1. Introduction
Nanomaterials have found extensive applications across various fields, Especially in the field of medicine. The shortcomings of traditional medical materials, for example: traditional medical materials have issues with biocompatibility, insufficient strength and elasticity to meet the needs of medical devices or implants, inadequate degradation and stability, and problems with toxicity and side effects. The advantages of nanomaterials over traditional materials include: better biocompatibility, mechanical properties, degradability, lower toxicity, and their multifunctionality (e.g., magnetic nanoparticles providing high precision in diagnostics, which enhances efficiency). Due to these unique properties of nanomaterials, that address challenges in targeted delivery and precision medicine, making them key to treating complex diseases such as cancer and neurodegenerative disorders. The definition of nanomaterials depends on their size (1-100 nanometres) and their unique surface-to-volume ratio, Due to the fact that nanomaterials also possess characteristics such as high strength and toughness, fluorescent properties, photothermal effects, photosensitive properties, reactivity, and environmental responsiveness. which endue them distinctive mechanical, optical, and chemical properties. The widespread application of nanomaterials in the medical field has driven the development of medical technology and provided strong support for precision medicine and personalized treatment. This review explores the primary applications of nanomaterials in medicine, their potential advantages, and current challenges. This article mainly discusses drug delivery and the application of nanoparticles, medical imaging, diagnosis and treatment, tissue engineering and regenerative medicine, and the future potential and challenges of nanomaterials.
2. Application of Nanomaterials in Drug Delivery Systems
2.1. Nanocarrier Types and Designs
2.1.1. Metal nanoparticles
Gold nanoparticles: Gold nanoparticles have unique optical properties that can be used for targeted drug delivery and as photothermal agents for cancer treatment. Their surfaces can be modified to attach ligands, thereby enhancing specificity, this targeted approach helps to reduce off-target effects and improve therapeutic efficiency in drug delivery applications. [1] For example, Gold nanoparticles are particularly useful in cancer treatment because they can enhance the delivery of drugs to tumor sites due to their high surface area, which allows for a higher drug loading capacity. At the same time, the surface of gold nanoparticles can be customized with molecular markers targeting cancer cells, thereby increasing the selective uptake of these cells. Moreover, the unique optical properties of AuNPs enable them to act as contrast agents in imaging, helping to precisely track drug delivery within the body. [2]
The core is gold-containing gold nanoparticles, which are a low-toxicity agent with excellent optical, plasmonic, and magnetic properties, as well as a large surface area. [3] These monolayers can contain targeted drugs or other drugs such as small molecules, peptides, proteins, and antibodies. [4]
Iron oxide nanoparticles: Iron oxide nanoparticles are magnetic and have extensive applications in magnetic resonance imaging and targeted drug delivery, particularly in the field of oncology. MRI is a non-invasive medical imaging technology that utilizes a strong magnetic field, radiofrequency waves and a computer to generate detailed images of the inside of the human body.
For example, recent research on IONPs (iron oxide nanoparticles) in MRI and drug delivery applications has emphasized their superparamagnetic properties, which make them effective T2-weighted MRI contrast agents. IONPs enhance imaging by reducing proton relaxation times, thereby increasing the contrast between different tissues in MRI. [5] IONPs are a class of nanoparticles based on iron oxides (e.g., Fe3O4 or γ-Fe2O3) compositions that typically have diameters in the range of 1 to 100 nm.
Additionally, when exposed to an external magnetic field, drugs can be targeted for release, guiding them to specific areas within the body, reducing side effects and improving therapeutic outcomes. Coatings such as silica, dextran, chitosan, and Polyethylene Glycol can improve the stability of nanoparticles, prevent aggregation, and provide sites for drug attachment, further enhancing targeted drug delivery capabilities. This allows IONPs to be guided to specific locations, thereby reducing the side effects of drugs. [6]
2.1.2. Polymer Nanoparticles
Biodegradable polymers: Polymers such as lactic acid and glycolic acid help control drug release, minimize side effects, and enhance biocompatibility.
