1. Introduction
The drug delivery system (DDS) refers to a technological system that comprehensively controls the distribution of drugs in the body, regulating dosage, timing, and location. Its goal is to deliver the drug to the required location in time, in order to improve the utilization of drug and therapeutic effects while reducing economic costs and minimizing side effects. According to a document issued by the U.S. FDA in 2014, nanomaterials are defined as particles with dimensions smaller than 100 nm, or materials with dimensions smaller than 1 μm that exhibit properties like nanoparticles. Nanomaterials are widely used in DDSs [1]. Due to their amphiphilic nature, efficient and stable long-distance drug loading, and targeted release capabilities, liposomes were used as early as 1995 in the drug Doxil [2]. Plasma proteins, as the most prominent proteins in human plasma, can transport various inorganic ions and small organic molecules within the body. They also possess a relatively long half-life, specificity for inflammatory sites, and exhibit almost no toxicity or immunogenicity. Therefore, the binding of nano albumin particles to drugs has been continuously explored. In 2005, the FDA approved the drug albumin-bound paclitaxel, Abraxane, for the treatment of breast cancer and other diseases [3]. Magnetic iron oxide nanoparticles, which can both target drug delivery and serve as a contrast agent for MRI, improving imaging efficiency, have also been applied in DDS. In 2009, the FDA approved the nano iron oxide-based drug Ferumoxytol [4].
Nowadays, new nanomaterials are constantly being explored for their application in DDS. Nanogels are hydrogel nanoparticles composed of crosslinked polymers. Due to their responsiveness to stimuli such as pH and temperature, they exhibit excellent targeting capabilities and significantly reduce the cytotoxicity of drugs. In recent years, nanogels have been extensively studied and are anticipated to be applied in the treatment of inflammatory diseases and cancer [5]. Metal organic frameworks (MOFs) are coordination polymers composed of metal ions and organic compounds. They are characterized by high porosity and surface area, as well as excellent biocompatibility and non-toxicity. As early as 2006, there were reports on the use of MOFs for drug loading, and over the past decade, various MOFs based on synthetic and natural polymers have been developed, showing great potential in DDS [6].
In conclusion, the research on nanomaterials in DDS holds significant importance, as it can markedly improve drug targeting and utilization while reducing drug toxicity. Emerging nanomaterials, as drug carriers, can precisely deliver drugs to the target sites, minimizing drug loss during transport and reducing the impact on healthy cells. They also have the ability to easily penetrate target cells, increasing the efficiency of drug usage. Moreover, these novel materials are easily labeled and imaged, allowing researchers to observe the effects of drugs, thus promoting continuous advancements in the biomedical field.
2. Nanomaterials that have been widely applied
2.1. Liposome nanoparticles
In 1965, British scientists Bangham and Standish discovered that phospholipid molecules spontaneously form bilayer vesicles in water, which they named liposomes. A liposome is an artificial membrane structure; when amphiphilic phospholipid molecules are in water, their hydrophobic tails gather on the inside, while the hydrophilic heads are exposed to water, forming a bilayer vesicle structure (Figure 1). This unique structure allows liposome to serve as effective drug carriers, with lipophilic drugs positioned within the hydrophobic area of the bilayer, and hydrophilic drugs stored in the aqueous center of the vesicle [7].
Liposome can be prepared by traditional methods which include the thin-film hydration method and reverse-phase evaporation, as well as newer techniques like crossflow injection and supercritical fluid methods [8,9]. Among these, the first method is commonly used. In this method, to begin with, dissolve the lipids with an organic solution in a round-bottom flask. The solvent is then evaporated to create a thin lipid film. Hydrating this thin lipid film leads to the formation of liposome. Drugs can be encapsulated in liposome using either passive or active loading methods [10]. In passive loading, hydrophilic drugs are first dissolved in water, and part of the drug-containing aqueous phase is captured as the liposome form. However, this method has relatively low efficiency, and active loading yields better results. In active loading, an ion or EDTA gradient is created to actively transport the drug into the lipid bilayer [11].
