1. Introduction
NASA’s criteria for drinkable water, to ensure the crew’s comfort while consuming it, mandates that it should be devoid of any free gas at a temperature of 38 ℃ (100 ℉) and a pressure of 101.3 kPa (14.7 psia). Small water bubbles might make the crew feel uncomfortable and change how the water tastes [1]. Unfortunately, there was no acceptable separator to remove the gas from the CWC-I drinking water supply. The CWC-I, used to store a supply of potable water, utilized a gas-permeable bladder. This would lead to the formation of bubbles, which over time would grow to such an extent that the number of bubbles would no longer be acceptable. In addition, there may still be bubbles in drinking water due to gas dissolution, microgravity environment, chemical reactions and microbial metabolism [2]. Thus, the degassing process in the water recovery and management system is essential. By degassing, not only can the longevity of machine become longer, but also make better the feeling of drinking water for astronaut.
Typical gases that need to be removed are oxygen, nitrogen, carbon dioxide and volatile organic compounds. These gases come out of solution in the cosmic environment with reduced gravity. Early Space Shuttle water harvesting systems used gravity-dependent processes that were not effective in zero gravity. It was necessary to develop additional techniques for the ISS. Typical bubble removal methods tested include centrifugal separation [3] and membrane degassing using hollow fibres [1]. The current ISS system uses a combination of hollow fibre membrane and centrifugal separators to efficiently remove bubbles from wastewater. Researchers are still working to improve degassing efficiency, as full bubble removal remains challenging.
This article will first discuss the current situation about dealing with bubbles in international space station, then providing some future directions of the development of the bubble-remove techniques by analyzing literature review. The objective of this article is to introduce the current state of the bubble-removing process in the international space station, highlight the drawbacks of the existing method, and subsequently offer future directions for the advancement of bubble-removing techniques.
2. General description
Degassing is necessary to remove free gases from the water as bubbles can disturb the crew and change the taste of the water. At the same time, the incomplete gas/liquid separation in the water electrolysis system not only has a negative effect on the downstream subsystems, but also leads to a high H2O content, since the water required for the electrolysis and the product gas are in constant contact with each other [4]. Additionally, some conceptual units of the Rotary Battery/Separator (RSA), which acts as a battery and phase separator for the OGA (Oxygen Generator Assembly) in the International Space Station’s zero-gravity environment, require degassing prior to use [3]. Degassing research is also important for the development and optimization of systems such as a drinking water purifier and a water reclamation and management system for the International Space Station. Understanding the causes of out-gassing and developing effective out-gassing methods can ensure the production of potable water that meets NASA specifications and crew comfort requirements. Common degassers include membrane degassers, centrifugal separators, and hollow fiber separators [2,3].
The determination of a separation method for life support system design is contingent upon the attributes of gas and liquid flow, phase classification, liquid properties, liquid type, and the intended purpose of liquid separation. Various studies have been conducted on the removal of bubbles, as the operational efficiency of the liquid separator directly determines the effectiveness and dependability of the water recovery system. Ohya et al. [5] proposed the utilization of a strainer to separate bubbles from the liquid. Imai and Yano [6, 7] introduced a technique involving the use of an electric field to transport bubbles and suggested a cyclone separator. Wakayama demonstrated the transfer of bubbles using a magnetic field [8]. There are multiple categorizations of separators accessible, but for zero-gravity operations, two kinds are suitable: stationary separators that utilize the material’s wetting capability through surface tension forces, and rotational separators that depend on inertial forces. Stationary separators provide several primary merits, including strong dependability, minimal energy consumption, user-friendly nature, and the production of separated liquid of excellent quality. Nevertheless, a noteworthy disadvantage of current stationary separators in water reclamation systems is their relatively restricted lifespan. This is particularly applicable when dealing with gas and liquid streams containing contaminated and bio-genetic liquids, as the device can become obstructed due to excessive growth of micro-flora. However, this issue can be addressed by selecting appropriate materials and implementing a primary filter. In addition, thoroughly crafted stationary separators can not only withstand bio-fouling conditions but also enhance their performance [9-12].