Lactic acid and glycolic acid nanoparticles are often used in drug delivery systems because they can encapsulate therapeutics and release them in a controlled manner. These polymers degrade into non-toxic byproducts such as lactic acid and glycolic acid within the body, which are metabolized naturally by the body and do not cause harm. This controlled degradation allows for precise release rates, making them suitable carriers for drugs that require extended release or targeted delivery to specific tissues. [7]
PLGA (glycolic acid) nanoparticles have been shown to be excellent carriers for the in vivo and in vitro transport of biomolecules, such as transporting RNA, DNA, peptides, vitamins, proteins, and drugs. glycolic acid has been demonstrated to transport DNA,[8] and glycolic acid nanoparticles extravasate through tumor vasculature, delivering the payload to cells via the enhanced permeability and retention (EPR) effect, thus achieving drug delivery to tumor tissues through the EPR effect. [9]
2.1.3. Nanostructure
Quantum dots: Quantum dots are increasingly used in the field of medical imaging due to their fluorescent properties, which make them suitable for imaging. They offer many advantages over traditional organic dyes and fluorescent compounds, but there are concerns regarding cytotoxicity. Factors such as water solubility, toxicity, and stability need to be considered. [10] Additionally, the quantum dots also wisely use in the other medicinal field, such as cancer diagnostics and treatment and drug delivery.
Quantum dots use in term of cancer diagnostics and treatment, quantum dots improve tumor detection through targeted imaging, exploiting their ability to bind specific biomarkers. Additionally, functionalized quantum dots can be used in photodynamic and photothermal therapies to destroy cancer cells with minimal damage to surrounding tissues. In term of drug delivery, the quantum dots deliver drugs precisely to targeted cells, reducing systemic side effects. Their functional surface allows for versatile drug conjugation and controlled release, making them ideal for complex therapeutic strategies. [11]
2.2. Targeted drug delivery system
Common nanomaterials used for drug delivery include liposomes, polymeric nanoparticles, and metallic nanoparticles such as gold and iron oxide. For example, liposomes can coat both hydrophilic and hydrophobic drugs, preventing drug degradation and ensuring controlled drug release. Polymer nanoparticles are biodegradable, thereby reducing harm to the human body. Metal nanoparticles can be functionalized with ligands to target specific cells, and iron oxide nanoparticles can be magnetically guided to deliver drugs to tumors.
Research on nanoparticle drug carriers that utilize the enhanced permeability and retention (EPR) effect and ligand-based surface modifications for active targeting has shown promising results in targeted therapy. The EPR effect allows nanoparticles to accumulate in tissues with high vascular permeability (such as tumors), thereby facilitating passive targeting, while surface modifications with ligands (e.g., antibodies, peptides, or aptamers) enable active targeting of specific cell receptors.
2.3. Clinical application cases of nanomedicine delivery systems
Drug delivery methods for heart disease: Liposomes, silica nanoparticles, dendrimers, cerium oxide nanoparticles, micelles, TiO2 nanoparticles, stents with nanocoatings, microbubbles, and polymer-drug conjugates can all be used for drug delivery. Magnetic liposomes (ML) are formed by combining magnetic nanoparticles with liposomes. Drugs are loaded onto nanoparticles for effective delivery within cells, and experiments have been conducted on mice with myocardial infarction. [12]
3. The application of nanomaterials in medical imaging
3.1. The application of nanomaterials in MRI imaging
One example is the efficacy of superparamagnetic iron oxide nanoparticles (SPIONs) in enhancing magnetic resonance imaging (MRI) contrast. Due to its super magnetic properties, it causes local magnetic field inhomogeneity and shortens the T2 and T2* relaxation times. This results in a low-intensity signal that effectively contrasts strongly with the surrounding tissue. Likewise, gadolinium-based nanoparticles can enhance T1-weighted images due to their strong paramagnetic properties, resulting in brighter imaging contrast. [13]
3.2. The application of nanomaterials in computed tomography imaging
Nanomaterials are used for computed tomography imaging, including gold and bismuth nanoparticles, which have excellent contrast due to their high atomic numbers. For instance, gold nanoparticles have a strong ability to absorb X-rays. Nanomaterials are typically functionalized with targeting ligands or polymers, allowing them to specifically accumulate in diseased tissues, thereby enhancing the specificity and effectiveness of imaging. For example, functionalization with peptides or antibodies helps guide these nanoparticles to cancer cells, thereby enhancing image-guided precision therapy. Research also focuses on combining computed tomography imaging with therapeutic applications, where nanoparticles act as drug carriers or as agents in photothermal or photodynamic therapy, thus achieving simultaneous diagnosis and treatment. [14, 15]
Gold nanoparticles and iodine-based nanomaterials are often used as contrast agents, For example, gold nanoparticles have a high atomic number, which makes them ideally suited for enhancing contrast in CT imaging, especially in cancer diagnostics. In addition, iron oxide nanoparticles are used for targeted imaging and functional studies, contributing to accurate disease monitoring.
