1. Introduction
The development of human society is facing various problems, among which environmental issues are closely related to people’s . Excessive carbon dioxide emissions are a significant factor contributing to global warming. Global warming results in melting polar glaciers and rising sea levels, among other issues. However, from the perspective of green chemistry, carbon dioxide contains abundant carbon atoms. By fully utilizing carbon dioxide as a raw material, it can be converted into needed resources and reused. This not only addresses the issue of excessive carbon dioxide emissions, but also contributes to enhancing resource diversity, thereby promoting the development of sustainable chemistry. [1] With the development of green chemistry, the conversion schemes of carbon dioxide have become more diversified. The catalytic conversion of carbon dioxide is mainly achieved through techniques such as thermal catalysis, photocatalysis, electrocatalysis, solar photothermal synergistic catalysis, and biocatalysis. The main carbon dioxide conversions include methane, methanol, formic acid, carbon monoxide, and olefins.[2] Catalysts play a crucial role in the conversion process, making high conversion efficiency and high selectivity catalysts key research areas in carbon dioxide conversion. Biocatalytic conversion of carbon dioxide involves using living cells or enzymes as catalysts to synthesize complex organic molecules from carbon dioxide. Biocatalysts have advantages such as high selectivity, green and mild operating conditions, and easy facilitation of carbon-carbon coupling, aiding in converting carbon dioxide into high-value-added products. However, due to the high chemical inertness of carbon dioxide, its activation and the breaking of the C=O bond pose a challenge. Drawing inspiration from natural photosynthesis, researchers proposed an electroenzymatic coupling strategy for the catalytic conversion of carbon dioxide. This method employs an electrochemical system, combined with immobilized or non-immobilized enzymes, using electrical energy to drive the enzymatic cleavage of the C=O bond and convert carbon dioxide into high-value compounds.[3] This method has higher efficiency and can yield a variety of products, which is significant for converting carbon dioxide.
2. Enzyme-electric conversion
2.1. Existing CO₂ conversion technologies
Carbon dioxide conversion methods predominantly include thermal catalysis, photocatalysis, electrocatalysis, and biocatalysis. Thermal catalysis is notable for its high rates, but it requires a significant amount of energy, leading to the production of simple products that can be easily scaled up. While clean and mild, photocatalysis yields simple products with relatively low efficiency. Electrocatalysis also offers clean and mild conditions with high efficiency, leading to the production of simple products that are easily scalabole. Although efficient and capable of generating complex products, Biocatalysis necessitates additional energy input. Enzyme catalysis faces challenges such as the high cost of sacrificial donors and the low efficiency of NADH regeneration. Innovations in photochemical and electrochemical NADH regeneration methods, and advanced non-NADH systems present new solutions to these challenges. Artificial electroactive media and cofactor-free systems enhance the feasibility and sustainability of enzymatic CO₂ conversion to formate. Given the multifunctional properties of enzyme catalysis, potential applications include carbon upgrading via multi-enzyme cascades and whole-cell catalysis. [4] The electrolyte-coupled catalysis CO₂ reduction system leverages renewable electrical energy to drive enzymatic CO₂ reduction, facilitating the conversion of electrical to chemical energy and achieving greater efficiency and diversity of products. CO₂, as an inert gas, is typically unreactive and requires catalysts to reduce the reaction energy barrier. The primary catalysts include noble and transition metal salts and oxides on substrates like metal-organic frameworks, molecular sieves, activated carbon, zirconia, alumina, and magnesia. Additionally, some non-metallic, novel, and bulk catalysts have demonstrated catalytic activity for CO₂ conversion.[5]
2.2. Enzyme-electrocatalysis
Enzyme-catalyzed CO2 conversion typically involves the activation of CO2 molecules at the enzyme's active site, lowering the subsequent reaction energy barriers and enhancing process efficiency. The key lies in the enzyme's ability to stabilize the transition state of the reaction, which is often the rate-limiting step. For example, formate dehydrogenase catalyzes the conversion of CO2 to formate by transferring electrons from donor molecules such as NADH to CO2. This electron transfer is facilitated by the enzyme's active site, which simultaneously binds the donor and CO2, optimally positioning them for the reaction.
In electroenzymatic systems, electrodes play a crucial role as sites for electron transfer, providing the electrons needed for reduction reactions. Enzymes can be immobilized on electrodes through various techniques such as adsorption, covalent attachment, or encapsulation. The choice of immobilization method affects the enzyme's stability and activity and the efficiency of electron transfer. Additionally, the electrochemical environment can be finely tuned to optimize the performance of enzyme conversion. Parameters such as pH, temperature, and applied potential significantly affect the enzyme's activity and selectivity. Electrochemical techniques also allow for real-time monitoring and control of the reaction, providing insights into reaction kinetics and mechanisms.
