1. Introduction
In recent years, the global average obesity rate from the World Health Organization (WHO) rises from 13.1% in 2020 to 14% in 2025 [1]. The obesity rate in Asia is relatively low, and the obesity rate in China is 6.2% [1], which is much lower than the global average, but shows a significant increasing trend [2].
Based on the study of the relationship between intestinal flora and obesity, the composition and function of intestinal flora play a key role in the occurrence and development of obesity [3-7]. The structure of the gut flora in obese populations differs significantly from that of normal weight populations, with obese populations having a lower diversity of gut flora [8-11].
Previous studies systematically analyzed the relationship between gut flora and obesity in North American, South American, and European populations, besides differences in the study population, sample size, and assay methods may cause inconsistencies in the conclusions of different studies on the Firmicutes to Bacteroidetes ratio. The relative abundance of Firmicutes and Actinobacteria was higher in the obese group, and the abundance of Bacteroidetes was lower in the obese group [12]. However, these findings may be influenced by geographic and gender differences. Therefore, the relationship between gut flora and obesity in Asian populations still needs to be further analysed.
2. Methods
2.1. Search strategy and selection criteria
We identified studies published from January 1st, 2015, to June, 2025, through systematic literature searches. Electronic searches were done with PubMed, China Knowledge Network (CNKI), Wanfang Database, and Wipu Information. The English search was based on the search terms "intestinal flora," and "obesity," and "Asia," and the Chinese search was based on the combination of the subject terms "chang dao jun qun" and "fei pang" so as to collect the published research studies on the relationship between intestinal flora and obesity in Asian populations since 2015. Cross-sectional, case-control, or cohort studies thereof were eligible if they: (1) published studies on the relationship between intestinal flora and obesity in Asian adults since 2015; (2) had full-text articles that could be downloaded; (3) revealed correlation coefficient, and 95% confidence intervals (95% CI).
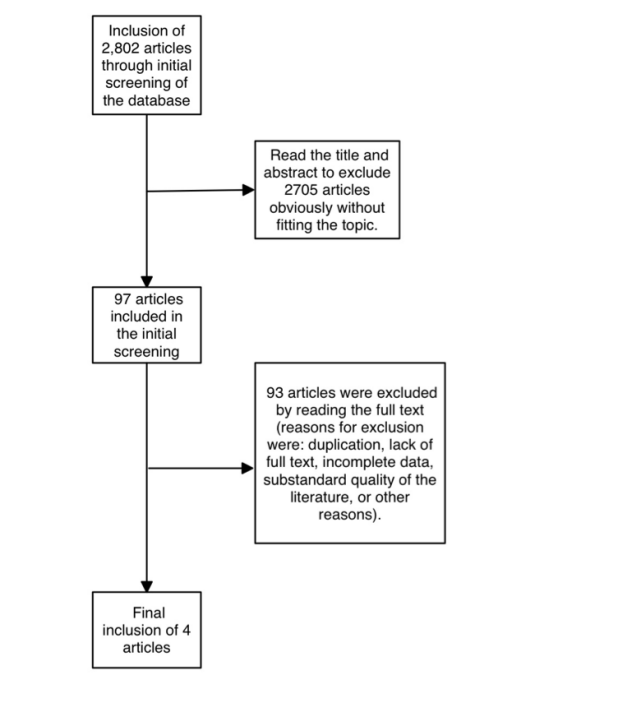
The flow diagram describes the inclusion and exclusion criteria (Figure 1). We excluded studies: (1) literature reviews; (2) the age of the study population was less than 18 years old; (3) only the abundance value of intestinal flora in the obese population was stated, and the specific effect value was not stated; (4) the literature was duplicated, the quality of the literature was substandard, and full texts were not available to be utilized; (5) the STROBE score was lower than 16. One researcher independently reviewed the literature, excluded irrelevant literature, read the full text to include literature that met the criteria, and extracted the title, study place, design, year of publication, age of participants, obesity definition of the literature, the correlation coefficient and the 95% confidence interval (95% CI) of each intestinal flora.
