1. Introduction
Olefin represents a significant category of organic compounds, extensively utilized within the chemical sector. The Methanol-to-Gasoline (MTG) process was pioneered in 1977, while the Methanol-to-Olefins (MTO) process was initially identified in 1981.[1] This manuscript primarily examines the evolution of the MTO (Methanol-to-Olefins) process as a standalone technology for the synthesis of two significant products, ethylene and propylene. Additionally, it analyzes the UOP/Hydro methodology for the conversion of methanol to olefins utilizing the SAPO-34 catalyst[2]
The methanol to gasoline (MTG) process is critical to the methanol to olefin (MTO) process because studies of the conversion of methanol to oxygenates and the alkylation of methanol with isobutane using ZSM-5 catalysts have revealed the presence of unexpected hydrocarbons in the gasoline fraction. This discovery spurred the development of the MTG process. Subsequently, Mobil pioneered the development of the MTO process using molecular sieve catalysts. Both processes represented major technological breakthroughs in the field of synthetic fuels. Today, UOP/Hydro is the world's leading commercial technology provider and licensor and is widely recognised in the industry.[1]
The significance of this research paper is to elucidate the development and realisation of MTO, as well as the underlying reaction principles, along with a brief overview of the reaction mechanisms associated with UOP technology. Furthermore, it aims to highlight the practical applications of MTO in various industrial settings, examining its impact on efficiency and sustainability. By analysing recent advances in catalyst development and process optimisation, this paper seeks to contribute to the debate surrounding the future of hydrocarbon conversion technologies. In addition, a comparative analysis with other approaches will be presented, highlighting the advantages and potential limitations of MTO in the context of global energy demand.
2. The Development of MTO
The methanol-to-gasoline (MTG) process, pioneered by Mobil researchers in 1977, alongside the methanol-to-olefins (MTO) process, developed by Union Carbide in 1981, represent significant milestones in the domain of synthetic fuels research, emerging approximately five decades after the inception of the Fischer-Tropsch synthesis. These technologies were conceived in the United States as a strategic response to the energy crisis of the 1970s, characterized by the oil embargo, volatile gasoline prices, and erratic supply chains. Both MTG and MTO are recognized as essential components of the "synfuels factory," ready for deployment as technological and economic conditions evolve.
The methanol-to-gasoline conversion process was serendipitously uncovered by Mobil Oil Corporation (now ExxonMobil) when two separate research teams independently explored two unrelated chemical pathways. One team focused on the conversion of methanol to ethylene oxide, while the other investigated the reaction between methanol and isobutylene to synthesize neopentane. The methylation of isobutylene deviated from the expected reaction pathway outlined by Mobil Oil. The secondary team at Mobil’s Central Research Labs established that the conversion of methanol was both comprehensive and accurate through the application of the ZSM-5 catalyst[3].
\( CH3OH→[ \) CH2]+H2O(1)
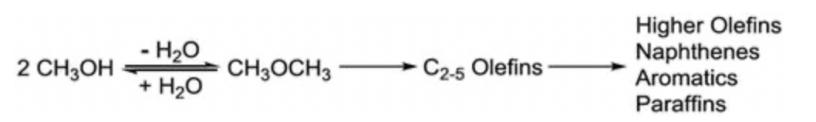
Figure 1: A general reaction scheme for the methanol-to-gasoline (MTG) process.
The methanol conversion process is nearly entirely balanced, with an end result specificity of 56 percent for hydrocarbons and 44 percent for water. The reaction primarily produces light olefins rather than petrol [4].
3. The UOP/Hydro Technologies of MTG
Four main methanol-to-olefin (MTO) technologies are used in the Chinese coal-to-olefin industry. The most important and popular technology currently available to MTO licensors is the joint innovation of UOP (currently Honeywell UOP, headquartered in Des Plaines, Illinois, USA) and Norsk Hydro (located in Oslo, Norway).
In the UOP/Norsk Hydro process, methanol is evaporated by preheating before entering the MTO reactor to facilitate the production of polyolefins, as shown in Figure 2. The reactor operates in the gas phase at temperatures of 340 to 540°C and pressures of 0.1 to 0.3 MPa. The reactor effluent, which contains ethylene, propylene, heavier fatty acids, and other liquids, is then cooled. Water is removed from the gaseous product stream and the remaining effluent is sent to a light olefin recovery unit. At this stage, ethylene (C2) and propylene (C3) are separated, while the heavier olefins (C4 to C6) are processed into C3 (propylene) or C4 (butadiene) olefins. Propylene is used to improve olefin recovery, while C4 olefins are used as an energy source for the MTO process or alternative processes.