The applications of CO2 electroenzymatic conversion are diverse, with significant potential in sustainable energy and chemical production. One of the main applications is the production of renewable fuels. For example, as a key product of CO2 reduction, methanol can be used as a fuel or for synthesizing various chemicals. The electroenzymatic method provides a sustainable approach to methanol production, utilizing CO2 as a carbon source and renewable electricity as the energy input. Another important application is the synthesis of fine chemicals and pharmaceuticals. Electroenzymatic processes can produce high-value chemicals with high specificity under mild conditions. For instance, the electroenzymatic reduction of CO2 can efficiently produce valuable chemicals such as formic acid used in industrial processes. Compared to traditional chemical synthesis, this method has the advantages of low energy consumption and minimal environmental impact.
Additionally, CO2 electroenzymatic conversion can play a role in carbon capture and utilization (CCU) strategies. By converting captured CO2 into valuable products, this technology provides economic incentives for CO2 capture, thereby increasing its attractiveness to industries that emit large amounts of CO2. This method helps in reducing greenhouse gas emissions and promotes a circular economy by transforming waste CO2 into useful products.[6]
In conclusion, the mechanisms and applications of CO2 electroenzymatic conversion offer promising avenues for addressing environmental challenges and promoting sustainable development. The integration of enzymes and chemical processes offers a unique approach to utilizing CO2 as a resource, paving the way for innovative energy and chemical production solutions.
2.3. Key enzymes in CO₂ electroconversion electron transfer mechanisms
The electrochemical conversion of carbon dioxide (CO₂) relies on specific enzymes that can efficiently catalyze the transformation of CO₂ under mild conditions. Enzymes are crucial in the electrochemical conversion of CO₂, by significantly reducing the activation energy of the reaction and enhancing both selectivity and efficiency. Common multi-enzyme systems include formate dehydrogenase and carbon monoxide dehydrogenase.[7]
Table 1: Carbon Dioxide Conversion Formulas.
Formate dehydrogenase (NAD(P)H dependent): | \( CO₂ + NADH → HCOO⁻ + NAD⁺ \) |
Carbon monoxide dehydrogenase (Ni/Fe dependent): | \( CO₂ + 2H⁺ + 2e⁻ → CO + H₂O \) |
Formate dehydrogenase (FDH) is an enzyme that reduces CO₂ to formate (HCOO⁻). Its active center typically contains metals such as molybdenum, tungsten, or nickel, which play crucial roles in the catalytic process. FDH's application in CO₂ electrochemical conversion is primarily reflected in its efficient electron transfer capability and high selectivity. In electrochemical environments, FDH typically combines with electrodes through various immobilization methods to form stable enzyme-electrode complexes. Standard immobilization methods include physical adsorption, covalent bonding, and polymer embedding. Immobilized FDH can maintain high activity and remain stable over extended periods. Additionally, the choice of electrode material significantly has a significant impact on the catalytic performance of FDH. Commonly used materials include gold and electrochemically modified carbon.Carbon monoxide dehydrogenase (CODH) is an enzyme capable of catalyzing the reduction of CO₂ to carbon monoxide (CO). Its active center typically contains nickel and iron, which play key roles in the enzyme's catalytic cycle. CODH's application in CO₂ electrochemical conversion is mainly reflected in its efficient CO production capability and tolerance to reaction conditions. In practical applications, CODH is combined with electrode materials to achieve efficient CO₂ conversion through electrochemical driving. CODH's immobilization methods are similar to those of FDH, with common techniques including physical adsorption, chemical bonding, and encapsulation in nanomaterials.
The electron transfer mechanism plays a critical role in the enzymatic electrochemical conversion of CO₂. Depending on the enzyme, they can be classified into NAD(P)H-independent and NAD(P)H-dependent oxidoreductases. They exhibit different electron transfer characteristics when interacting with electrodes.[8]
NAD(P)H-independent oxidoreductases have significant applications in CO₂ electrochemical conversion. Figure1 demonstrates the catalytic conversion of cofactor-independent system.These enzymes do not rely on cofactors like NAD(P)H but transfer electrons directly through the electrode. This direct electron transfer (DET) mechanism has high electron transfer efficiency, effectively reducing the reaction overpotential.