2.2. Statistical analysis
We divided the intestinal flora into different subgroups by the phylum to which they belonged and analyzed them together and separately. Statistical tests were performed using stata18.0, and the correlation coefficient and 95% of each study were used as effect indicators. Heterogeneity was assessed by fisher z-test with test level α = 0.1, and the heterogeneity was assessed by I2. If there was no heterogeneity among studies (P ≥ 0. 1, I2 ≤ 50% ), a fixed-effects model was used for the merger, and if there was statistically significant heterogeneity among studies (P < 0. 1, I2 > 50% ), a random-effects model was used.
3. Results
Table 1 shows the basic data of the included articles, including the region, population, study type, type of gut microbiota, and corresponding effect values. Different regions have different definitions of obesity. The included studies defined obesity as BMI ≥ 24 kg/m² and BMI ≥ 25 kg/m², which are roughly similar. A meta-analysis was performed on the effect values of gut microbiota and BMI.
|
Study ID |
Region |
n |
Intestinal flora |
Definition of obesity |
Results |
Types of research |
STROBE |
|
Yanrong Lv 2019 [13] |
LanZhou |
Total:28; obeisty:10; normal:18. age:18-27 (mean:22) |
Porphyromonadaceae, Acidaminococcaceae, Rikenellaceae, Desulfovibrionaceae , Blautia, Anaerotruncus, Parabacteroides, Alistipes |
BMI ≥24 kg/m2 |
Porphyromonadaceae, Acidaminococcaceae, Rikenellaceae, Desulfovibrionaceae with BMI (r –0·396, P 0·037; r –0·412, P 0·029; r –0·459, P 0·014; r –0·404, P 0·033) Blautia, Anaerotruncus, Parabacteroides and Alistipes with BMI (r –0·419, P 0·026; r –0·465, P 0·013; r –0·416, P 0·028; r –0·380, P 0·046) |
Cross-sectional study |
22 |
|
Yeojun Yun 2017 [14] |
Korea |
Total:1274; obeisty:745; normal:529. age: (mean:45.7) |
Acidaminococcus, Megasphaera, Christensenellaceae unknown genus, Clostridiales unknown family unknown genus |
BMI ≥25kg/m2 |
Acidaminococcus (r 0.378, P 0.002); Megasphaera (r 0.355, P 0.002); Christensenellaceae unknown genus (r -0.055, P 0.003); Clostridiales unknown family unknown genus (r -0.063, P 0.004); Akkermansia (r -0.225, P 0.038) |
Cross-sectional study |
24 |
|
LIU Ying 2022 [15] |
JiLin |
Total:132; obeisty:57; normal:65. age:>20 (mean:56.64) |
Bacteroidetes, Prevotella, Actinobacteria, Bifidobacterium, Lactobacillus, Enterococcus, Streptococcus |
BMI ≥24 kg/m2 |
Bacteroidetes and Prevotella (r 0.403, P<0.01; r 0.420, P<0.01); Actinobacteria and Bifidobacterium (r -0.251, P<0.01; r -0.436, P<0.01); Bifidobacterium (r -0.361, SE 0.109, P 0.001) Lactobacillus (r -0.369, SE 0.127, P 0.004); Enterococcus (beta -0.362, SE 0.117, P 0.002); Streptococcus (beta -0.352, SE 0.134, P 0.008) |
Cross-sectional study |
18.5 |
|
Yoshikuni Sugimura 2022 [16] |
Japen |
Total:848 age:19-93 (mean:50) |
Blauti, Dorea,Eisenbergiella |
ASM/BW |
Blautia (beta -0.0017, P 0.006); Dorea(bata 0.0056, P 00.016); Eisenbergiella (beta -0.0769, P 0.034) |
Cross-sectional study |
22 |
3.1. Meta-analysis results
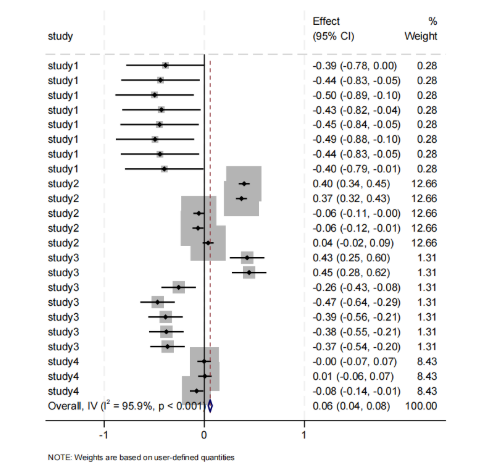
The relationship between gut microbiota and obesity is shown in Figure 2. 17 studies showed statistically significant results. Although most studies indicated a negative correlation between gut microbiota and obesity, due to the high weighting of Yeojun Yun [14], the overall result showed a weak positive correlation (Effect = 0.06, 95% CI [0.04, 0.08]). High heterogeneity and significant variability among studies prompted subgroup analysis.
