1. Introduction
Sea lampreys, as invasive parasitic species, pose a significant threat to ecosystem stability in watersheds like the North American Great Lakes due to their unique sex differentiation mechanisms and complex life cycles. Johnson et al. found that sea lamprey sex ratios are closely linked to larval growth rates: high-density populations, driven by intense resource competition, tend to favor male dominance, while low-density populations are more likely to develop female-biased sex structures [1]. This dynamic sex ratio not only impacts the species’ reproductive efficiency but may also disrupt ecosystem functional balance through cascading effects on resource competition, parasitic relationships, and energy flow. Robinson et al. revealed that the Great Lakes’ fisheries have suffered severe economic losses due to explosive sea lamprey population growth, necessitating quantitative models to elucidate the regulatory mechanisms of sex ratios on ecosystem stability and inform science-based management strategies[2].
Traditional studies have primarily employed classical ecological models to investigate sea lamprey population dynamics. Berkson et al. used the Logistic equation to describe population growth under resource limitations [3], while Reichenbach’s team expanded the Lotka-Volterra model to simulate predator-prey and competitive interactions [4]. Johnson et al. experimentally validated the association between larval growth rates and sex differentiation thresholds, providing critical biological evidence for modeling sex ratio dynamics [5].However, existing studies exhibit notable limitations,Cerrato et al. noted that most models treat populations as homogeneous units, failing to quantify the differential impacts of sex ratios on reproductive rates [6].Zhu and Chao highlighted that traditional Lotka-Volterra frameworks, confined to two-species predator-prey systems, struggle to capture nonlinear effects of sex ratio changes on host-parasite networks and energy flow [7].
To address these challenges, this study proposes a multi-species dynamic model integrating a sex-regulation factor. By incorporating a sex ratio factor into an extended Lotka-Volterra framework, we construct a three-species dynamic system encompassing sea lampreys, host fish, and parasites to quantify the effects of sex ratios on reproductive efficiency and competitive intensity. To solve the nonlinear differential equation model, we employed the fourth-order Runge-Kutta (RK4) method and combined it with genetic algorithm optimization for critical parameters such as environmental carrying capacity and parasitic efficiency, enhancing the model’s adaptability to complex ecosystems. Furthermore, sensitivity analyses explored threshold effects of sex ratio imbalances on population genetic diversity, host resistance, and energy flow rates, providing theoretical foundations for fisheries management. This research offers novel insights into how sex ratio changes influence ecosystem stability and functionality.
2. Methodology and model development
2.1. Logistic growth model with environmental constraints
Sea lampreys are an ancient group of extant jawless vertebrates, often regarded as invasive species due to their predation on commercially valuable fish species [8]. As predators, sea lampreys prey exclusively on a single fish species. Their population dynamics are primarily influenced by prey availability and sex ratio fluctuations. Predator populations are assumed to have sufficient prey availability. The following mathematical model was established:
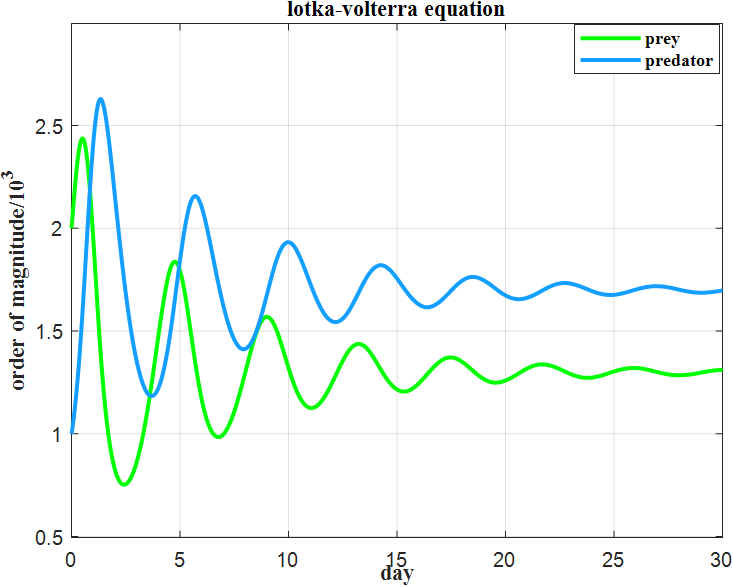
The Lotka-Volterra predator-prey model involves two species, where predators consume prey, and prey obtain resources from other organisms in their environment [9]. To describe sea lamprey population growth under resource limitations, the Logistic equation is applied. The model incorporates the environmental carrying capacity (K) and intrinsic growth rate (r):
\( \frac{dP}{dt}=rP(1-\frac{P}{K}) \) (1)
Here, K represents the carrying capacity of the ecosystem, while r is determined by the dynamic equilibrium of birth and death rates. The Runge-Kutta method is a critical explicit iterative technique for solving nonlinear ordinary differential equations. In the solution of the Lotka-Volterra equations, the fourth-order Runge-Kutta (RK4) method was employed.