In DDSs, liposome can transport drugs to specific parts of the body. This process mainly involves liposome carrying drugs that, through antibodies on their surfaces, specifically bind to receptors on the surfaces of target cells, forming complexes that are then endocytosed by the cells to release the drugs [12]. Liposomes can also reduce the cytotoxicity of drugs. For example, when free doxorubicin is encapsulated in liposome, its irreversible cardiotoxicity is significantly reduced [13].
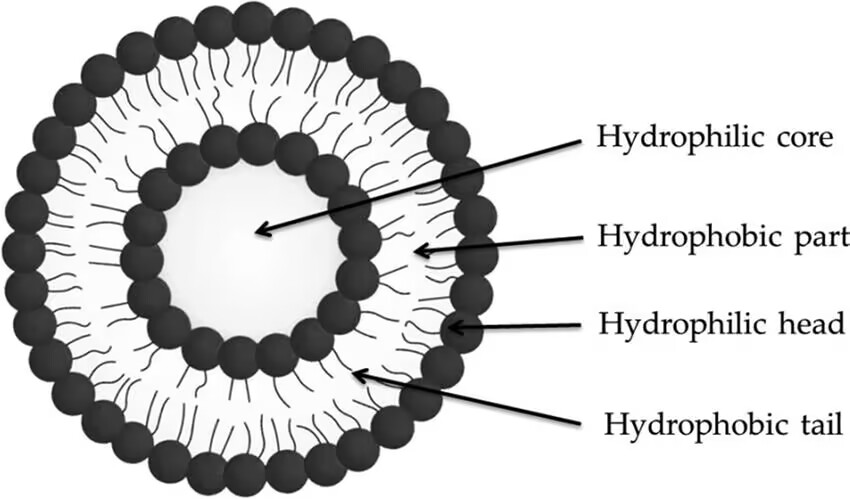
Figure 1: Structure of Liposome [14].
2.2. Albumin nanoparticles
Albumin is the main protein in human plasma, typically accounting for 50% of the total plasma protein [15]. It is a globular monomeric protein composed of 585 amino acid residues, containing 35 cysteine residues that form sulfhydryl groups and 17 disulfide bonds [16]. In the human body, it plays a role in maintaining plasma osmotic pressure [17]. Additionally, as a non-specific carrier, it can reversibly bind to poorly soluble small organic molecules and inorganic ions, enabling their transport in plasma. This property endows albumin-based nanoparticles with features such as biodegradability, good tolerance, high biocompatibility, the ability to bind compounds, and no adverse reactions in serum [18].
Common methods for preparing albumin nanoparticles include desolvation [19], emulsification [20], and thermal gelation [21]. The desolvation method involves adding an organic solvent, such as ethanol, to an albumin aqueous solution, reducing the solubility of albumin and initiating precipitation. At this point, a crosslinking agent is added to solidify the protein, forming nanoparticles [19]. The emulsification method chemically treats albumin by emulsifying it in an oil phase containing emulsifiers and then adding a crosslinking agent to stabilize the nanoparticles [18]. In thermal gelation, the protein solution is continuously heated and stirred to ultimately form a gel [21]. This method does not require the crosslinking agents.
2.3. Magnetic iron oxide nanoparticles (IONs)
Iron oxide is a common compound found in nature, primarily consisting of α-Fe₂O₃, γ-Fe₂O₃, and Fe₃O₄. Among these, α-Fe₂O₃ and Fe₃O₄ are widely used as magnetic nanomaterials in medicine [22]. Magnetic iron oxide nanoparticles possess a surface rich in hydroxyl groups, which facilitates chemical modification. Additionally, when the particle size is below the critical value (generally less than 20 nm), they exhibit superparamagnetic properties, meaning they can be rapidly magnetized under an external magnetic field [23]. These characteristics enable their application in DDSs, where they can be guided by an external magnetic field to deliver drugs to target sites. Furthermore, their surfaces can be functionalized to release drugs in response to stimuli such as pH changes, and they can also be utilized for magnetic resonance imaging (MRI) [24].