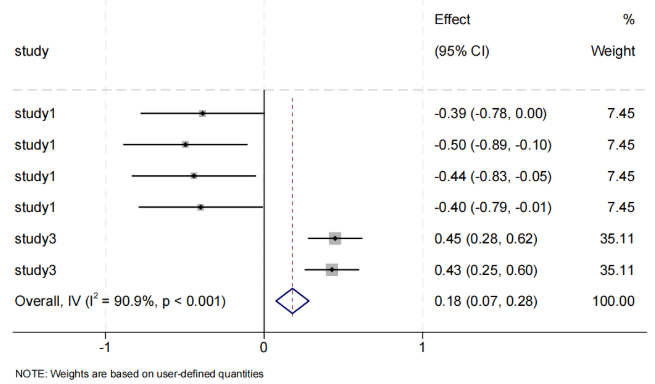
The studies were divided into three subgroups. The relationship between Bacteroidetes gut microbiota and obesity is shown in Figure 3. All six microbiota groups reported statistically significant results. Porphyromonadaceae, Rikenellaceae, Parabacteroides, and Alistipes showed a negative correlation with BMI, while Prevotella showed a positive correlation with BMI. The phylum Bacteroidetes and the overall analysis showed a positive correlation (Effect = 0.18, 95% CI [0.07, 0.28]). Due to high heterogeneity and significant differences among studies, results should be interpreted with caution.
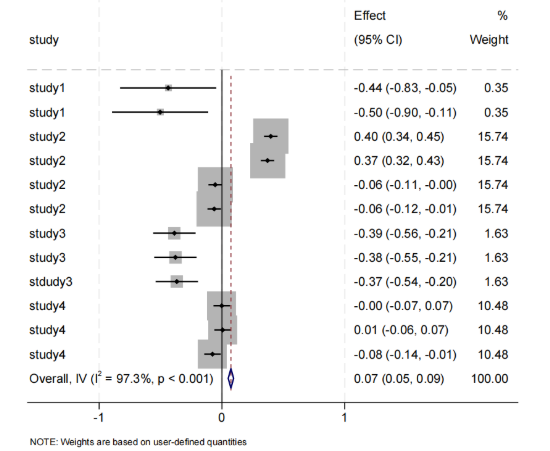
The relationship between the intestinal microbiota of the Firmicutes family and obesity had a positive correlation (Figure 4). Seven bacterial groups were reported to have statistical significance. Acidaminococcaceae, Blautia, Lactobacillus, Enterococcus, and Streptococcus were negatively correlated with BMI, while Acidaminococcus and Megasphaera were positively correlated with BMI, and the phylum Firmicutes were weakly positively correlated (Effect = 0.07, 95% CI [0.05, 0.09]). There was high heterogeneity, with significant differences between studies, and the results require further discussion.
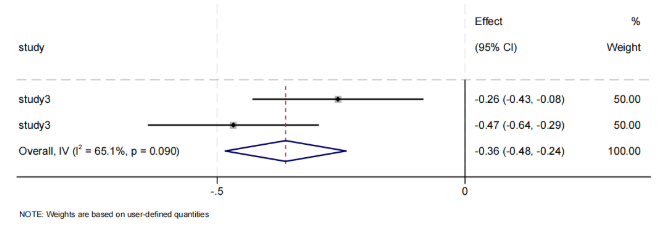
The relationship between Actinobacteria gut microbiota and obesity is shown in Figure 5. Two types of microbiota were statistically significant, both showing negative correlations. The Actinobacteria phylum also showed a negative correlation with obesity (Effect = -0.36, 95% CI [-0.48, -0.24]). There was heterogeneity.