4. Application of nanomaterials in diagnosis and treatment
4.1. Nanosensor and biomarker detection
Nano sensors utilize the unique electrical, optical, and magnetic properties of nanomaterials to significantly enhance detection sensitivity, thereby enabling the identification of biological molecules at very low concentrations. This capability is useful in detecting cancers, infectious diseases, and more. For instance, some Nano sensors use nanoparticles such as quantum dots or gold nanoparticles for optical detection, providing high signal amplification and stability. Other devices use nanostructured materials in electrochemical sensors to increase electron transfer rates, thereby enhancing the sensitivity and selectivity of biomarker detection. Functionalizing these nanoparticles with specific ligands or antibodies can achieve targeted recognition of biomarkers. [16, 17]
4.2. The application of nanomaterials in cancer treatment
In cancer treatment, nanomaterials such as nanoparticles, liposomes, and polymeric nanocarriers can overcome challenges associated with traditional therapies. These materials utilize the enhanced permeability and retention effect to more effectively target tumor sites. Specifically, NPs can be functionalized to deliver chemotherapeutic drugs directly to tumor cells, thereby reducing side effects and enhancing drug efficacy. For instance, nanoparticles coated with specific ligands, such as monoclonal antibodies, can selectively target cancer cells, thus enhancing the therapeutic effect. For neurodegenerative diseases such as Alzheimer's and Parkinson's, the use of nanomaterials to improve drug delivery is being explored. [18, 19]
5. Nanomaterials in tissue engineering and regenerative medicine
5.1. Bone and cartilage regeneration
Materials such as nanofibers and nanoparticles are capable of building bone tissue scaffolds. By loading growth factors or drugs, these scaffold materials can promote cell proliferation and thus repair damaged tissues.
Nanofibers produced by electrospinning technology provide a scaffold structure that is very similar to the natural bone extracellular matrix, which aids in cell adhesion, proliferation, and differentiation. Electrospun nanofibers can be made from synthetic polymers, natural materials, or composites, and can be combined with bioactive molecules to promote osteogenesis, vascularization, and inflammation control.
Nanoparticles also enhance scaffold performance or act as drug delivery carriers. For instance, hydroxyapatite nanoparticles embedded in biodegradable polymers can mechanically reinforce scaffolds and support osteoblast growth. Additionally, carbon-based nanoparticles are utilized due to their inherent biocompatibility and structural properties that promote cell interactions. [20]
5.2. Nerve regeneration
Electrically conductive nanomaterials such as carbon nanotubes and conductive polymer nanomaterials have made progress in neural tissue engineering. It has been shown that these materials can support the adhesion and proliferation of nerve cells and promote the growth of neuronal axons, which is expected to be used in the treatment of nerve injuries.
Carbon nanotubes, graphene, and graphene derivatives are materials that inherently possess high conductivity, biocompatibility, and structural properties. These materials can facilitate electrical stimulation in neural tissue, which greatly aids in guiding the growth of nerve cells and enhancing cell communication in damaged areas. For instance, the unique structure of graphene allows it to support the adhesion, proliferation, and differentiation of neuronal cells, making it an important component in neural engineering scaffolds.
Various forms of carbon allotropes exist, such as carbon nanotubes, graphene, and fullerenes, which exhibit unique properties due to the distinctive arrangement of carbon atoms. The versatility of carbon nanomaterials encompasses a range of sizes and shapes, allowing for precise tuning of their physical and chemical characteristics. Carbon and carbon-based materials possess unique mechanical, electrical, thermal, tribological, and biological properties across different carbon allotropes, making them widely applied. Carbon-based materials have excellent biocompatibility. Furthermore, carbon nanomaterials have tunable electrical conductivity, which has been applied in areas such as bionic neurons. Graphene and carbon nanotubes stand out due to their exceptional material properties compared to other allotropes. [21] The Figure 1 illustrates the application of various materials in various organs or tissues, as well as the extent of material use over time.