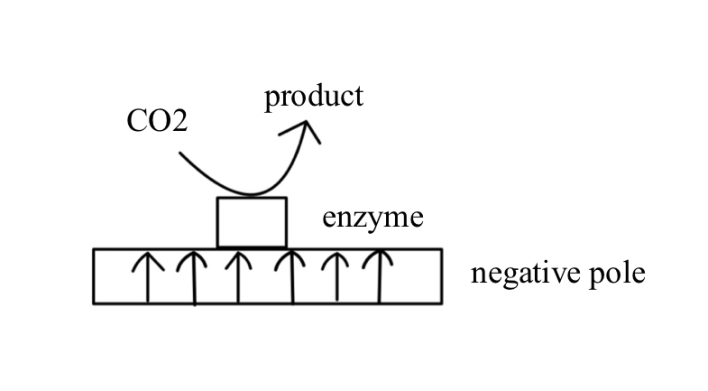
Figure 1: Cofactor-independent System.
NAD(P)H-dependent oxidoreductases also have important applications in CO₂ electrochemical conversion. Figure2 shows the catalytic conversion of the Cofactor-dependent system. These enzymes facilitate the transfer of electrons through cofactors such as NAD(P)H, utilizing a relatively complex mechanism that typically requires mediated electron transfer (MET). In the electrochemical systems of NAD(P)H-dependent oxidoreductases, the selection of electron mediators is crucial. Electron mediators refer to molecules or materials that facilitate the transfer of electrons between the electrode and the enzyme. Common electron mediators include metal complexes (such as ferranide), organic dyes (such as methylene blue), and polymers (such as polypyrrole). Choosing the appropriate electron mediator can significantly enhance electron transfer efficiency, improving overall catalytic performance. The stability and regeneration capability of electron mediators are also important factors to consider when making a selection.
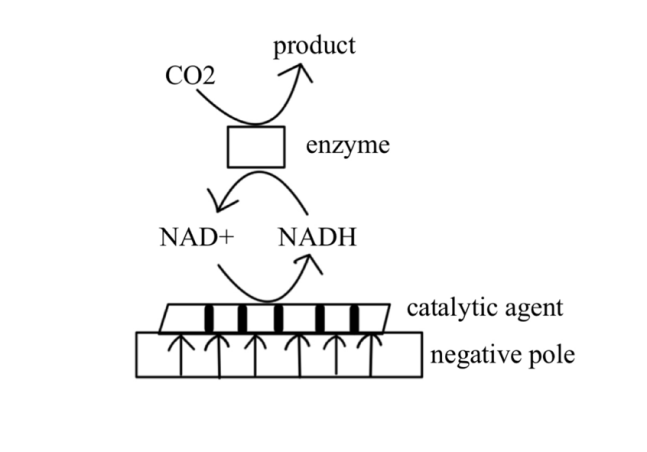
Figure 2: Cofactor-dependent System.
3. Innovations in Enzyme-electrocatalysis
Enzymatic-electrocatalysis still encounters several challenges. Recent advancements involve the integration of multi-enzyme systems and the introduction of artificial cofactors. Emerging trends include photoelectrochemical enzyme systems and enzyme immobilization techniques. Recently, Liu Jian’s team at the Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences, optimized a novel photo/electro-enzyme coupling pathway, synthesizing high-value chemicals. The team synthesized Rh-ZnIn2S4 with asymmetric interlayer polarization properties, facilitating the efficient and selective conversion of terminal aromatic alcohols. Additionally, the researchers achieved optimal NADH regeneration efficiency, as reported in the literature, providing favorable kinetic conditions for coupling with formate dehydrogenase. These combined achievements established a novel pathway that utilizes formate generated from CO2 electroreduction to mediate NADH regeneration, thereby driving enzymatic reactions for the continuous synthesis of high-value chemicals. The study employs a bismuth-based electrocatalyst, which stably reduces CO2 to formate with high current density and selectivity. The in-situ generated formate is used for NADH regeneration via a Rh complex, combined with dehydrogenases immobilized on the substrate, enabling the efficient continuous synthesis of target chemicals. The enzyme-based turnover numbers (TON) reached 1.8×10^6 to 3.1×10^6, surpassing currently reported levels.
Xing focuses on utilizing polarized ZnIn2S4 nanoflowers, in combination with Rh complexes, to efficiently regenerate NADH and selectively produce valuable chemicals like benzaldehyde. This system offers a promising route for achieving carbon-neutral industrial processes. Rh complex-modified ZIS (M-ZIS) demonstrated outstanding performance in NADH regeneration and the selective oxidation of benzyl alcohol to benzaldehyde. The results exhibited nearly 100% selectivity for benzaldehyde, alongside high NADH regeneration efficiency, underscoring the system’s potential for industrial applications. The study also explored the substrate scope, demonstrating the broad applicability of this synergistic system across various aromatic alcohols.
