comparing them with non-AD brains, this research aims to identify the primary causative factors responsible for the progression of AD. The use of advanced techniques such as 3D microscopy, multiplex fluorescent immunohistochemistry, MRI, and PET imaging allows for a detailed analysis of the spatial distribution of AD biomarkers, including amyloid-β and tau proteins, as well as the glial responses, particularly in astrocytes and microglia. The findings are expected to reveal early-stage alterations in neuronal and glial cells that may serve as key indicators of disease onset. By enhancing our understanding of these initial changes, this research contributes to the broader effort of developing more effective therapeutic strategies aimed at inhibiting the early progression of AD.
References
[1]. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017 Sep 16;390(10100):1211-1259. doi: 10.1016/S0140-6736(17)32154-2. Erratum in: Lancet. 2017 Oct 28;390(10106):e38. doi: 10.1016/S0140-6736(17)32647-8. PMID: 28919117; PMCID: PMC5605509.
[2]. https://www.alz.org/media/Documents/alzheimers-facts-and-figures.pdf
[3]. Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, Cummings J, van der Flier WM. Alzheimer's disease. Lancet. 2021 Apr 24;397(10284):1577-1590. doi: 10.1016/S0140-6736(20)32205-4. Epub 2021 Mar 2. PMID: 33667416; PMCID: PMC8354300.
[4]. Karch CM, Goate AM. Alzheimer's disease risk genes and mechanisms of disease pathogenesis. Biol Psychiatry. 2015 Jan 1;77(1):43-51. doi: 10.1016/j.biopsych.2014.05.006. Epub 2014 May 17. PMID: 24951455; PMCID: PMC4234692.
[5]. Tan Z, Garduño BM, Aburto PF, Chen L, Ha N, Cogram P, Holmes TC, Xu X. Cognitively impaired aged Octodon degus recapitulate major neuropathological features of sporadic Alzheimer's disease. Acta Neuropathol Commun. 2022 Dec 19;10(1):182. doi: 10.1186/s40478-022-01481-x. PMID: 36529803; PMCID: PMC9761982.
[6]. Chouliaras L, O'Brien JT. The use of neuroimaging techniques in the early and differential diagnosis of dementia. Mol Psychiatry. 2023 Oct;28(10):4084-4097. doi: 10.1038/s41380-023-02215-8. Epub 2023 Aug 22. PMID: 37608222; PMCID: PMC10827668.
[7]. Gong X, Zhang H, Liu X, Liu Y, Liu J, Fapohunda FO, Lü P, Wang K, Tang M. Is liquid biopsy mature enough for the diagnosis of Alzheimer's disease? Front Aging Neurosci. 2022 Aug 5;14:977999. doi: 10.3389/fnagi.2022.977999. PMID: 35992602; PMCID: PMC9389010.
[8]. Malhotra S, Miras MCM, Pappolla A, Montalban X, Comabella M. Liquid Biopsy in Neurological Diseases. Cells. 2023 Jul 22;12(14):1911. doi: 10.3390/cells12141911. PMID: 37508574; PMCID: PMC10378132.
[9]. https://www.labcorp.com/tests/484415/alzheimer-s-disease-evaluation-profile-csf
[10]. https://app.jove.com/cn/v/56774/an-improved-method-for-collection-of-cerebrospinal-fluid-from-anesthetized-mice
[11]. ZHANG Lu-Lin, YUAN Yu, LIANG Mei-Yu, LIU Ming-Xin, WANG Dong-Xia, XIE Jun-Xia, SONG Ning. A glass micropipette vacuum technique of cerebrospinal fluid sampling in C57BL/6 mice. Acta Physiol Sin 2023; 75 (2): 197-204 (in Chinese with English abstract).
[12]. Graff-Radford J, Yong KXX, Apostolova LG, Bouwman FH, Carrillo M, Dickerson BC, Rabinovici GD, Schott JM, Jones DT, Murray ME. New insights into atypical Alzheimer's disease in the era of biomarkers. Lancet Neurol. 2021 Mar;20(3):222-234. doi: 10.1016/S1474-4422(20)30440-3. PMID: 33609479; PMCID: PMC8056394.