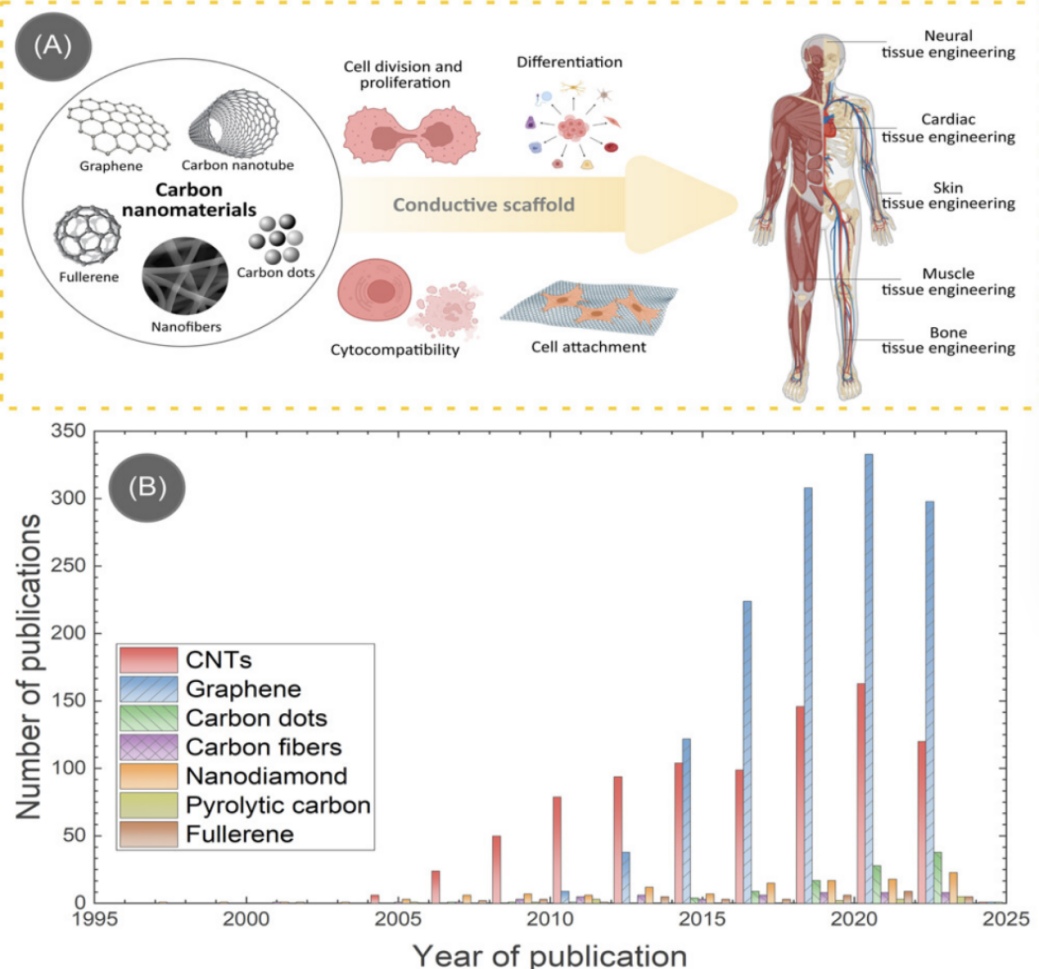
Figure 1: A. Carbon nanomaterials and their use in various tissue engineering applications. B. number of publications on carbon-based tissue engineering scaffolds for different carbon allotropes, as devised from the Web of Science. [21]
5.3. Cardiovascular tissue regeneration
Nanocomposites, such as nanofibrous scaffolds containing bioactive substances, have been used for vascular and cardiac tissue regeneration. They not only have good mechanical properties, but also promote cell proliferation and differentiation through the slow release of growth factors.
These nanomaterials can provide a structural support that facilitates cell attachment and growth, while also delivering essential biochemical cues to guide stem cell differentiation towards cardiomyocyte lineages. This approach is advancing both scaffold designs and stem cell-based therapies for cardiovascular regeneration. [22]
Nanofiber material, such as polylactic acid and polylactic acid - supplements, widely used in the manufacture of scaffolds that facilitate the regeneration of the heart. These nanofibers are able to promote cardiomyocyte decay, ischemia and urination by mimicking the structure of the natural extracellular matrix. One of the studies showed that the use of polylactic acid - supplements nanofiber scaffolds promoted the regeneration of cardiomyocytes in a cardiac model and improved the functional recovery of cardiac cell regeneration. [23]
6. Future trends and challenges in nanomedicine
6.1. Recent research progress in nanomedicine
The advancement of nanomedicine has propelled the development of nanoparticles as drug carriers, enhancing the precision and effectiveness of drug delivery. These nanocarriers, which include liposomes, micelles, and polymer nanoparticles, can be designed to target specific tissues or cells, reducing side effects and enhancing therapeutic outcomes. For instance, researchers are working on developing sustained-release drug formulations to decrease the treatment frequency for patients with diseases such as cancer.