Wang developed a continuous electro-enzyme pathway for producing valuable chemicals[10]. This process utilizes formate from electrochemical CO2 reduction to regenerate NADH, thereby driving multiple enzymatic reactions. The study reported high efficiency (~90%) in converting CO2 to formate, along with significant yields of target chemicals like alcohols, lactic acid, and glutamic acid. The CO2 reduction reaction employed a bismuth-based electrocatalyst, which efficiently converts CO2 to formate. The form is then utilized with immobilized redox enzymes for NADH regeneration. This system includes enzymes such as alcohol dehydrogenase (ADH), lactate dehydrogenase (LDH), and glutamate dehydrogenase (GDH), facilitating the high-yield production of chemicals. This breakthrough represents a significant step towards achieving carbon-neutral chemical production and holds promise for large-scale industrial applications.
4. Conclusion
The global environment is currently facing significant challenges, with a particular focus on the issue of global warming driven by excessive CO₂ emissions. To address the environmental impact of carbon dioxide, there is a growing emphasis on CO₂ conversion. Common catalytic methods for this purpose include photocatalysis, electrocatalysis, and enzymatic catalysis. To achieve more efficient conversion, scientists have adopted coupled catalysis as an innovative research approach. Combining enzymatic catalysis and electrocatalysis for CO₂ conversion is an innovative method to harness valuable resources. Enzyme-electrocatalytic carbon dioxide conversion merges enzymatic and electrocatalytic processes, offering an efficient and clean solution for CO₂ utilization. The electron transfer mechanisms in enzyme-electrocatalytic systems are examined, particularly focusing on the roles of NAD(P)H-dependent and non-NAD(P)H-dependent oxidoreductases. The challenges and limitations of these systems in practical applications are also evaluated. Current challenges are analyzed, and recent advancements in the field are illustrated through case studies. Successful applications of enzyme-electrocatalytic systems in CO₂ conversion are demonstrated, including discussions on formic acid-mediated electroenzymatic synthesis and other innovative methods. An innovative photo/electro-enzymatic catalysis pathway has been developed, enabling the continuous synthesis of high-value chemicals. This approach enhances NADH and enzyme activity, significantly increasing the potential for large-scale electro-enzymatic synthesis. These technologies hold great potential for sustainable chemistry and environmental protection. Despite current challenges, the potential benefits for sustainable development and environmental protection render this a promising research area.
References
[1]. He, L.-N., Li, H.-R., Xie, W.-J., Wu, A.-G., Zhao, L., Zhang, Y.-K., Yao, X., & Chen, J.-M. (2024). Fundamental science for carbon dioxide valorization and its transformation pathways. Chinese Science Bulletin. https://doi.org/10.1360/TB-2024-0186
[2]. Pan, G. F., Ma, Y. Y., Xu, D. H., & others. (2023). Research status on catalyst of CO2 catalytic conversion. Journal of Environmental Engineering Technology, 13(1), 79-84. https://doi.org/10.12153/j.issn.1674-991X.20210763
[3]. Lü, Y., Su, H., Qin, P., et al. (2023). Advances in electrocatalytic-enzymatic hybrid systems for CO2 reduction. Chinese Journal of Bioprocess Engineering, 21(5), 471-484. https://doi.org/10.3969/j.issn.1672-3678.2023.05.001
[4]. Chen, H., Huang, Y., Sha, C., Mohammadi Moradian, J., Yong, Y.-C., & Fang, Z. (2023). Enzymatic carbon dioxide to formate: Mechanisms, challenges, and opportunities. Renewable and Sustainable Energy Reviews, 178, 113271. https://doi.org/10.1016/j.rser.2023.113271
[5]. Luan, L., Ji, X., Guo, B., Cai, J., Dong, W., Huang, Y., & Zhang, S. (2023). Bioelectrocatalysis for CO2 reduction: Recent advances and challenges to develop a sustainable system for CO2 utilization. Biotechnology Advances, 63, 108098. https://doi.org/10.1016/j.biotechadv.2023.108098