[13]. Kumar A, Nader MA, Deep G. Emergence of Extracellular Vesicles as "Liquid Biopsy" for Neurological Disorders: Boom or Bust. Pharmacol Rev. 2024 Feb 13;76(2):199-227. doi: 10.1124/pharmrev.122.000788. PMID: 38351075; PMCID: PMC10877757.
[14]. Assfaw AD, Schindler SE, Morris JC. Advances in blood biomarkers for Alzheimer disease (AD): A review. Kaohsiung J Med Sci. 2024 Jun 18. doi: 10.1002/kjm2.12870. Epub ahead of print. PMID: 38888066.
[15]. Oruganti M, Gaidhani S. Routine bleeding techniques in laboratory rodents[J]. International Journal of Pharmaceutical Sciences and Research, 2011, 2(3): 516.
[16]. Rose SE, Williams CA, Hailey DW, Mishra S, Kirkland A, Keene CD, Garden GA, Jayadev S, Young JE. Advancements in high-resolution 3D microscopy analysis of endosomal morphology in postmortem Alzheimer's disease brains. Front Neurosci. 2024 Jan 16;17:1321680. doi: 10.3389/fnins.2023.1321680. PMID: 38292900; PMCID: PMC10824887.
[17]. Muñoz-Castro C, Noori A, Magdamo CG, Li Z, Marks JD, Frosch MP, Das S, Hyman BT, Serrano-Pozo A. Cyclic multiplex fluorescent immunohistochemistry and machine learning reveal distinct states of astrocytes and microglia in normal aging and Alzheimer's disease. J Neuroinflammation. 2022 Feb 2;19(1):30. doi: 10.1186/s12974-022-02383-4. PMID: 35109872; PMCID: PMC8808995.
[18]. Miller MB, Huang AY, Kim J, Zhou Z, Kirkham SL, Maury EA, Ziegenfuss JS, Reed HC, Neil JE, Rento L, Ryu SC, Ma CC, Luquette LJ, Ames HM, Oakley DH, Frosch MP, Hyman BT, Lodato MA, Lee EA, Walsh CA. Somatic genomic changes in single Alzheimer's disease neurons. Nature. 2022 Apr;604(7907):714-722. doi: 10.1038/s41586-022-04640-1. Epub 2022 Apr 20. PMID: 35444284; PMCID: PMC9357465.
Cite this article
Liu,Y. (2025). Characterizing Initial Neuropathological Changes in Degus’ Brains During Alzheimer’s Disease Onset. Theoretical and Natural Science,78,167-175.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Biological Engineering and Medical Science
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017 Sep 16;390(10100):1211-1259. doi: 10.1016/S0140-6736(17)32154-2. Erratum in: Lancet. 2017 Oct 28;390(10106):e38. doi: 10.1016/S0140-6736(17)32647-8. PMID: 28919117; PMCID: PMC5605509.
[2]. https://www.alz.org/media/Documents/alzheimers-facts-and-figures.pdf
[3]. Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, Cummings J, van der Flier WM. Alzheimer's disease. Lancet. 2021 Apr 24;397(10284):1577-1590. doi: 10.1016/S0140-6736(20)32205-4. Epub 2021 Mar 2. PMID: 33667416; PMCID: PMC8354300.
[4]. Karch CM, Goate AM. Alzheimer's disease risk genes and mechanisms of disease pathogenesis. Biol Psychiatry. 2015 Jan 1;77(1):43-51. doi: 10.1016/j.biopsych.2014.05.006. Epub 2014 May 17. PMID: 24951455; PMCID: PMC4234692.
[5]. Tan Z, Garduño BM, Aburto PF, Chen L, Ha N, Cogram P, Holmes TC, Xu X. Cognitively impaired aged Octodon degus recapitulate major neuropathological features of sporadic Alzheimer's disease. Acta Neuropathol Commun. 2022 Dec 19;10(1):182. doi: 10.1186/s40478-022-01481-x. PMID: 36529803; PMCID: PMC9761982.
[6]. Chouliaras L, O'Brien JT. The use of neuroimaging techniques in the early and differential diagnosis of dementia. Mol Psychiatry. 2023 Oct;28(10):4084-4097. doi: 10.1038/s41380-023-02215-8. Epub 2023 Aug 22. PMID: 37608222; PMCID: PMC10827668.