The overall gut microbiota is positively correlated with obesity, with the Bacteroidetes and Firmicutes phyla positively correlated with BMI, and the Actinobacteria phylum negatively correlated with BMI. All meta-analysis results exhibit heterogeneity (P < 0.1, I² > 50%), and a random-effects model was used.
4. Discussion
We found that when analyzing the relationship between the overall gut microbiota and obesity, the results showed both positive and negative correlations, and the same study showed two types of correlations in the microbiota. This is largely related to differences in the microbiota, population, and methods studied. The human gut microbiota is highly diverse, and different bacterial communities exert varying effects on human health. Therefore, In order to determine the accurate relationship between gut microbiota and obesity, we conducted separate analyses of the relationship between different bacterial groups and obesity.
Previous studies found that in obese populations, the abundance of the Firmicutes phylum increases, the abundance of the Bacteroidetes phylum decreases, and the ratio of Firmicutes to Bacteroidetes increases [17-20]. However, our meta-analysis of Asian populations revealed that the Bacteroidetes phylum also showed a positive correlation with obesity. This may be because previous studies were primarily conducted in European countries, and differences in geographical location, population distribution, and dietary structure can all influence the results. Traditional European diets are typically rich in dietary fiber, which promotes the growth of Bacteroidetes. Bacteroidetes can break down fiber to produce short-chain fatty acids, which help regulate appetite and energy metabolism [21]. However, in recent years, the Westernization of Asian diets has led to increased intake of high-fat and high-saturated fat foods among Asian populations, increasing the abundance of Bacteroidetes but reducing their ability to metabolize dietary fiber, resulting in increased energy absorption [22]. Additionally, different regions have different criteria for defining obesity. The World Health Organization defines obesity as a BMI ≥ 30 kg/m² [1], while the studies we included defined obesity as a BMI ≥ 24 kg/m² or 25 kg/m², leading to differences in basal metabolism and gut microbiota distribution among the obese population studied. Studies on the Bacteroidetes phylum presented in Yanrong Lv [13] and LIU Ying [15], which reached completely opposite conclusions. Yanrong Lv [13] found a negative correlation between the Bacteroidetes phylum and obesity, consistent with previous studies, but the sample size was small and the weighting was low. Therefore, LIU Ying [15] concluded the abundance of the Bacteroidetes phylum increases in obese populations with a large sample size. The study population had an older average age in LIU Ying’ s study, which may have been associated with metabolic diseases or other health issues, leading to reduced gut microbiota diversity and abundance, decreased beneficial bacteria, and increased opportunistic pathogens such as Bacteroidetes [23, 24]. The detection method used in this study was cultivation plus mass spectrometry, which may result in underestimation of Bacteroidetes due to potential missed detections. Both studies only adjusted for some variables (age, gender) and did not fully control for other confounding factors, which may lead to inaccuracies in the research. Currently, the core consensus still holds that the abundance of Bacteroidetes is reduced in obese populations in Asian populations, so our conclusions still require further investigation.