[6]. Zhang, Z. X., Zhang, Y. W., Zhang, X. J., & Wang, L. (2024). Technical progress related to the production of chemicals through catalytic conversion of carbon dioxide. Petroleum Refinery Engineering(07), 9-12. https://doi.org/10.20138/j.cnki.issn1002-106X.2024.07.003
[7]. Zhang, X., Guo, S.-X., Gandionco, K. A., Bond, A. M., & Zhang, J. (2020). Electrocatalytic carbon dioxide reduction: From fundamental principles to catalyst design. Materials Today Advances, 7, 100074. https://doi.org/10.1016/j.mtadv.2020.100074
[8]. Wu, R., Ma, C., & Zhu, Z. (2020). Enzymatic electrosynthesis as an emerging electrochemical synthesis platform. Current Opinion in Electrochemistry, 19, 1-7. https://doi.org/10.1016/j.coelec.2019.08.004
[9]. Xing, F., Xue, X., Li, J., Liu, J., Wang, W., Dong, W., Yuan, H., & Liu, J. (2024). Sustainable photocatalytic biological cofactor regeneration fueled by selective alcohol oxidation over polarized ZnIn2S4. ACS Catalysis, 14(15), 11366-11377. https://doi.org/10.1021/acscatal.4c01703
[10]. Wang, C., Dong, W., Zhang, P., Ma, Y., Han, Z., Zou, Y., Wang, W., Li, H., Hollmann, F., & Liu, J. (2024). Formate-mediated electroenzymatic synthesis via biological cofactor NADH. Angewandte Chemie International Edition, n/a(n/a), e202408756. https://doi.org/10.1002/anie.202408756
Cite this article
Yu,X. (2025). Research and Significance of Electroenzymatic Conversion of Carbon Dioxide. Applied and Computational Engineering,123,248-254.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 5th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. He, L.-N., Li, H.-R., Xie, W.-J., Wu, A.-G., Zhao, L., Zhang, Y.-K., Yao, X., & Chen, J.-M. (2024). Fundamental science for carbon dioxide valorization and its transformation pathways. Chinese Science Bulletin. https://doi.org/10.1360/TB-2024-0186
[2]. Pan, G. F., Ma, Y. Y., Xu, D. H., & others. (2023). Research status on catalyst of CO2 catalytic conversion. Journal of Environmental Engineering Technology, 13(1), 79-84. https://doi.org/10.12153/j.issn.1674-991X.20210763
[3]. Lü, Y., Su, H., Qin, P., et al. (2023). Advances in electrocatalytic-enzymatic hybrid systems for CO2 reduction. Chinese Journal of Bioprocess Engineering, 21(5), 471-484. https://doi.org/10.3969/j.issn.1672-3678.2023.05.001
[4]. Chen, H., Huang, Y., Sha, C., Mohammadi Moradian, J., Yong, Y.-C., & Fang, Z. (2023). Enzymatic carbon dioxide to formate: Mechanisms, challenges, and opportunities. Renewable and Sustainable Energy Reviews, 178, 113271. https://doi.org/10.1016/j.rser.2023.113271
[5]. Luan, L., Ji, X., Guo, B., Cai, J., Dong, W., Huang, Y., & Zhang, S. (2023). Bioelectrocatalysis for CO2 reduction: Recent advances and challenges to develop a sustainable system for CO2 utilization. Biotechnology Advances, 63, 108098. https://doi.org/10.1016/j.biotechadv.2023.108098
[6]. Zhang, Z. X., Zhang, Y. W., Zhang, X. J., & Wang, L. (2024). Technical progress related to the production of chemicals through catalytic conversion of carbon dioxide. Petroleum Refinery Engineering(07), 9-12. https://doi.org/10.20138/j.cnki.issn1002-106X.2024.07.003
[7]. Zhang, X., Guo, S.-X., Gandionco, K. A., Bond, A. M., & Zhang, J. (2020). Electrocatalytic carbon dioxide reduction: From fundamental principles to catalyst design. Materials Today Advances, 7, 100074. https://doi.org/10.1016/j.mtadv.2020.100074
[8]. Wu, R., Ma, C., & Zhu, Z. (2020). Enzymatic electrosynthesis as an emerging electrochemical synthesis platform. Current Opinion in Electrochemistry, 19, 1-7. https://doi.org/10.1016/j.coelec.2019.08.004
[9]. Xing, F., Xue, X., Li, J., Liu, J., Wang, W., Dong, W., Yuan, H., & Liu, J. (2024). Sustainable photocatalytic biological cofactor regeneration fueled by selective alcohol oxidation over polarized ZnIn2S4. ACS Catalysis, 14(15), 11366-11377. https://doi.org/10.1021/acscatal.4c01703
[10]. Wang, C., Dong, W., Zhang, P., Ma, Y., Han, Z., Zou, Y., Wang, W., Li, H., Hollmann, F., & Liu, J. (2024). Formate-mediated electroenzymatic synthesis via biological cofactor NADH. Angewandte Chemie International Edition, n/a(n/a), e202408756. https://doi.org/10.1002/anie.202408756