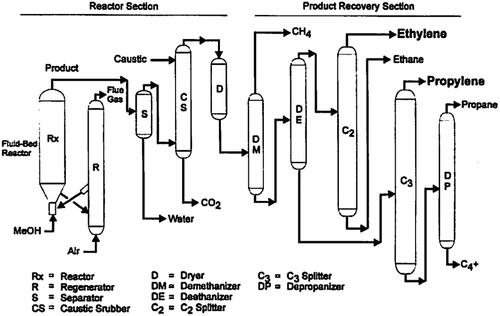
Figure 2: Process flow diagram of the UOP/hydro MTO process
4. The Catalyst of UOP/Hydro Technology
The methanol-to-hydrocarbon (MTH) conversion process represents a serendipitous yet pivotal advancement in petrochemical synthesis, initially demonstrated by Mobil Oil Corporation through the implementation of ZSM-5 zeolite catalyst, which facilitated complete methanol conversion with quantitative yields. A significant milestone was achieved in the 1980s when Union Carbide researchers synthesized SAPO-34, a silicoaluminophosphate molecular sieve catalyst, which demonstrated exceptional selectivity in converting methanol to light olefins, specifically ethylene and propylene. The commercialized UOP/Hydro MTO (Methanol-to-Olefins) technology, employing SAPO-34-based catalytic systems, achieves remarkable olefin selectivity with yields approaching 80% while maintaining near-complete methanol conversion efficiency.
The ongoing research in this domain has further expanded the understanding of catalyst design and optimization, leading to the exploration of alternative materials and reaction conditions. Recent studies have highlighted the potential of using metal-modified zeolites and hybrid catalysts to enhance the selectivity and activity of the MTH process. Additionally, the integration of advanced characterization techniques has provided deeper insights into the reaction mechanisms at play, allowing for the fine-tuning of catalyst properties to maximize performance. As the demand for sustainable and efficient chemical processes grows, the MTH conversion technology continues to evolve, promising to play a crucial role in the future of petrochemical production and the development of renewable feedstock pathways.
The growing accessibility of substantial reserves of natural gas has intensified interest in the catalytic methanol-to-olefins (MTO) process, which presents a viable alternative to conventional thermal cracking methods for olefin synthesis. A range of molecular sieves, including ZSM-5, zeolite beta, chabazite, and silicoaluminophosphate SAPO-34, have demonstrated exceptional catalytic efficacy in the MTO reaction. SAPO-34, which possesses a framework structure akin to that of natural chabazite, is characterized by its narrow pore dimensions (0.43 nm) and cavity sizes (1.1 nm × 0.65 nm). In comparison to ZSM-5, SAPO-34 exhibits superior selectivity for light olefins, specifically ethylene and propylene. Nonetheless, it faces a critical drawback: rapid deactivation due to coke deposition, which can entirely obstruct the internal channels of SAPO-34 crystals.
Chen et al. explored the impact of SAPO-34 crystal dimensions on the selectivity and deactivation mechanisms in the methanol-to-olefins (MTO) reaction by analyzing crystal fractions of varying sizes obtained through sedimentation techniques. For larger crystals (2.5 μm), the reactions were constrained by the diffusion of methanol (MeOH) and the intermediate dimethyl ether (DME). Additionally, the rate of coking increased with the enlargement of the crystal size. In contrast, SAPO-34 fractions with crystalline dimensions below 500 nm exhibited remarkable stability and a prolonged operational lifespan during the MTO process.
The van Heyden group successfully synthesized SAPO-34 crystals with dimensions below 300 nm from colloidal solutions that included tetraethylammonium ion as a structure-directing agent (SDA). They achieved the synthesis of 100 nm crystals utilizing a microwave oven under hydrothermal conditions. The rate of phosphoric acid addition to the precursor solution emerged as the primary determinant influencing the formation of colloidal solutions. Yao et al. employed a polymer-assisted dry gel conversion technique to effectively produce SAPO-34 nanocrystals from a precursor gel containing polyacrylamide. This methodology also facilitated the simultaneous growth of larger SAPO-34 crystals, reaching sizes of several micrometers.