3. Analysis on degassing problem
3.1. Membrane Based Gas-Liquid Separator for the Space Station Water Processor
3.1.1. General introduction. The space station’s water processor’s membrane-based gas-liquid separator was created in 2001. The membrane separator’s original goal was to stop microbiological transmission, which was a problem for onboard water systems. Scientists saw the separator’s potential to save crew time spent eliminating the tiny bubbles and realized its usefulness. The membrane-based gas-liquid separator therefore comes into being [2]. To achieve the “no free gas” criterion, the ultimate gas-liquid phase separation is supposed to be carried out at an elevated temperature as a result of the operating pressure of the system (2 psig, 14 kPa). Additionally, the gas-liquid s is required to remove any extra oxygen that was introduced during the catalytic reactor’s operation. According to the derived specifications, the gas-liquid separator is required to operate within a temperature range of 17 ℃ to 88 ℃ (63 ℉ to 190 ℉) and remove 99.75% of the free gas, as per the operation standard. The membrane and potting technique that was discovered proved to be highly suitable for achieving phase separation in a Water Processor. This technology possesses several important features, including outstanding chemical inertness, compatibility with high temperatures, the tubular fiber structure, and the encapsulated inner layer.
By utilizing the gas-liquid separator component and orbital replacement unit design, the available resources are optimally utilized to passively eliminate 99.75% of liberated gas detected in a dual-phase inlet flow within a microgravity setting. This is accomplished through precise temperature control, minimal heat loss, and the application of a specific volume of sweeping gas in a condensed configuration [1].
3.1.2. Product description. Figure 1 depicts the gas-liquid separator configuration, comprising four tubular fiber modules, each encompassing a minimum of 1300 hydrophobic tubular fiber membranes. These modules are housed within Inconel enclosures, which are securely sealed with O-rings at both ends. As the biphasic flow progresses through each module in sequence, the permeate gases are simultaneously gathered from each individual module. Fitted with a encompassing heating element, every Inconel enclosure guarantees the upkeep of the suitable temperature. The modules, secured to an end plate, showcase a link for the shared permeate output. Additionally, each module features a central sweep gas port that regulates condensation. By incorporating an opening in each distribution leg, uniform delivery of sweep gas to the modules is ensured [1].
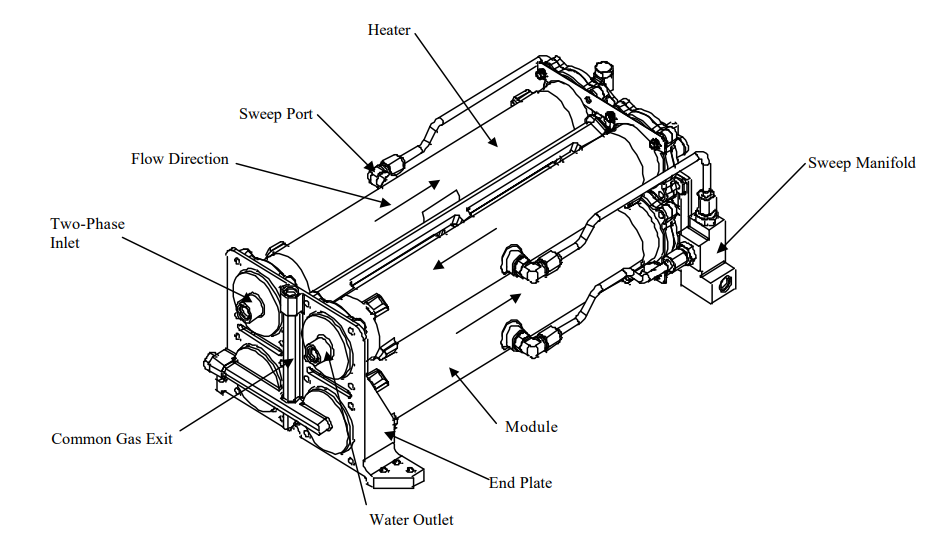
Figure 1. Gas-liquid separator assembly [1].