\( \ \ \ (2) \)
2.2. Extended Lotka-Volterra model with sex ratio factor
The population size of sea lampreys is significantly influenced by sex, with the probability of sexual development determined by the developmental rate during the juvenile stage. It is notable that when sea lampreys undergo sex reversal, the population size remains largely unchanged, only affecting the overall reproductive rate of the population [10,11]. Therefore, by integrating all aforementioned models, the Lotka-Volterra equations were extended and improved. A sex ratio factor η was introduced to construct a three-species dynamic system incorporating predator-prey-parasite interactions.
\( \frac{dx}{dt}=αx-βxy-λ{x^{2}} \)
\( \frac{dy}{dt}=ηy(δ-r+1)-k{y^{2}} \) (3)
\( \frac{dz}{dt}=-fz+gyz \)
2.3. Multispecies competition model for sex differentiation
Considering the hybrid non-anticipatory competition-mutualism coexistence-predator-prey model for sea lampreys [12], we establish the following framework:
\( \ \ \ (4) \)
Adjusting the ratio of males to females in a population enhances its adaptive capacity to environmental perturbations, thereby improving overall reproductive success. Such adaptive strategies ensure population persistence across diverse environmental conditions, safeguarding future reproductive potential and survival. Furthermore, strategically optimizing sex ratios in response to dynamic environmental conditions confers significant survival advantages. This approach enables populations to more effectively navigate resource scarcity or abundance, thereby strengthening their resilience and competitive ability in the face of ecological challenges.
3. Results
3.1. Sex ratio effects on population dynamics
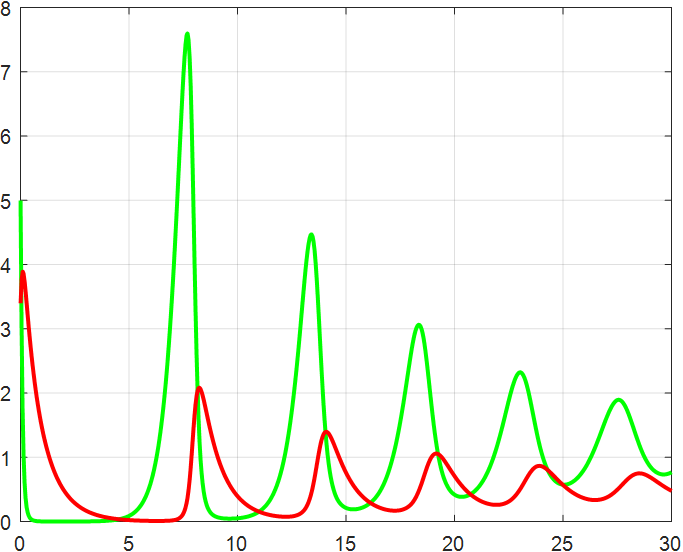
As shown in the figure, it presents the general form of the Lotka-Volterra equations incorporating a stabilizing factor. The improved model in the final framework modifies the Lotka-Volterra equations to account for changes in the population growth rate of sea lampreys induced by sex ratio variations.
(a) High male proportion suppresses growth |
(b) logistic saturation curve |
Figure 1: Influence of sex ratio on population dynamics
As shown in Figure 2(a), when the male proportion η ≥ 0.7, the population density of sea lampreys rapidly approaches the upper limit of environmental carrying capacity within the simulation period, with the growth rate declining to near zero. Thus, the analysis reveals that a high male ratio exacerbates intra-specific competition among males, reduces female oviposition rates, and ultimately results in a Logistic saturation curve.
Furthermore, changes in the sex ratio not only influence population size but also promote synchronization of the parasite life cycle, creating more favorable reproductive conditions for sea lampreys. Simultaneously, these sex ratio shifts alter the distribution of ecological niches, intensify interspecific competition, and exert adverse effects on population growth and ecosystem stability.