The synthesis methods for magnetic iron oxide nanoparticles primarily include co-precipitation, thermal decomposition, and sol-gel reactions. The co-precipitation method involves adjusting a mixture of ferrous (Fe²⁺) and ferric (Fe³⁺) ions to alkaline conditions under nitrogen at room temperature. The key to this method lies in controlling the particle size, as smaller sizes correspond to narrower Curie temperature ranges and improved magnetic properties [25]. The thermal decomposition method involves decomposing organometallic iron compounds in high-boiling organic solvents to produce magnetic iron oxides. Common iron organometallic compounds include Fe(CO)₅, Fe(CuP)₃, and Fe(acac)₃ [26]. The sol-gel method generates a sol of nanoparticles through hydroxylation and condensation of molecular precursors in solution, followed by heat treatment to obtain crystalline structures. Experiments have shown that heat treatment at 400°C can yield iron oxide nanoparticles with diameters ranging from 6 to 15 nm [27].
3. Nanomaterials that are still in the research stage
3.1. pH-responsive magnetic nanogel
Nowadays, magnetic nano-metallic particles have been utilized in applications such as magnetic resonance imaging (MRI) and magnetically guided drug delivery, exemplified by nanomagnetic iron oxide [28]. To address numerous challenges in magnetically controlled manipulation, biosensing, and therapeutic applications, integrating responsive nanogels into magnetic metal nano-platforms offers significant advantages, such as enhanced stability, high drug-loading capacity, and environmental responsiveness [29].
Wu et al. incorporated Ni-Ag bimetallic nanoparticles into the copolymer gel layer p(EG-MAA), to synthesize a multifunctional nanogel, Ni-Ag@p(EG-MAA). They treated B16F10 mouse melanoma cells with a mixture of nanocapsules containing the drug 5-FU (NAG-1 and NAG-2) and free 5-FU solution. It was observed that a significant number of cells survived even at high concentrations of the nanogel, whereas cell viability was notably reduced in the presence of the free 5-FU solution, as shown in Figure 2. It indicates that the nanogel effectively reduces the cytotoxicity of the drug. Additionally, it was found that the IC50 values (half maximal inhibitory concentration) of 5-FU-loaded NAG-1 and NAG-2 were much lower than the values which they thought should be. These results suggest that the mixed nanogels provide enhanced anticancer activity, improving the efficacy of the drug [29].
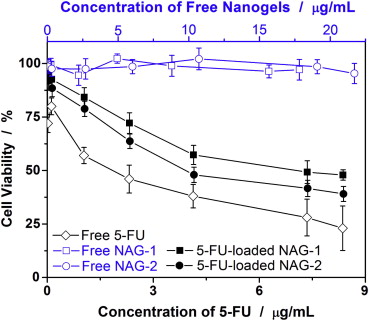
Figure 2: Comparison of B16F10 cell viability following treatments with free 5-FU solutions, free hybrid nanogels, and 5-FU-loaded hybrid nanogels, respectively [29].
Fattachi et al. created a pH-responsive magnetic nanogel which is CuFe2O₄@PMAA@Lig-ADH nanocarrier. This nanogel carrier was capable of loading curcumin (CUR) and delivering it to breast cancer cells. During the experiment, they found that observed that the cytotoxicity of the drug encapsulated in the nanogel carrier was significantly lower compared to its free form. Furthermore, the IC50 values were remarkably low [30]. This result aligns with the findings of Wu et al., indicating that the nanogels hold great potential to deliver anticancer drugs, as they can reduce both the cytotoxicity and the required dosage of the drugs.
3.2. MOFs
Metal-organic frameworks (MOFs) are crystal formed by coordinating organic ligands with metal ions. They are characterized by their ultra-high porosity and large internal surface area [31,32]. Due to these properties, MOFs can load a higher amount of drugs, achieving a locally high concentration during drug release [33]. Some MOFs release drugs in response to stimuli such as pH changes or ultraviolet light, while others release drugs through the adjustment of functional groups to facilitate release from the pores [34].