The results of the analysis of the Firmicutes phylum were consistent with those in Western populations. This change in microbial community structure was believed to be closely related to alterations in energy metabolism. The Firmicutes phylum tended to extract more energy from food, thereby promoting fat accumulation, and thus showed a positive correlation with obesity [18, 25]. The results exhibited strong heterogeneity, with Yeojun Yun [14] having the largest sample size and the highest weight, thus its positive correlation conclusion dominated the overall results. Although the definition of obesity and testing population in Yoshikuni Sugimura [16] were different with another three studies, it had no statistical significance and was not included in the discussion. The conclusions of the other two studies were that the Firmicutes phylum was negatively correlated with obesity, contrary to Yeojun Yun [14]. This may be because Yeojun Yun [14] focused on Koreans, while the other two studies were conducted in China. Differences in geography and diet can lead to heterogeneity in research results. Yanrong Lv [13] had absence of control over dietary variables and metabolic parameters, as well as the small sample size, which led to the opposite conclusion. The Firmicutes phylum in LIU Ying [15] included beneficial bacteria such as Lactobacillus, Enterobacter, and Streptococcus. Differences in the functional subpopulations may also lead to variations in study results. The Gordon team first proposed that the gut microbiota acts as an environmental regulator of fat storage and obesity, and found that the abundance of Clostridium in the gut of obese individuals increases, while the abundance of Lactobacillus decreases [26], leading to the negative correlation conclusion. Yeojun Yun [14] adjusted dietary factors during testing, used a large sample size, and employed the Illumina MiSeq platform, resulting in higher statistical power. Therefore, the findings align with the general consensus.
The results for the Actinobacteria phylum aligned with the general Western conclusion, showing a negative correlation with obesity. Bifidobacterium is the core genus of Actinobacteria, possessing probiotic functions. It can secrete extracellular polysaccharides (EPS) and short-chain fatty acids (SCFA), promote mucin synthesis, strengthen tight junctions in the intestinal epithelium, and reduce lipopolysaccharide (LPS) entry into the bloodstream [27, 28]. However, only one article analyzed this, so its heterogeneity and accuracy cannot be assessed at this time.
5. Conclusion
Overall, our analysis revealed a certain association between gut microbiota and obesity. There was a weak positive correlation between gut microbiota and obesity in general, but there was significant heterogeneity among studies, and different microbiota had different functions. Therefore, we focused more on the individual associations between different microbiota and obesity. In our study, we observed that the Firmicutes phylum aligned with Western populations, with abundance increasing as BMI increased. The association between Actinobacteria and obesity had been studied less in Asian populations and cannot currently be used for extrapolation. Therefore, further research is needed to investigate the association between gut microbiota and obesity in Asian populations.
References
[1]. World Obesity Federation. Global Obesity Observatory (2023).
[2]. Chu D T , Singh V .Obesity and hypertension in Asia: Current status and challenges. LRHP 2021; 15: 100243.
[3]. Martel J , Ojcius D M , Chang C J. Anti-obesogenic and antidiabetic effects of plants and mushrooms. NAT REV ENDOCRINOL 2017; 13(3): 149-160.
[4]. Gao Y, Liu W, Ma X. The role of intestinal microbiota and its metabolites in the occurrence and intervention of obesity. Front Microbiol 2025; 16: 1559178.
[5]. YANG Qi-Hang, PU R, CHEN Zi-Yang. Role and mechanism of intestinal flora metabolites in the regulation of obesity. Chinese Journal of Tissue Engineering CJTER 2024; 28(2).
[6]. Proença IM, Allegretti JR, Bernardo WM. Fecal microbiota transplantation improves metabolic syndrome parameters: systematic review with meta-analysis based on randomized clinical trials. Nutr Res 2020; 83: 1-14.
[7]. Dao MC, Everard A, Aron-Wisnewsky J. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 2016; 65(3): 426-36.
[8]. YU Miao, XU Argenta Zhao, YANG Xiaoying. Advances in the mechanism and regulation of obesity mediated by intestinal flora. Food Science 2023; 44(15): 339-350.
[9]. Le Chatelier E, Nielsen T, Qin J. Richness of human gut microbiome correlates with metabolic markers. Nature 2013; 29: 541-6.
[10]. Muheyati D, Han J, Lv M, Jielili M. Composition of gut microbiota in obese and normal-weight Uygur adults and its association with adipocyte-related factors. Sci Rep 2024; 14(1): 24649.
[11]. Liu R, Hong J, Xu X, Feng Q. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat Med 2017; 23(7): 859-868.
[12]. Pinart M, Dötsch A, Schlicht K, Laudes M. Gut Microbiome Composition in Obese and Non-Obese Persons: A Systematic Review and Meta-Analysis. Nutrients 2021; 14(1): 12.