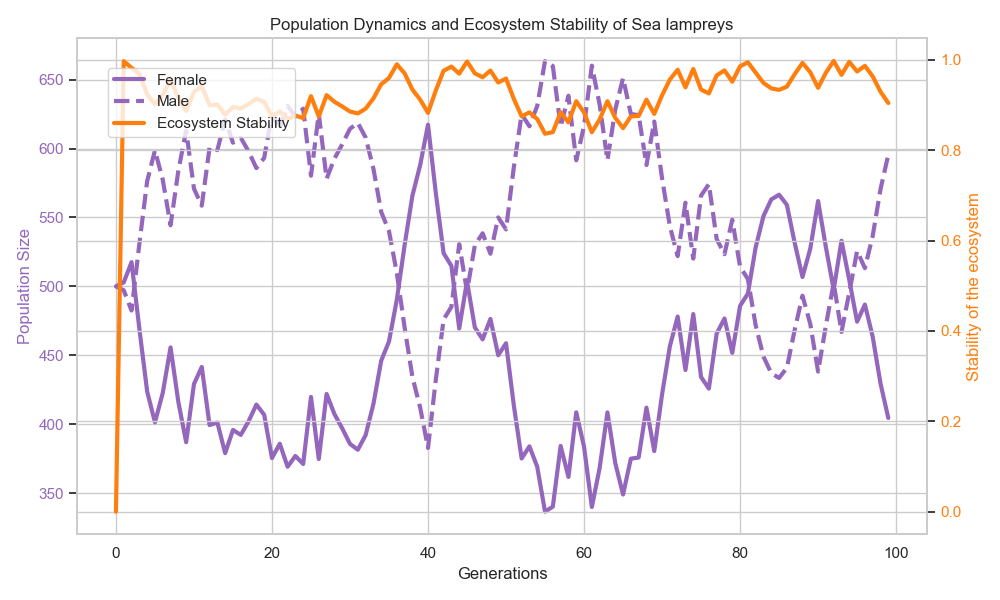
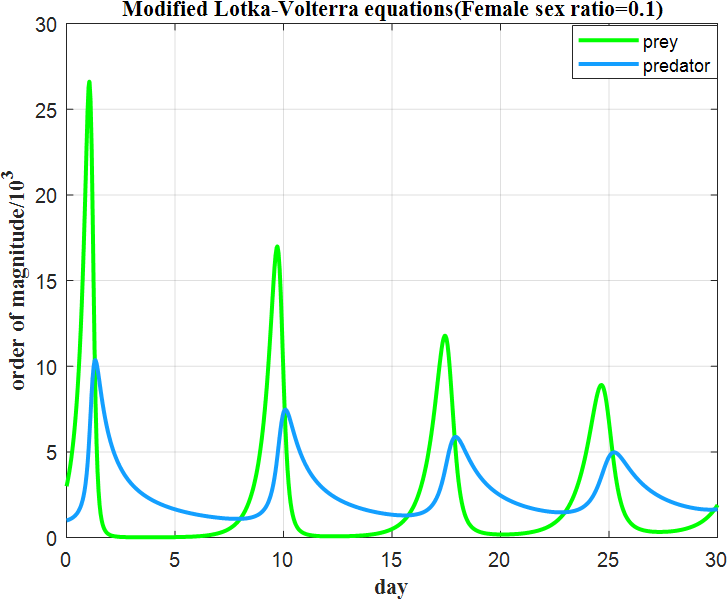
Figure 2: Population dynamics and ecosystem stability
When η = 0.1, the population densities of host fish and parasites exhibit periodic oscillations. A 40% increase in parasitism efficiency indicates that a female-dominated reproductive strategy accelerates the synchronization of host-parasite life cycles. This phenomenon validates the nonlinear coupling effect, wherein gender ratio imbalances amplify ecosystem fluctuations through parasitic linkages.