The latest advancements in materials nanomedicine involve the use of various nanomaterials such as liposomes, polymeric nanoparticles, and inorganic nanoparticles to enhance drug delivery, imaging, and therapeutic effects, applied in areas such as cancer treatment, immunotherapy, and gene therapy. These nanomaterials can provide targeted drug delivery and controlled release, thereby achieving precise treatment and reducing side effects. Major developments include multifunctional nanomaterials for simultaneous therapy and diagnosis, as well as engineered nanoparticles that can provide genetic material for regenerative medicine.
6.2. Scientific and technological challenges faced by nanomedicine
Although the nanoparticles currently under study have reduced toxicity in medicine, the potential toxicity of organ accumulation can still have long-term effects on the human body. Therefore, the next direction of research is functional nanomedicines with enhanced passive targeting, active targeting, and stimulus responsiveness. [24] For example, metal nanoparticles (gold nanoparticles and silver nanoparticles) is widely used in cancer treatment. The research shows the gold nanoparticles may cause cytotoxicity and accumulate in organs such as the liver, hepatic and other organs, causing adverse reactions. And the silver nanoparticles have an effect on the oxidative reactions of cells, which may lead to DNA damage.
Uncontrollability of drug release: Although nanoparticles enable emergency drug release, the control of drug release remains a challenge in practical applications. For example, although PLGA nanoparticles have good biodegradability in clinical applications, the control of drug release remains a challenge in practical applications. The rate of drug release cannot be accurately controlled. This leads to too fast or too slow release of the drug in some cases, affecting the efficacy of the treatment.
7. Conclusion
Nanomaterials have transformative potential in medicine, revolutionizing drug delivery, diagnostics, therapy and regenerative medicine. Nanocarriers such as liposomes and polymer nanoparticles enable targeted drug delivery, improving efficacy and minimizing side effects. Stimulus-responsive nanomaterials can control drug release based on environmental triggers and improve therapeutic precision. In medical diagnostics and therapeutics, quantum dots and magnetic nanoparticles improve imaging sensitivity for early and accurate disease detection. Multifunctional nanoparticles combine diagnostics and therapeutics (therapeutic diagnostics), allowing for simultaneous treatment and monitoring, thereby simplifying patient care. In regenerative medicine, nanomaterials can be used as scaffolds that mimic extracellular matrix to promote tissue repair and regeneration in bone, cartilage and skin. Nanoparticles can also deliver growth factors or genetic material to advance gene therapy and enable precise cellular repair mechanisms. These innovations address major medical challenges such as cancer treatment, immunotherapy and gene therapy by providing targeted delivery and controlled release. Despite the promise of nanomaterials, they face a number of challenges, such as potential long-term toxicity due to organ accumulation. Regulatory oversight and clinical trials are critical to ensure safety. Recent advances have focused on enhancing passive and active targeting as well as stimulus response functions to improve therapeutic efficacy. Future research must address scalability and toxicity issues to pave the way for safer and more efficient nanomedicine solutions. Nanomaterials will redefine modern healthcare, providing innovative approaches to treatment and diagnosis.
References
[1]. Abdullah Abdelkawi, Aliyah Slim, Zaineb Zinoune and Yashwant Pathak. Surface Modification of Metallic Nanoparticles for Targeting Drugs. Coatings, 2023, 13(9), 1660.
[2]. Hang Li, Hui Xu, Shuo Yao, Shengnan Wei, Yi Liu, Xuening Shi, Wei Zhao & Chao Zhao. Target-inhibited MCOF-Apt-AuNPs self-assembly for multicolour colorimetric detection of Salmonella Typhimurium. Nature, npj Science of Food, 2024, 8, 78.
[3]. Ekaterina Y. Lukianova-Hleb, Daniel S. Wagner, Malcolm K. Brenner, Dmitri O. Lapotko. Cell-specific transmembrane injection of molecular cargo with gold nanoparticle-generated transient plasmonic nanobubbles. Biomaterials, 2012, 33, 21, 5441-5450.
[4]. Mohamed Yafout, Amine Ousaid, Youssef Khayati, Ibrahim Sbai El Otmani. Gold nanoparticles as a drug delivery system for standard chemotherapeutics: A new lead for targeted pharmacological cancer treatments. Scientific African, 2021, 11, e00685.