3.1.3. Possible future development. Due to fouling, membranes are vulnerable to performance loss. To reduce this impact, the membrane’s skinned structure and chemical compatibility should be combined with the flow-through design. An operational flight configuration catalytic reactor will be operated downstream of a flight-like GLS component for an extended period of time during testing [1].
3.2. Centrifugal gas-liquid separator
Utilizing powerful centrifugal force, a centrifugal decanter is used to separate mixes with minor density variations. In low-gravity situations, centrifugal force separation is deemed effective as a result of the notable disparity in density within the gas-liquid system [8].
3.2.1. Design and working principle. There are two kinds of centrifugal gas-liquid separator, the separator A and separator B, which are shown in the figure 2 and figure 3. A two-dimensional ring pipe serves as separator A. When there are bubbles in the liquid, centrifugal force will cause the bubbles to condense inside the pipe. In Separator B, a cyclone separator, bubbles in a gas-liquid two-phase fluid are drawn into a vortex that has formed around the cylinder’s axis by centrifugal force [8].
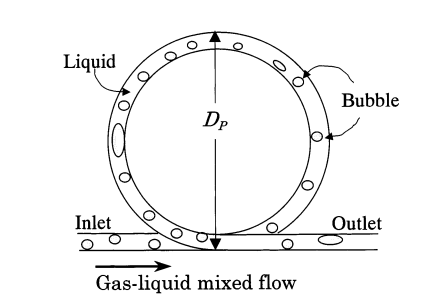
Figure 2. Centrifugal separator A [8].
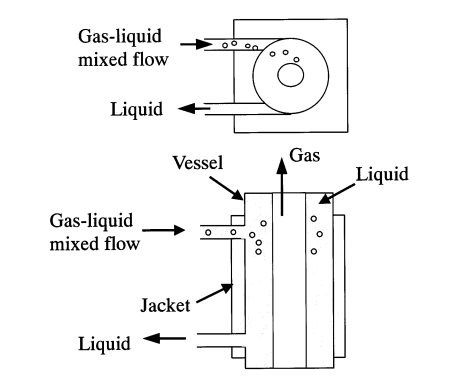
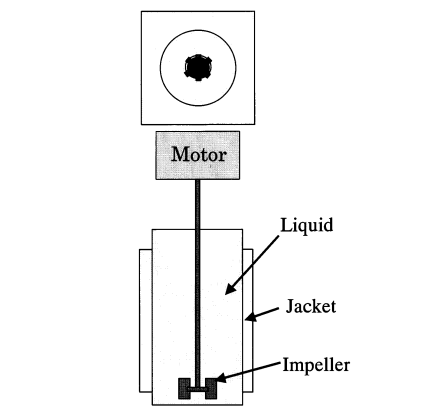
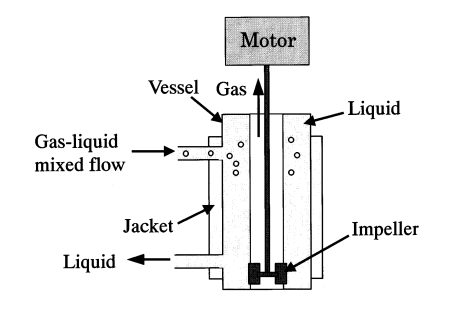
Figure 3. Centrifugal separators B (B-1, B-2 and B-3) [8].