Li et al. developed a Sr-based MOF for loading ketoprofen to treat osteoarthritis. Using high-performance liquid chromatography (HPLC), they determined that the Sr/PTA-MOF exhibited a high drug loading capacity of 36%. Furthermore, it was observed that different concentrations of MOFs had minimal impact on chondrocyte activity, as depicted in Figure 3 [35]. Therefore, MOFs are excellent drug carriers with high loading capacity and low toxicity.
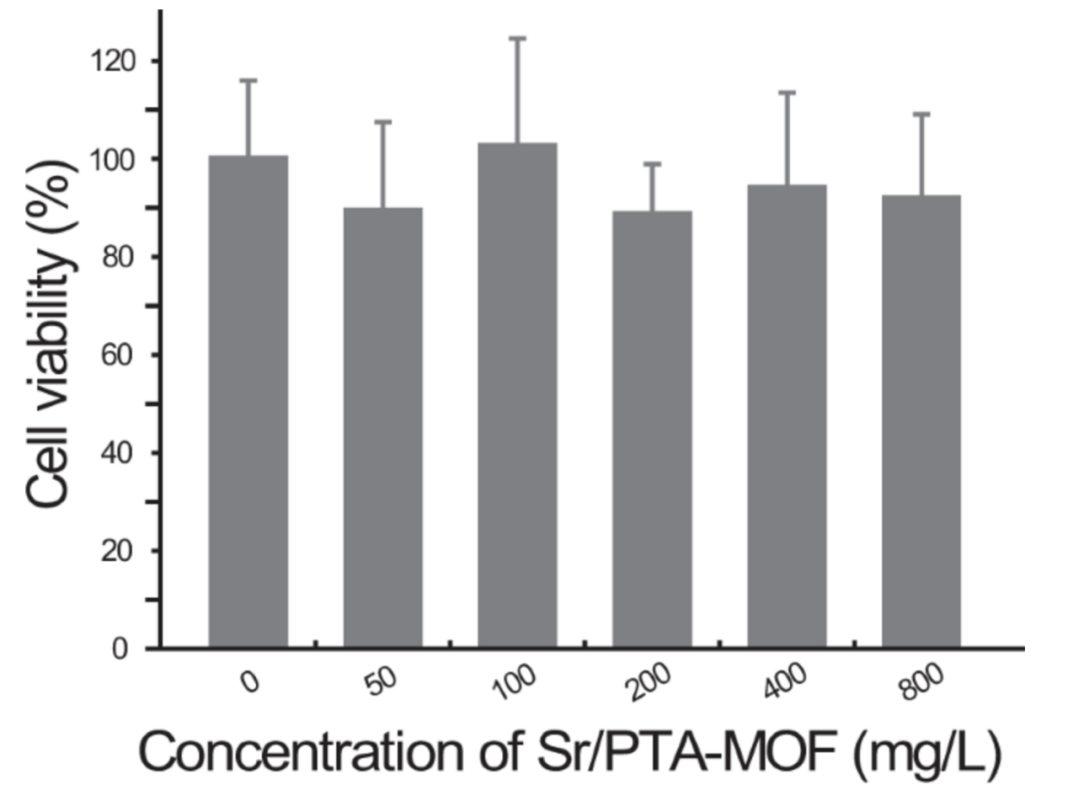
Figure 3: MTT cytotoxicity assay of Sr/PTA-MOF towards chondrocyte at various concentrations [35].