[7]. Gong X, Zhang H, Liu X, Liu Y, Liu J, Fapohunda FO, Lü P, Wang K, Tang M. Is liquid biopsy mature enough for the diagnosis of Alzheimer's disease? Front Aging Neurosci. 2022 Aug 5;14:977999. doi: 10.3389/fnagi.2022.977999. PMID: 35992602; PMCID: PMC9389010.
[8]. Malhotra S, Miras MCM, Pappolla A, Montalban X, Comabella M. Liquid Biopsy in Neurological Diseases. Cells. 2023 Jul 22;12(14):1911. doi: 10.3390/cells12141911. PMID: 37508574; PMCID: PMC10378132.
[9]. https://www.labcorp.com/tests/484415/alzheimer-s-disease-evaluation-profile-csf
[10]. https://app.jove.com/cn/v/56774/an-improved-method-for-collection-of-cerebrospinal-fluid-from-anesthetized-mice
[11]. ZHANG Lu-Lin, YUAN Yu, LIANG Mei-Yu, LIU Ming-Xin, WANG Dong-Xia, XIE Jun-Xia, SONG Ning. A glass micropipette vacuum technique of cerebrospinal fluid sampling in C57BL/6 mice. Acta Physiol Sin 2023; 75 (2): 197-204 (in Chinese with English abstract).
[12]. Graff-Radford J, Yong KXX, Apostolova LG, Bouwman FH, Carrillo M, Dickerson BC, Rabinovici GD, Schott JM, Jones DT, Murray ME. New insights into atypical Alzheimer's disease in the era of biomarkers. Lancet Neurol. 2021 Mar;20(3):222-234. doi: 10.1016/S1474-4422(20)30440-3. PMID: 33609479; PMCID: PMC8056394.
[13]. Kumar A, Nader MA, Deep G. Emergence of Extracellular Vesicles as "Liquid Biopsy" for Neurological Disorders: Boom or Bust. Pharmacol Rev. 2024 Feb 13;76(2):199-227. doi: 10.1124/pharmrev.122.000788. PMID: 38351075; PMCID: PMC10877757.
[14]. Assfaw AD, Schindler SE, Morris JC. Advances in blood biomarkers for Alzheimer disease (AD): A review. Kaohsiung J Med Sci. 2024 Jun 18. doi: 10.1002/kjm2.12870. Epub ahead of print. PMID: 38888066.
[15]. Oruganti M, Gaidhani S. Routine bleeding techniques in laboratory rodents[J]. International Journal of Pharmaceutical Sciences and Research, 2011, 2(3): 516.
[16]. Rose SE, Williams CA, Hailey DW, Mishra S, Kirkland A, Keene CD, Garden GA, Jayadev S, Young JE. Advancements in high-resolution 3D microscopy analysis of endosomal morphology in postmortem Alzheimer's disease brains. Front Neurosci. 2024 Jan 16;17:1321680. doi: 10.3389/fnins.2023.1321680. PMID: 38292900; PMCID: PMC10824887.
[17]. Muñoz-Castro C, Noori A, Magdamo CG, Li Z, Marks JD, Frosch MP, Das S, Hyman BT, Serrano-Pozo A. Cyclic multiplex fluorescent immunohistochemistry and machine learning reveal distinct states of astrocytes and microglia in normal aging and Alzheimer's disease. J Neuroinflammation. 2022 Feb 2;19(1):30. doi: 10.1186/s12974-022-02383-4. PMID: 35109872; PMCID: PMC8808995.
[18]. Miller MB, Huang AY, Kim J, Zhou Z, Kirkham SL, Maury EA, Ziegenfuss JS, Reed HC, Neil JE, Rento L, Ryu SC, Ma CC, Luquette LJ, Ames HM, Oakley DH, Frosch MP, Hyman BT, Lodato MA, Lee EA, Walsh CA. Somatic genomic changes in single Alzheimer's disease neurons. Nature. 2022 Apr;604(7907):714-722. doi: 10.1038/s41586-022-04640-1. Epub 2022 Apr 20. PMID: 35444284; PMCID: PMC9357465.