[5]. Samuel D. Oberdick, Kalina V. Jordanova, John T. Lundstrom, Giacomo Parigi, Megan E. Poorman, Gary Zabow & Kathryn E. Keenan. Iron oxide nanoparticles as positive T1 contrast agents for low-field magnetic resonance imaging at 64 mTSamuel D. Oberdick, Kalina V. Jordanova, John T. Lundstrom, Giacomo Parigi, Megan E. Poorman, Gary Zabow & Kathryn E. Keenan. Scientific Reports, 2023, 13, 11520.
[6]. Ruirui Qiao, Changkui Fu, Helen Forgham, Ibrahim Javed, Xumin Huang, Jiayuan Zhu, Andrew K. Whittaker, Thomas P. Davis. Magnetic iron oxide nanoparticles for brain imaging and drug delivery. Advanced Drug Delivery Reviews, 2023, 197, 114822.
[7]. Bret D. Ulery, Lakshmi S. Nair, Cato T. Laurencin. Biomedical applications of biodegradable polymers. Polymer Physics, 2011, 12, 49, 832-864.
[8]. Hemin Nie, Lai Yeng Lee, Hui Tong, Chi-Hwa Wang. PLGA/Chitosan composites from a combination of spray drying and supercritical fluid foaming techniques: New carriers for DNA delivery. Journal of Controlled Release, 2008, 3, 129, 207-214
[9]. Sarbari Acharya, Sanjeeb K. Sahoo. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Advanced Drug Delivery Reviews, 2011, 3, 63, 170-183.
[10]. Angela M. Wagner, Jennifer M. Knipe, Gorka Orive, Nicholas A. Peppas. Quantum dots in biomedical applications. Acta Biomaterialia, 2019, 94, 44-63
[11]. Aisha Hamidu, William G. Pitt, and Ghaleb A. Husseini. Recent Breakthroughs in Using Quantum Dots for Cancer Imaging and Drug Delivery Purposes. Nanomaterials, 2023, 13 (18), 2566.
[12]. Obaid Afzal, Abdulmalik S. A. Altamimi, Muhammad Shahid Nadeem, Sami I. Alzarea, Waleed Hassan Almalki, Aqsa Tariq, Bismillah Mubeen, Bibi Nazia Murtaza, Saima Iftikhar, Naeem Riaz and Imran Kazmi. Nanoparticles in Drug Delivery: From History to Therapeutic Applications. Nanomaterials, 2022, 12(24), 4494
[13]. Sofia Caspani, Ricardo Magalhães, João Pedro Araújo and Célia Tavares Sousa. Magnetic Nanomaterials as Contrast Agents for MRI. Materials, 2020, 13(11), 2586.
[14]. Deepak Gupta, Indrajit Roy, Sona Gandhi. Metallic nanoparticles for CT-guided imaging of tumors and their therapeutic applications. Open Nano, 2023, 12, 100146.
[15]. Naim Aslan, Burhan Ceylan, Mümin Mehmet Koç, Fehim Findik. Metallic nanoparticles as X-Ray computed tomography (CT) contrast agents: A review. Journal of Molecular Structure, 2020, 1219, 128599.
[16]. Esmaeil Heydari-Bafrooei, Ali A. Ansafi. Nanomaterials-based biosensing strategies for biomarkers diagnosis, a review. Biosensors and Bioelectronics: X, 2023, 13, 100245.
[17]. Masoud Khazaei, Marzieh Sadat Hosseini, Ali Moshfegh Haghighi, Majid Misaghi. Nanosensors and their applications in early diagnosis of cancer. Sensing and Bio-Sensing Research, 2023, 41, 100569.
[18]. Mahwash Mukhtar, Muhammad Bilal, Abbas Rahdar, Mahmood Barani, Rabia Arshad, Tapan Behl, Ciprian Brisc, Florin Banica and Simona Bungau. Nanomaterials for Diagnosis and Treatment of Brain Cancer: Recent Updates. Chemosensors, 2020, 8(4), 117.
[19]. Mominur Rahman, Rezaul Islam, Shopnil Akash, Harun-Or-Rashid, Tanmay Kumar Ray, Saidur Rahaman, Mahfuzul Islam, Fazilatunnesa Anika, Kawser Hosain, Farjana Islam Aovi, Hassan A. Hemeg, Abdur Rauf, Polrat Wilairatana, Recent advancements of nanoparticles application in cancer and neurodegenerative disorders: At a glance. ScienceDirect, Biomedicine & Pharmacotherapy, 2022, 153, 113305.