4. Conclusion
With the development of technology, the use of nanotechnology in DDSs has become increasingly widespread. This review first discusses three nanomaterials that are already widely used: liposomes, albumin, and nano-iron oxide. These materials can reduce drug toxicity, improve targeting, and be used in imaging. However, these existing materials also have some limitations. For instance, liposomes have relatively low drug loading capacity, and their product Doxil exhibits low tolerance and some adverse side effects. Therefore, further development is needed for nanomaterials used in DDSs. In the later sections of this review, nanogels, which significantly reduce drug cytotoxicity, and MOFs, which offer high drug loading capacity, are introduced. It is hoped that these nanomaterials, currently in the research phase, can soon be applied in clinical practice and actual production.
References
[1]. U.S. Food and Drug Administration. (2011). Considering whether an FDA-regulated product involves the application of nanotechnology: Guidance for industry: Draft guidance. U.S. Food and Drug Administration. Available at: http://www.fda.gov/RegulatoryInformation/Guidances/ucm257698.htm. Accessed 14.11.13.
[2]. Barenholz, Y. (2012). Doxil® — The first FDA-approved nano-drug: Lessons learned. Journal of Controlled Release, 160(2), 117–134.
[3]. Kundranda, M. N., & Niu, J. (2015). Albumin-bound paclitaxel in solid tumors: Clinical development and future directions. Drug Design, Development and Therapy, 9, 3767–3777.
[4]. Trujillo-Alonso, V., Pratt, E. C., Zong, H., et al. (2019). FDA-approved ferumoxytol displays anti-leukaemia efficacy against cells with low ferroportin levels. Nature Nanotechnology, 14, 616–622.
[5]. Jha, A., Rama, A., Ladani, B., Verma, N., Kannan, S., & Naha, A. (2021). Temperature and pH-responsive nanogels as intelligent drug delivery systems: A comprehensive review. Journal of Applied Pharmaceutical Science, 11(12), 1–16.
[6]. Maranescu, B., & Visa, A. (2022). Applications of metal-organic frameworks as drug delivery systems. International Journal of Molecular Sciences, 23(8), 4458.
[7]. Allen, T. M. (1997). Liposomes. Drugs, 54(Suppl 4), 8–14.
[8]. Guimarães, D., Cavaco-Paulo, A., & Nogueira, E. (2021). Design of liposomes as drug delivery system for therapeutic applications. International Journal of Pharmaceutics, 601, 120571.
[9]. Wagner, A., & Vorauer-Uhl, K. (2011). Liposome technology for industrial purposes. Journal of Drug Delivery, 2011, 591325.
[10]. Akbarzadeh, A., Rezaei-Sadabady, R., Davaran, S., et al. (2013). Liposome: Classification, preparation, and applications. Nanoscale Research Letters, 8, 102.
[11]. Guimarães, D., Cavaco-Paulo, A., & Nogueira, E. (2021). Design of liposomes as drug delivery system for therapeutic applications. International Journal of Pharmaceutics, 601, 120571.
[12]. Kalepu, S., KT, S., Betha, S., & Mohan, M. (2013). Liposomal drug delivery system - A comprehensive review. International Journal of Drug Development & Research, 5, 62–75.
[13]. Allen, T. M., & Cullis, P. R. (2013). Liposomal drug delivery systems: From concept to clinical applications. Advanced Drug Delivery Reviews, 65(1), 36–48.
[14]. Nikoo, M., Niknia, N., Rahmanian, N., & Wani, T. (2019). Nanoscale encapsulation. In Nanotechnology (pp. 1–8). CRC Press.
[15]. Quinlan, G. J., Martin, G. S., & Evans, T. W. (2005). Albumin: Biochemical properties and therapeutic potential. Hepatology, 41(6), 1211–1219.
[16]. Hirose, M., Tachibana, A., & Tanabe, T. (2010). Recombinant human serum albumin hydrogel as a novel drug delivery vehicle. Materials Science and Engineering: C, 30(5), 664–669.
[17]. van de Wouw, J., Joles, J. A., & Allen, J. (2022). Albumin is an interface between blood plasma and cell membrane, and not just a sponge. Clinical Kidney Journal, 15(4), 624–634.