3.2.2. Separation efficiency. Separator A works well for separating bigger bubbles, however it might not be the best choice for tiny bubble separation. The gas-liquid flow velocity, stirring rate, impeller diameter, and other variables may have an impact on the separation effect of separator B. Larger gas-liquid flow rates, stirring speeds, and impeller diameters may facilitate the bubble’s separation from the liquid more effectively because they may result in a shorter transport path to the inner vortex [8]. The disadvantage of separator A is that it is difficult to completely separate small bubbles in the gas-liquid mixture. Although the utilization of centrifugal force is efficient in segregating sizable bubbles, attaining full separation of diminutive bubbles poses a challenge.
3.3. Gas-Liquid Separator for Water Electrolysis
Water electrolysis, known as the process of utilizing electrical energy to split water into hydrogen and oxygen, is employed for this purpose. In addition to producing H2 and O2, moisture will also be present. Therefore, it is crucial to separate gas and liquid in the water electrolysis system [4]. Water supply to the electrolysis cell in the prior arrangement required water circulation in order to remove the produced H2 and cool the cell. Prior research did note certain issues brought on by water circulation, though. Later, a straightforward system was built by designing an electrolysis device with a gas/liquid separation function. Not only may the system’s size and mass be considerably decreased, but by minimizing pressure drops and preserving thermal efficiency, more power can be saved [4].
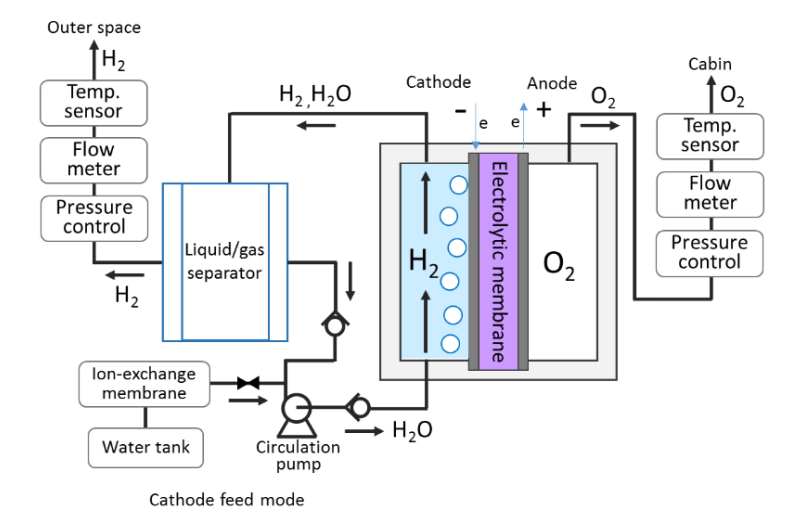
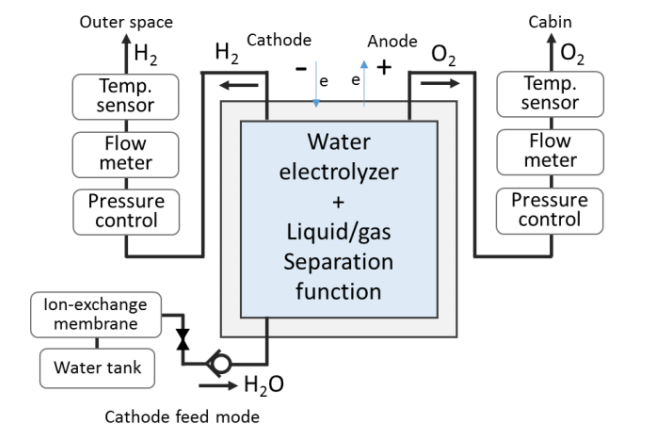
Figure 4. The water electrolysis systems (left: without gas-liquid separator, right: with gas-liquid separator) [4].
However, pressure changes inside the electrolytic cell frequently happen when the unit has an initial moisture content. In order for the hydrogen gas to be released through the PTFE hydrophobic membrane smoothly, maintain the initial moisture level inside the electrolytic cell unit low.
Therefore, for reliable gas/liquid separation, it is important to study the contact between gas and water inside the electrolysis unit.