[20]. Samira Farjaminejad, Rosana Farjaminejad and Franklin Garcia-Godoy. Nanoparticles in Bone Regeneration: A Narrative Review of Current Advances and Future Directions in Tissue Engineering. Journal of Functional Biomaterials, 2024 , 15 (9), 241.
[21]. Genevieve Abd, Raquel S. Díaz, Anju Gupta, Tagbo H. R. Niepa, Kunal Mondal, Seeram Ramakrishna, Ashutosh Sharma, Andrés D. Lantada and Monsur Islam. Carbon nanomaterials-based electrically conductive scaffolds for tissue engineering applications. Med Comm – Biomaterials and Applications, 2024, 2, 3.
[22]. Hossein Omidian, Niloofar Babanejad, Luigi X Cubeddu. Nanosystems in Cardiovascular Medicine: Advancements, Applications, and Future Perspectives. MDPI, Pharmaceutics, 2023, 15 (7), 1935
[23]. Baolin Guo, Bo Lei, Peng Li, Peter X. Ma. Functionalized scaffolds to enhance tissue regeneration. Regenerative Biomaterials, 2015, 1, 2, 47-57.
[24]. Chenyang Zhang, Liang Yan, Xin Wang, Shuang Zhu, Chunying Chen, Zhanjun Gu, Yuliang Zhao. Progress, challenges, and future of nanomedicine. Nanotoday, 2020, 35, 101008.
Cite this article
Liu,K. (2025). Aapplication of Nanomaterials in the Medical Field. Applied and Computational Engineering,126,116-124.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 5th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Abdullah Abdelkawi, Aliyah Slim, Zaineb Zinoune and Yashwant Pathak. Surface Modification of Metallic Nanoparticles for Targeting Drugs. Coatings, 2023, 13(9), 1660.
[2]. Hang Li, Hui Xu, Shuo Yao, Shengnan Wei, Yi Liu, Xuening Shi, Wei Zhao & Chao Zhao. Target-inhibited MCOF-Apt-AuNPs self-assembly for multicolour colorimetric detection of Salmonella Typhimurium. Nature, npj Science of Food, 2024, 8, 78.
[3]. Ekaterina Y. Lukianova-Hleb, Daniel S. Wagner, Malcolm K. Brenner, Dmitri O. Lapotko. Cell-specific transmembrane injection of molecular cargo with gold nanoparticle-generated transient plasmonic nanobubbles. Biomaterials, 2012, 33, 21, 5441-5450.
[4]. Mohamed Yafout, Amine Ousaid, Youssef Khayati, Ibrahim Sbai El Otmani. Gold nanoparticles as a drug delivery system for standard chemotherapeutics: A new lead for targeted pharmacological cancer treatments. Scientific African, 2021, 11, e00685.
[5]. Samuel D. Oberdick, Kalina V. Jordanova, John T. Lundstrom, Giacomo Parigi, Megan E. Poorman, Gary Zabow & Kathryn E. Keenan. Iron oxide nanoparticles as positive T1 contrast agents for low-field magnetic resonance imaging at 64 mTSamuel D. Oberdick, Kalina V. Jordanova, John T. Lundstrom, Giacomo Parigi, Megan E. Poorman, Gary Zabow & Kathryn E. Keenan. Scientific Reports, 2023, 13, 11520.
[6]. Ruirui Qiao, Changkui Fu, Helen Forgham, Ibrahim Javed, Xumin Huang, Jiayuan Zhu, Andrew K. Whittaker, Thomas P. Davis. Magnetic iron oxide nanoparticles for brain imaging and drug delivery. Advanced Drug Delivery Reviews, 2023, 197, 114822.
[7]. Bret D. Ulery, Lakshmi S. Nair, Cato T. Laurencin. Biomedical applications of biodegradable polymers. Polymer Physics, 2011, 12, 49, 832-864.
[8]. Hemin Nie, Lai Yeng Lee, Hui Tong, Chi-Hwa Wang. PLGA/Chitosan composites from a combination of spray drying and supercritical fluid foaming techniques: New carriers for DNA delivery. Journal of Controlled Release, 2008, 3, 129, 207-214
[9]. Sarbari Acharya, Sanjeeb K. Sahoo. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Advanced Drug Delivery Reviews, 2011, 3, 63, 170-183.