[18]. Elzoghby, A. O., Samy, W. M., & Elgindy, N. A. (2012). Albumin-based nanoparticles as potential controlled release drug delivery systems. Journal of Controlled Release, 157(2), 168–182.
[19]. Langer, K., Balthasar, S., Vogel, V., Dinauer, N., von Briesen, H., & Schubert, D. (2003). Optimization of the preparation process for human serum albumin (HSA) nanoparticles. International Journal of Pharmaceutics, 257(1–2), 169–180.
[20]. Patil, G. V. (2003). Biopolymer albumin for diagnosis and in drug delivery. Drug Development Research, 58, 219–247.
[21]. Yu, S., Yao, P., Jiang, M., & Zhang, G. (2006). Nanogels prepared by self-assembly of oppositely charged globular proteins. Biopolymers, 83, 148–158.
[22]. Kumar, C. S. S. R., & Mohammad, F. (2011). Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Advanced Drug Delivery Reviews, 63, 789–808.
[23]. Laurent, S., Forge, D., Port, M., Roch, A., Robic, C., Vander Elst, L., & Muller, R. N. (2008). Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chemical Reviews, 108(6), 2064–2110.
[24]. Vangijzegem, T., Stanicki, D., & Laurent, S. (2018). Magnetic iron oxide nanoparticles for drug delivery: Applications and characteristics. Expert Opinion on Drug Delivery, 16(1), 69–78.
[25]. Shcherbakov, V. P., Fabian, K., Sycheva, N. K., McEnroe, S. A. (2012). Size and shape dependence of the magnetic ordering temperature in nanoscale magnetic particles. Geophysical Journal International, 191(3), 954–964.
[26]. Hasany, S. F., Abdurahman, N. H., Sunarti, A. R., & Jose, R. (2013). Current nanoscience. Current Nanoscience, 9(5), 561–575.
[27]. Laurent, S., Forge, D., Port, M., Roch, A., Robic, C., Vander Elst, L., & Muller, R. N. (2008). Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chemical Reviews, 108(6), 2064–2110.
[28]. Ganapathe, L. S., Mohamed, M. A., Yunus, R. M., & Berhanuddin, D. D. (2020). Magnetite (Fe3O4) nanoparticles in biomedical application: From synthesis to surface functionalization. Magnetochemistry, 6(4), 68.
[29]. Wu, W., Shen, J., Gai, Z., Hong, K., Banerjee, P., Zhou, S. (2011). Multi-functional core-shell hybrid nanogels for pH-dependent magnetic manipulation, fluorescent pH-sensing, and drug delivery. Biomaterials, 32(36), 9876–9887.
[30]. Fattahi, N., Aghaz, F., Rezaei, A., et al. (2024). pH-responsive magnetic CuFe2O4-PMAA nanogel conjugated with amino-modified lignin for controlled breast cancer drug delivery. Scientific Reports, 14, 25987.
[31]. He, S., Wu, L., Li, X., Sun, H., Xiong, T., Liu, J., Huang, C., Xu, H., Sun, H., Chen, W., Gref, R., Zhang, J. (2021). Metal-organic frameworks for advanced drug delivery. Acta Pharmaceutica Sinica B, 11(8), 2362–2395.
[32]. Zhou, H. C., Long, J. R., & Yaghi, O. M. (2012). Introduction to metal-organic frameworks. Chemical Reviews, 112(2), 673–674.
[33]. Luo, Z., Jiang, L., Yang, S., Li, Z., Soh, W. M. W., Zheng, L., Loh, X. J., & Wu, Y. (2019). Light-induced redox-responsive smart drug delivery system by using selenium-containing polymer@MOF shell/core nanocomposite. Advanced Healthcare Materials, 8(15), 1900406.
[34]. Harrison, L. D., Walton, S. P., & Chan, C. (2021). Metal-organic frameworks for drug delivery: A design perspective. ACS Applied Materials & Interfaces, 13(10), 1–13.
[35]. Li, Z., Peng, Y., Xia, X., et al. (2019). Sr/PTA metal organic framework as a drug delivery system for osteoarthritis treatment. Scientific Reports, 9, 17570.