[13]. Lv Y, Qin X, Jia H, Chen S. The association between gut microbiota composition and BMI in Chinese male college students, as analysed by next-generation sequencing. Br J Nutr 2019; 122(9): 986-995.
[14]. Yun Y, Kim HN, Kim SE. Comparative analysis of gut microbiota associated with body mass index in a large Korean cohort. BMC Microbiol 2017; 17(1): 151.
[15]. Liu Ying, Tan Yinfeng, Zhang Jinyue. Analysis of differences in gut microbiota between overweight/obese and normal-weight populations. Chinese Clinical Research 2022.
[16]. Sugimura Y, Kanda A, Sawada K. Association between Gut Microbiota and Body Composition in Japanese General Population: A Focus on Gut Microbiota and Skeletal Muscle. Int J Environ Res Public Health 2022; 19(12): 7464.
[17]. Pinart M, Dötsch A, Schlicht K. Gut Microbiome Composition in Obese and Non-Obese Persons: A Systematic Review and Meta-Analysis. Nutrients 2021; 14(1): 12.
[18]. Visconti, A., Le Roy, C.I., Rosa, F. et al. Interplay between the human gut microbiome and host metabolism. Nat Commun 2019; 10: 4505.
[19]. Hiippala K, Jouhten H, Ronkainen A. The Potential of Gut Commensals in Reinforcing Intestinal Barrier Function and Alleviating Inflammation. Nutrients 2018; 10(8): 988.
[20]. Sanna S, van Zuydam NR, Mahajan A. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat Genet 2019; 51(4): 600-605.
[21]. Sonnenburg JL, Bäckhed F. Diet-microbiota interactions as moderators of human metabolism. Nature 2016; 535(7610): 56-64.
[22]. Murphy EF, Cotter PD, Hogan A. Divergent metabolic outcomes arising from targeted manipulation of the gut microbiota in diet-induced obesity. Gut 2013; 62(2): 220-6.
[23]. Adriana Florinela Cӑtoi, Andreea Corina, Niki Katsiki. Gut microbiota and aging-A focus on centenarians. BBA - Molecular Basis of Disease 2020; 7: 0925-4439.
[24]. D’Amelio, P., Sassi, F. Gut Microbiota. Immune System, and Bone. Calcif Tissue Int 2018; 102: 415–425.
[25]. Ridlon JM, Ikegawa S, Alves JM. Clostridium scindens: a human gut microbe with a high potential to convert glucocorticoids into androgens. J Lipid Res 2013; 54(9): 2437-49.
[26]. ZHAO Li-Ping, FEI Na. Research progress on the relationship between intestinal flora and obesity. CMI 2013; 8(02): 67-71.
[27]. Cani PD, Bibiloni R, Knauf C. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008; 57(6): 1470-81.
[28]. Everard A, Belzer C, Geurts L. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A 2013; 110(22): 9066-71.
Cite this article
Zou,J. (2025). Association Between Intestinal Flora and Obesity Among Asian Adults Based on Meta-analysis. Theoretical and Natural Science,124,101-108.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of ICBioMed 2025 Symposium: AI for Healthcare: Advanced Medical Data Analytics and Smart Rehabilitation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. World Obesity Federation. Global Obesity Observatory (2023).
[2]. Chu D T , Singh V .Obesity and hypertension in Asia: Current status and challenges. LRHP 2021; 15: 100243.
[3]. Martel J , Ojcius D M , Chang C J. Anti-obesogenic and antidiabetic effects of plants and mushrooms. NAT REV ENDOCRINOL 2017; 13(3): 149-160.
[4]. Gao Y, Liu W, Ma X. The role of intestinal microbiota and its metabolites in the occurrence and intervention of obesity. Front Microbiol 2025; 16: 1559178.
[5]. YANG Qi-Hang, PU R, CHEN Zi-Yang. Role and mechanism of intestinal flora metabolites in the regulation of obesity. Chinese Journal of Tissue Engineering CJTER 2024; 28(2).