[10]. Angela M. Wagner, Jennifer M. Knipe, Gorka Orive, Nicholas A. Peppas. Quantum dots in biomedical applications. Acta Biomaterialia, 2019, 94, 44-63
[11]. Aisha Hamidu, William G. Pitt, and Ghaleb A. Husseini. Recent Breakthroughs in Using Quantum Dots for Cancer Imaging and Drug Delivery Purposes. Nanomaterials, 2023, 13 (18), 2566.
[12]. Obaid Afzal, Abdulmalik S. A. Altamimi, Muhammad Shahid Nadeem, Sami I. Alzarea, Waleed Hassan Almalki, Aqsa Tariq, Bismillah Mubeen, Bibi Nazia Murtaza, Saima Iftikhar, Naeem Riaz and Imran Kazmi. Nanoparticles in Drug Delivery: From History to Therapeutic Applications. Nanomaterials, 2022, 12(24), 4494
[13]. Sofia Caspani, Ricardo Magalhães, João Pedro Araújo and Célia Tavares Sousa. Magnetic Nanomaterials as Contrast Agents for MRI. Materials, 2020, 13(11), 2586.
[14]. Deepak Gupta, Indrajit Roy, Sona Gandhi. Metallic nanoparticles for CT-guided imaging of tumors and their therapeutic applications. Open Nano, 2023, 12, 100146.
[15]. Naim Aslan, Burhan Ceylan, Mümin Mehmet Koç, Fehim Findik. Metallic nanoparticles as X-Ray computed tomography (CT) contrast agents: A review. Journal of Molecular Structure, 2020, 1219, 128599.
[16]. Esmaeil Heydari-Bafrooei, Ali A. Ansafi. Nanomaterials-based biosensing strategies for biomarkers diagnosis, a review. Biosensors and Bioelectronics: X, 2023, 13, 100245.
[17]. Masoud Khazaei, Marzieh Sadat Hosseini, Ali Moshfegh Haghighi, Majid Misaghi. Nanosensors and their applications in early diagnosis of cancer. Sensing and Bio-Sensing Research, 2023, 41, 100569.
[18]. Mahwash Mukhtar, Muhammad Bilal, Abbas Rahdar, Mahmood Barani, Rabia Arshad, Tapan Behl, Ciprian Brisc, Florin Banica and Simona Bungau. Nanomaterials for Diagnosis and Treatment of Brain Cancer: Recent Updates. Chemosensors, 2020, 8(4), 117.
[19]. Mominur Rahman, Rezaul Islam, Shopnil Akash, Harun-Or-Rashid, Tanmay Kumar Ray, Saidur Rahaman, Mahfuzul Islam, Fazilatunnesa Anika, Kawser Hosain, Farjana Islam Aovi, Hassan A. Hemeg, Abdur Rauf, Polrat Wilairatana, Recent advancements of nanoparticles application in cancer and neurodegenerative disorders: At a glance. ScienceDirect, Biomedicine & Pharmacotherapy, 2022, 153, 113305.
[20]. Samira Farjaminejad, Rosana Farjaminejad and Franklin Garcia-Godoy. Nanoparticles in Bone Regeneration: A Narrative Review of Current Advances and Future Directions in Tissue Engineering. Journal of Functional Biomaterials, 2024 , 15 (9), 241.
[21]. Genevieve Abd, Raquel S. Díaz, Anju Gupta, Tagbo H. R. Niepa, Kunal Mondal, Seeram Ramakrishna, Ashutosh Sharma, Andrés D. Lantada and Monsur Islam. Carbon nanomaterials-based electrically conductive scaffolds for tissue engineering applications. Med Comm – Biomaterials and Applications, 2024, 2, 3.
[22]. Hossein Omidian, Niloofar Babanejad, Luigi X Cubeddu. Nanosystems in Cardiovascular Medicine: Advancements, Applications, and Future Perspectives. MDPI, Pharmaceutics, 2023, 15 (7), 1935
[23]. Baolin Guo, Bo Lei, Peng Li, Peter X. Ma. Functionalized scaffolds to enhance tissue regeneration. Regenerative Biomaterials, 2015, 1, 2, 47-57.
[24]. Chenyang Zhang, Liang Yan, Xin Wang, Shuang Zhu, Chunying Chen, Zhanjun Gu, Yuliang Zhao. Progress, challenges, and future of nanomedicine. Nanotoday, 2020, 35, 101008.