These studies highlight the critical significance of controlling the crystal size of SAPO-34 to enhance the catalyst's performance and extend its operational lifespan. The production of SAPO-34 crystals with a narrow size distribution has predominantly been achieved through product fractionation. Developing a more streamlined approach for regulating crystal size to achieve a tight distribution is essential for practical applications[5].
5. The Uses of Olefin
Olefins are vital precursors in the production of a wide array of chemical and polymer products, including plastics, detergents, adhesives, rubber, and food packaging materials. The principal categories of olefins encompass ethylene, propylene, and butadiene. Ethylene, being the most extensively produced olefin, plays a critical role across various industries. It is a key ingredient in the formulation of synthetic polymers, antifreeze, and fiber production. Ethylene also acts as a precursor for the synthesis of ethylene oxide, which is widely employed as a sterilizing agent for medical instruments and in the production of ethylene glycol. Furthermore, it is the primary constituent of polyethylene, which is utilized in products such as food packaging, grocery bags, wire insulation, toys, mulch, and a variety of household and kitchen items. Propylene, also known as propene, is predominantly used in the production of polypropylene, a plastic that accounts for 25% of global plastic manufacturing. Polypropylene is found in products such as food packaging films, pharmaceutical containers, plastic components, carpet fibers, and various textiles.
6. The Uses of Ethene
Propylene (C3H6), a volatile organic compound, exhibits the characteristics of a colourless gas with mild petrochemical olfactory properties under standard conditions. Modern information indicates that propylene emissions occur naturally through biomass combustion processes and anthropogenic activities, including vehicle and aircraft operations.
Propylene is the second most important industrial feedstock in contemporary petrochemical processing, second only to ethylene in terms of commercial significance. Approximately 67% of the world's propylene capacity is used in the synthesis of polypropylene, which produces versatile thermoplastic polymers used in a wide range of applications such as polymer films, synthetic fibres and industrial packaging materials. The compound is an important intermediate in the industrial synthesis of a wide range of high value-added chemicals, particularly propylene oxide, acrylonitrile, cumene, n-butyraldehyde and acrylic acid derivatives.
7. The Uses of Propene
Propylene, also known as propene, is an unsaturated hydrocarbon characterized by the molecular formula CH3CH=CH2. It features a single double bond and is recognized as the second simplest alkene within the hydrocarbon series. This chemical appears as a colorless gas with a faint odor reminiscent of petroleum.
Propylene is produced during the combustion processes of wildfires, cigarette smoke, and the exhaust emissions from vehicles and aircraft. Its discovery dates back to 1850, attributed to Captain John Williams Reynolds, who later attained the rank of Major General and was a protégé of A. W. von Hoffman. Reynolds identified propylene as the exclusive gaseous byproduct resulting from the thermal decomposition of amyl alcohol in the presence of chlorine and bromine.
In the petrochemical industry, propylene is the second most crucial feedstock after ethylene, serving as a fundamental precursor for a wide range of products[6]. Approximately two-thirds of the global propylene production is directed towards the synthesis of polypropylene, which finds extensive applications in films, fibers, containers, packaging, and closures. Additionally, propylene plays a vital role in the production of several key chemicals, including propylene oxide, acrylonitrile, cumene, butyraldehyde, and acrylic acid. In 2013, the global processing of propylene reached nearly 85 million metric tonnes.