4. Suggestion
After carefully examining the gas-liquid separator devices, the study discovered two types of separators for the water processor in space stations: the membrane-based gas-liquid separator and the centrifugal gas-liquid separation. There was also a gas-liquid separator application for water electrolysis. We discovered that the contact between gas and water is not completely stable and that fouling might cause membranes to lose performance. The optimal performance of the separator may be maintained by changing the membranes on a regular basis because they are prone to performance loss. The interface between gas and water inside the electrolysis unit has to be studied for reliable gas/liquid separation in water electrolysis. The literature review portions for centrifugal gas-liquid separation did not provide details on how to increase productivity. Future study in this area can be advanced by emphasizing that both stirring speed and impeller diameter will impact efficiency.
5. Conclusion
This passage first introduces two types of gas-liquid separator in the water recovery and management system: the membrane-based gas-liquid separator and the centrifugal gas-liquid separator. After that, the application of the bubble removing process in water electrolysis has also been introduced. For the membrane-based gas-liquid separator, it is required to operate within a temperature range of 17 ℃ to 88 ℃ (63 ℉ to 190 ℉) and remove 99.75% of the free gas, according to an operation standard derived from these specifications. However, due to fouling, membranes are vulnerable to performance loss. To reduce this impact, the membrane’s skinned structure and chemical compatibility should be combined with the flow-through design. The future experiments will be performed in the extended tests. For centrifugal gas-liquid separator, separator A and separator B both responsible for gas-liquid separation. Separator A functions as a two-dimensional ring pipe. The presence of bubbles within the liquid results in the condensation of said bubbles within the pipe due to centrifugal force. Separator B, known as a cyclone separator, utilizes centrifugal force to create a vortex around the axis of the cylinder, drawing in bubbles from a gas-liquid two-phase fluid. However, an inherent drawback of separator A is its limited ability to fully separate small bubbles within the gas-liquid mixture. While centrifugal force proves efficient in separating larger bubbles, achieving complete separation of smaller bubbles poses a challenge. For the gas-liquid separator in water electrolysis, by reducing pressure drops and maintaining thermal efficiency, not only can more power be conserved, but also the system’s size and mass can be significantly reduced. However, pressure changes inside the electrolytic cell frequently happen when the unit has an initial moisture content. Therefore, for reliable gas-liquid separation, it is important to study the contact between gas and water inside the electrolysis unit in the future.
References
[1]. C. Thibaud-Erkey, A. Lanzarone, A. Lurie, D. Snowdon, and K. S. Cheng, Jul. 09, 2001. “Development of a Membrane Based Gas-Liquid Separator for the Space Station Water Processor”
[2]. C. Brown and B. Tobias, vol. 5, pp. 4–640, 2020. “History, Current Operations and Management of Water Systems on the International Space Station”
[3]. D. Samplatsky and W. Clark Dean, SAE technical paper series, Jul. 2002, “Development of a Rotary Separator Accumulator for Use on the International Space Station”
[4]. M. Sakurai, Jul. 2017. “Study on Water Electrolysis for Oxygen Production -Reduction of Water Circulation and Gas-Liquid Separator”
[5]. H. Ohya, M. Oguti, A. Hakamaya, K. Matsumoto, and Y. Negishi, Japan Society of Aeronautical Space Sciences, vol. 39, pp. 109–115, Jan. 1991. “Gas-liquid separation with microporous hollow fiber membrane,”