Cite this article
Zheng,Z. (2025). The Application of Nanomaterials in Drug Delivery System. Applied and Computational Engineering,126,162-168.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 5th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. U.S. Food and Drug Administration. (2011). Considering whether an FDA-regulated product involves the application of nanotechnology: Guidance for industry: Draft guidance. U.S. Food and Drug Administration. Available at: http://www.fda.gov/RegulatoryInformation/Guidances/ucm257698.htm. Accessed 14.11.13.
[2]. Barenholz, Y. (2012). Doxil® — The first FDA-approved nano-drug: Lessons learned. Journal of Controlled Release, 160(2), 117–134.
[3]. Kundranda, M. N., & Niu, J. (2015). Albumin-bound paclitaxel in solid tumors: Clinical development and future directions. Drug Design, Development and Therapy, 9, 3767–3777.
[4]. Trujillo-Alonso, V., Pratt, E. C., Zong, H., et al. (2019). FDA-approved ferumoxytol displays anti-leukaemia efficacy against cells with low ferroportin levels. Nature Nanotechnology, 14, 616–622.
[5]. Jha, A., Rama, A., Ladani, B., Verma, N., Kannan, S., & Naha, A. (2021). Temperature and pH-responsive nanogels as intelligent drug delivery systems: A comprehensive review. Journal of Applied Pharmaceutical Science, 11(12), 1–16.
[6]. Maranescu, B., & Visa, A. (2022). Applications of metal-organic frameworks as drug delivery systems. International Journal of Molecular Sciences, 23(8), 4458.
[7]. Allen, T. M. (1997). Liposomes. Drugs, 54(Suppl 4), 8–14.
[8]. Guimarães, D., Cavaco-Paulo, A., & Nogueira, E. (2021). Design of liposomes as drug delivery system for therapeutic applications. International Journal of Pharmaceutics, 601, 120571.
[9]. Wagner, A., & Vorauer-Uhl, K. (2011). Liposome technology for industrial purposes. Journal of Drug Delivery, 2011, 591325.
[10]. Akbarzadeh, A., Rezaei-Sadabady, R., Davaran, S., et al. (2013). Liposome: Classification, preparation, and applications. Nanoscale Research Letters, 8, 102.
[11]. Guimarães, D., Cavaco-Paulo, A., & Nogueira, E. (2021). Design of liposomes as drug delivery system for therapeutic applications. International Journal of Pharmaceutics, 601, 120571.
[12]. Kalepu, S., KT, S., Betha, S., & Mohan, M. (2013). Liposomal drug delivery system - A comprehensive review. International Journal of Drug Development & Research, 5, 62–75.
[13]. Allen, T. M., & Cullis, P. R. (2013). Liposomal drug delivery systems: From concept to clinical applications. Advanced Drug Delivery Reviews, 65(1), 36–48.
[14]. Nikoo, M., Niknia, N., Rahmanian, N., & Wani, T. (2019). Nanoscale encapsulation. In Nanotechnology (pp. 1–8). CRC Press.
[15]. Quinlan, G. J., Martin, G. S., & Evans, T. W. (2005). Albumin: Biochemical properties and therapeutic potential. Hepatology, 41(6), 1211–1219.
[16]. Hirose, M., Tachibana, A., & Tanabe, T. (2010). Recombinant human serum albumin hydrogel as a novel drug delivery vehicle. Materials Science and Engineering: C, 30(5), 664–669.
[17]. van de Wouw, J., Joles, J. A., & Allen, J. (2022). Albumin is an interface between blood plasma and cell membrane, and not just a sponge. Clinical Kidney Journal, 15(4), 624–634.
[18]. Elzoghby, A. O., Samy, W. M., & Elgindy, N. A. (2012). Albumin-based nanoparticles as potential controlled release drug delivery systems. Journal of Controlled Release, 157(2), 168–182.