8. Conclusion
The UOP/Hydro MTO methodology offers a cost-effective strategy for converting commercially viable feedstocks, such as natural gas or coal, into valuable propylene and ethylene derivatives. Advancements in process technology—particularly the integration of the MTO process with olefin cracking—and enhancements in catalyst optimization have resulted in significant improvements in the overall efficiency of the process. These advancements not onl y boost yield but also reduce energy consumption, thereby addressing both economic and environmental challenges. Additionally, the implementation of advanced monitoring systems allows for real-time adjustments, optimizing operational parameters and enhancing product quality. As the industry transitions towards sustainable practices, the UOP/Hydro MTO process stands out as a pivotal technology in the shift towards eco-friendly chemical production methods. Future research is expected to focus on improving catalyst performance and exploring alternative feedstock sources to broaden the applicability of this innovative process. Furthermore, the integration of renewable energy sources into the MTO process is becoming increasingly common, promoting a more sustainable approach to feedstock conversion. The utilization of solar or wind energy enables facilities to significantly lower their carbon emissions while maintaining productivity. Moreover, collaborations between academia and industry are fostering innovation, leading to the development of advanced catalysts that exhibit enhanced selectivity and durability. These advancements not only improve the economic viability of the MTO process but also align with global sustainability goals. As regulatory frameworks evolve, the chemical industry is poised to adapt, ensuring compliance while leveraging the benefits of these cutting-edge technologies. The upcoming paradigm of chemical manufacturing is likely to emphasize circular economy principles, minimizing waste and encouraging resource reuse, thereby underscoring the importance of the UOP/Hydro MTO process in promoting a more sustainable future.
References
[1]. Sousa, Z. S. B., Luna, A. S., Zotin, F. M. Z., & Henriques, C. A. (2022). Methanol-to-olefin conversion over ZSM-5: Influence of zeolite chemical composition and experimental conditions on propylene formation. Chemical Engineering Communications, 209(5), 623-635.
[2]. Chen, J. Q., Bozzano, A., Glover, B., Fuglerud, T., & Kvisle, S. (2005). Recent advancements in ethylene and propylene production using the UOP/Hydro MTO process. Catalysis Today, 106(1–4), 103-107.
[3]. Awudu, I., & Zhang, J. (2012). Uncertainties and sustainability concepts in biofuel supply chain management: A review. Renewable and Sustainable Energy Reviews, 16(2), 1359-1368.
[4]. Xu, S., Zhi, Y., Han, J., Zhang, W., Wu, X., Sun, T., Wei, Y., & Liu, Z. (2017). Advances in catalysis for methanol-to-olefins conversion. In C. Song (Ed.), Advances in Catalysis (Vol. 61, pp. 37-122). Academic Press.
[5]. Nishiyama, N., Kawaguchi, M., Hirota, Y., Vu, D. V., Egashira, Y., & Ueyama, K. (2009). Size control of SAPO-34 crystals and their catalyst lifetime in the methanol-to-olefin reaction. Microporous and Mesoporous Materials, 362(1-2), 193-199.
[6]. Phung, T. K., Pham, T. L. M., Vu, K. B., & Busca, G. (2021). (Bio) Propylene production processes: A critical review. Journal of Environmental Chemical Engineering, 9(4), 105673.
Cite this article
Li,Z. (2025). A Review of the Methanol to Olefin Process. Applied and Computational Engineering,129,193-198.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 5th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Sousa, Z. S. B., Luna, A. S., Zotin, F. M. Z., & Henriques, C. A. (2022). Methanol-to-olefin conversion over ZSM-5: Influence of zeolite chemical composition and experimental conditions on propylene formation. Chemical Engineering Communications, 209(5), 623-635.
[2]. Chen, J. Q., Bozzano, A., Glover, B., Fuglerud, T., & Kvisle, S. (2005). Recent advancements in ethylene and propylene production using the UOP/Hydro MTO process. Catalysis Today, 106(1–4), 103-107.
[3]. Awudu, I., & Zhang, J. (2012). Uncertainties and sustainability concepts in biofuel supply chain management: A review. Renewable and Sustainable Energy Reviews, 16(2), 1359-1368.
[4]. Xu, S., Zhi, Y., Han, J., Zhang, W., Wu, X., Sun, T., Wei, Y., & Liu, Z. (2017). Advances in catalysis for methanol-to-olefins conversion. In C. Song (Ed.), Advances in Catalysis (Vol. 61, pp. 37-122). Academic Press.
[5]. Nishiyama, N., Kawaguchi, M., Hirota, Y., Vu, D. V., Egashira, Y., & Ueyama, K. (2009). Size control of SAPO-34 crystals and their catalyst lifetime in the methanol-to-olefin reaction. Microporous and Mesoporous Materials, 362(1-2), 193-199.
[6]. Phung, T. K., Pham, T. L. M., Vu, K. B., & Busca, G. (2021). (Bio) Propylene production processes: A critical review. Journal of Environmental Chemical Engineering, 9(4), 105673.