[6]. R. Imai and T. Yano, Nippon Kikai Gakkai Ronbunshu. B Hen (Transactions of the Japan Society of Mechanical Engineers. Part B), vol. 60, Dec. 1994. “Bubble transfer by electrostatic force; Seidenkiryoku wo riyoshita kiho iso,”
[7]. R. Imai and T. Yano, Nippon Kikai Gakkai Ronbunshu. B Hen (Transactions of the Japan Society of Mechanical Engineers. Part B), vol. 63, Jul. 1997. “Study of bubble separation utilizing centrifugal force under a reduced gravity condition; Bisho juryoku kankyoyo enshinryoku riyo kiho jokyo sochi ni kansuru kenkyu,”
[8]. H. Ahn, K. Tanaka, H. Tsuge, K. Terasaka, and K. Tsukada, Separation and Purification Technology, vol. 19, no. 1–2, pp. 121–129, Jun. 2000, “Centrifugal gas–liquid separation under low gravity conditions,”
[9]. K. A. A., Acta Astronautica, vol. 80, pp. 40–45, Nov. 2012, “A promising method of liquid separation in orbital station’s life support systems,”
[10]. E. Thomas, M. Weislogel, D. Klaus, Adv. Space Res. Design strategies for sustainable spacecraft fluid management systems 46 (2010) 761–767
[11]. E. Thomas, D. Muirhead, Wastewater fouling impact on capillary contact angle, Biofouling 2 (5) (2009) 445–454
[12]. M. Weislogel, E. Thomas, J. Graf, A Novel, Microgravity Sci. Technol. Device addressing design challenges for passive fluid phase separations aboard spacecraft 21 (3) (2009) 257–268
Cite this article
Li,S. (2024). Analysis on degassing at microgravity condition. Applied and Computational Engineering,58,176-181.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. C. Thibaud-Erkey, A. Lanzarone, A. Lurie, D. Snowdon, and K. S. Cheng, Jul. 09, 2001. “Development of a Membrane Based Gas-Liquid Separator for the Space Station Water Processor”
[2]. C. Brown and B. Tobias, vol. 5, pp. 4–640, 2020. “History, Current Operations and Management of Water Systems on the International Space Station”
[3]. D. Samplatsky and W. Clark Dean, SAE technical paper series, Jul. 2002, “Development of a Rotary Separator Accumulator for Use on the International Space Station”
[4]. M. Sakurai, Jul. 2017. “Study on Water Electrolysis for Oxygen Production -Reduction of Water Circulation and Gas-Liquid Separator”
[5]. H. Ohya, M. Oguti, A. Hakamaya, K. Matsumoto, and Y. Negishi, Japan Society of Aeronautical Space Sciences, vol. 39, pp. 109–115, Jan. 1991. “Gas-liquid separation with microporous hollow fiber membrane,”
[6]. R. Imai and T. Yano, Nippon Kikai Gakkai Ronbunshu. B Hen (Transactions of the Japan Society of Mechanical Engineers. Part B), vol. 60, Dec. 1994. “Bubble transfer by electrostatic force; Seidenkiryoku wo riyoshita kiho iso,”
[7]. R. Imai and T. Yano, Nippon Kikai Gakkai Ronbunshu. B Hen (Transactions of the Japan Society of Mechanical Engineers. Part B), vol. 63, Jul. 1997. “Study of bubble separation utilizing centrifugal force under a reduced gravity condition; Bisho juryoku kankyoyo enshinryoku riyo kiho jokyo sochi ni kansuru kenkyu,”
[8]. H. Ahn, K. Tanaka, H. Tsuge, K. Terasaka, and K. Tsukada, Separation and Purification Technology, vol. 19, no. 1–2, pp. 121–129, Jun. 2000, “Centrifugal gas–liquid separation under low gravity conditions,”
[9]. K. A. A., Acta Astronautica, vol. 80, pp. 40–45, Nov. 2012, “A promising method of liquid separation in orbital station’s life support systems,”
[10]. E. Thomas, M. Weislogel, D. Klaus, Adv. Space Res. Design strategies for sustainable spacecraft fluid management systems 46 (2010) 761–767
[11]. E. Thomas, D. Muirhead, Wastewater fouling impact on capillary contact angle, Biofouling 2 (5) (2009) 445–454
[12]. M. Weislogel, E. Thomas, J. Graf, A Novel, Microgravity Sci. Technol. Device addressing design challenges for passive fluid phase separations aboard spacecraft 21 (3) (2009) 257–268