[19]. Langer, K., Balthasar, S., Vogel, V., Dinauer, N., von Briesen, H., & Schubert, D. (2003). Optimization of the preparation process for human serum albumin (HSA) nanoparticles. International Journal of Pharmaceutics, 257(1–2), 169–180.
[20]. Patil, G. V. (2003). Biopolymer albumin for diagnosis and in drug delivery. Drug Development Research, 58, 219–247.
[21]. Yu, S., Yao, P., Jiang, M., & Zhang, G. (2006). Nanogels prepared by self-assembly of oppositely charged globular proteins. Biopolymers, 83, 148–158.
[22]. Kumar, C. S. S. R., & Mohammad, F. (2011). Magnetic nanomaterials for hyperthermia-based therapy and controlled drug delivery. Advanced Drug Delivery Reviews, 63, 789–808.
[23]. Laurent, S., Forge, D., Port, M., Roch, A., Robic, C., Vander Elst, L., & Muller, R. N. (2008). Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chemical Reviews, 108(6), 2064–2110.
[24]. Vangijzegem, T., Stanicki, D., & Laurent, S. (2018). Magnetic iron oxide nanoparticles for drug delivery: Applications and characteristics. Expert Opinion on Drug Delivery, 16(1), 69–78.
[25]. Shcherbakov, V. P., Fabian, K., Sycheva, N. K., McEnroe, S. A. (2012). Size and shape dependence of the magnetic ordering temperature in nanoscale magnetic particles. Geophysical Journal International, 191(3), 954–964.
[26]. Hasany, S. F., Abdurahman, N. H., Sunarti, A. R., & Jose, R. (2013). Current nanoscience. Current Nanoscience, 9(5), 561–575.
[27]. Laurent, S., Forge, D., Port, M., Roch, A., Robic, C., Vander Elst, L., & Muller, R. N. (2008). Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chemical Reviews, 108(6), 2064–2110.
[28]. Ganapathe, L. S., Mohamed, M. A., Yunus, R. M., & Berhanuddin, D. D. (2020). Magnetite (Fe3O4) nanoparticles in biomedical application: From synthesis to surface functionalization. Magnetochemistry, 6(4), 68.
[29]. Wu, W., Shen, J., Gai, Z., Hong, K., Banerjee, P., Zhou, S. (2011). Multi-functional core-shell hybrid nanogels for pH-dependent magnetic manipulation, fluorescent pH-sensing, and drug delivery. Biomaterials, 32(36), 9876–9887.
[30]. Fattahi, N., Aghaz, F., Rezaei, A., et al. (2024). pH-responsive magnetic CuFe2O4-PMAA nanogel conjugated with amino-modified lignin for controlled breast cancer drug delivery. Scientific Reports, 14, 25987.
[31]. He, S., Wu, L., Li, X., Sun, H., Xiong, T., Liu, J., Huang, C., Xu, H., Sun, H., Chen, W., Gref, R., Zhang, J. (2021). Metal-organic frameworks for advanced drug delivery. Acta Pharmaceutica Sinica B, 11(8), 2362–2395.
[32]. Zhou, H. C., Long, J. R., & Yaghi, O. M. (2012). Introduction to metal-organic frameworks. Chemical Reviews, 112(2), 673–674.
[33]. Luo, Z., Jiang, L., Yang, S., Li, Z., Soh, W. M. W., Zheng, L., Loh, X. J., & Wu, Y. (2019). Light-induced redox-responsive smart drug delivery system by using selenium-containing polymer@MOF shell/core nanocomposite. Advanced Healthcare Materials, 8(15), 1900406.
[34]. Harrison, L. D., Walton, S. P., & Chan, C. (2021). Metal-organic frameworks for drug delivery: A design perspective. ACS Applied Materials & Interfaces, 13(10), 1–13.
[35]. Li, Z., Peng, Y., Xia, X., et al. (2019). Sr/PTA metal organic framework as a drug delivery system for osteoarthritis treatment. Scientific Reports, 9, 17570.