[6]. Proença IM, Allegretti JR, Bernardo WM. Fecal microbiota transplantation improves metabolic syndrome parameters: systematic review with meta-analysis based on randomized clinical trials. Nutr Res 2020; 83: 1-14.
[7]. Dao MC, Everard A, Aron-Wisnewsky J. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 2016; 65(3): 426-36.
[8]. YU Miao, XU Argenta Zhao, YANG Xiaoying. Advances in the mechanism and regulation of obesity mediated by intestinal flora. Food Science 2023; 44(15): 339-350.
[9]. Le Chatelier E, Nielsen T, Qin J. Richness of human gut microbiome correlates with metabolic markers. Nature 2013; 29: 541-6.
[10]. Muheyati D, Han J, Lv M, Jielili M. Composition of gut microbiota in obese and normal-weight Uygur adults and its association with adipocyte-related factors. Sci Rep 2024; 14(1): 24649.
[11]. Liu R, Hong J, Xu X, Feng Q. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat Med 2017; 23(7): 859-868.
[12]. Pinart M, Dötsch A, Schlicht K, Laudes M. Gut Microbiome Composition in Obese and Non-Obese Persons: A Systematic Review and Meta-Analysis. Nutrients 2021; 14(1): 12.
[13]. Lv Y, Qin X, Jia H, Chen S. The association between gut microbiota composition and BMI in Chinese male college students, as analysed by next-generation sequencing. Br J Nutr 2019; 122(9): 986-995.
[14]. Yun Y, Kim HN, Kim SE. Comparative analysis of gut microbiota associated with body mass index in a large Korean cohort. BMC Microbiol 2017; 17(1): 151.
[15]. Liu Ying, Tan Yinfeng, Zhang Jinyue. Analysis of differences in gut microbiota between overweight/obese and normal-weight populations. Chinese Clinical Research 2022.
[16]. Sugimura Y, Kanda A, Sawada K. Association between Gut Microbiota and Body Composition in Japanese General Population: A Focus on Gut Microbiota and Skeletal Muscle. Int J Environ Res Public Health 2022; 19(12): 7464.
[17]. Pinart M, Dötsch A, Schlicht K. Gut Microbiome Composition in Obese and Non-Obese Persons: A Systematic Review and Meta-Analysis. Nutrients 2021; 14(1): 12.
[18]. Visconti, A., Le Roy, C.I., Rosa, F. et al. Interplay between the human gut microbiome and host metabolism. Nat Commun 2019; 10: 4505.
[19]. Hiippala K, Jouhten H, Ronkainen A. The Potential of Gut Commensals in Reinforcing Intestinal Barrier Function and Alleviating Inflammation. Nutrients 2018; 10(8): 988.
[20]. Sanna S, van Zuydam NR, Mahajan A. Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat Genet 2019; 51(4): 600-605.
[21]. Sonnenburg JL, Bäckhed F. Diet-microbiota interactions as moderators of human metabolism. Nature 2016; 535(7610): 56-64.
[22]. Murphy EF, Cotter PD, Hogan A. Divergent metabolic outcomes arising from targeted manipulation of the gut microbiota in diet-induced obesity. Gut 2013; 62(2): 220-6.
[23]. Adriana Florinela Cӑtoi, Andreea Corina, Niki Katsiki. Gut microbiota and aging-A focus on centenarians. BBA - Molecular Basis of Disease 2020; 7: 0925-4439.
[24]. D’Amelio, P., Sassi, F. Gut Microbiota. Immune System, and Bone. Calcif Tissue Int 2018; 102: 415–425.
[25]. Ridlon JM, Ikegawa S, Alves JM. Clostridium scindens: a human gut microbe with a high potential to convert glucocorticoids into androgens. J Lipid Res 2013; 54(9): 2437-49.
[26]. ZHAO Li-Ping, FEI Na. Research progress on the relationship between intestinal flora and obesity. CMI 2013; 8(02): 67-71.
[27]. Cani PD, Bibiloni R, Knauf C. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008; 57(6): 1470-81.
[28]. Everard A, Belzer C, Geurts L. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A 2013; 110(22): 9066-71.