3.2. Sensitivity analysis of key parameters
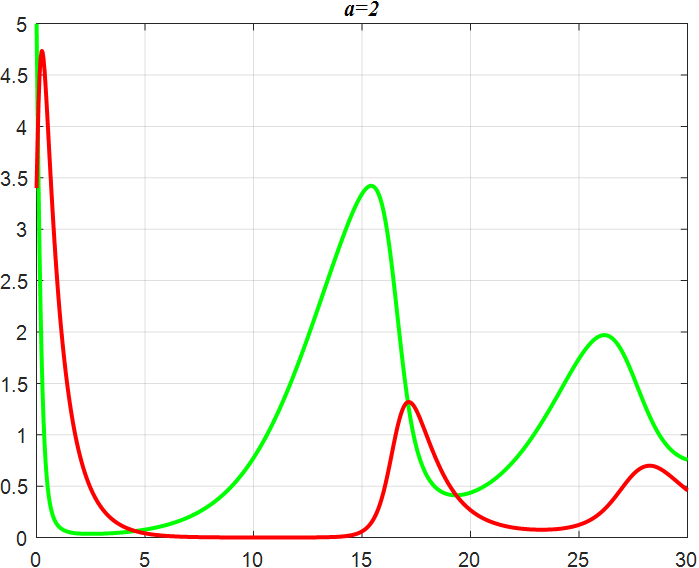
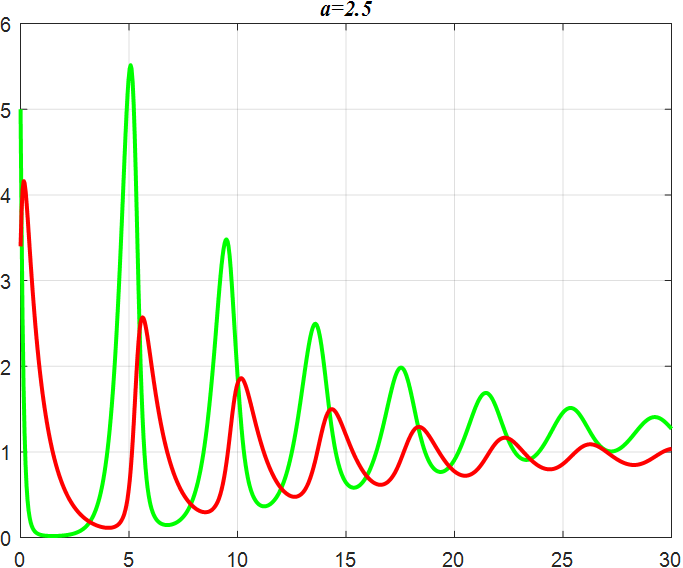
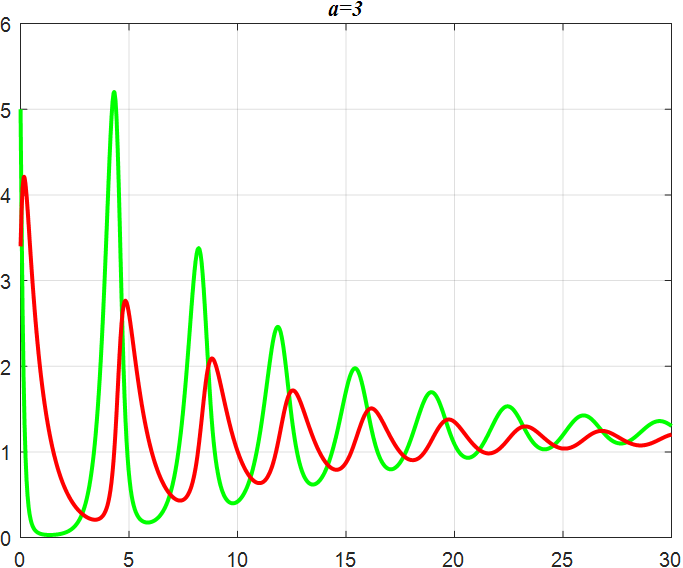
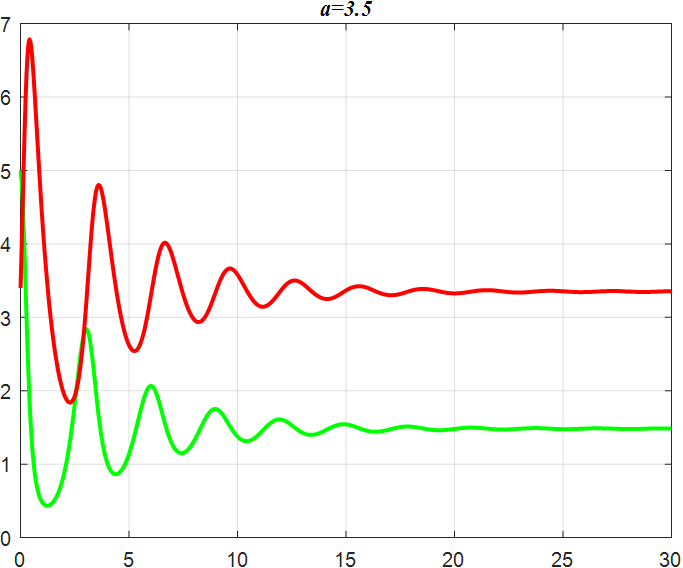
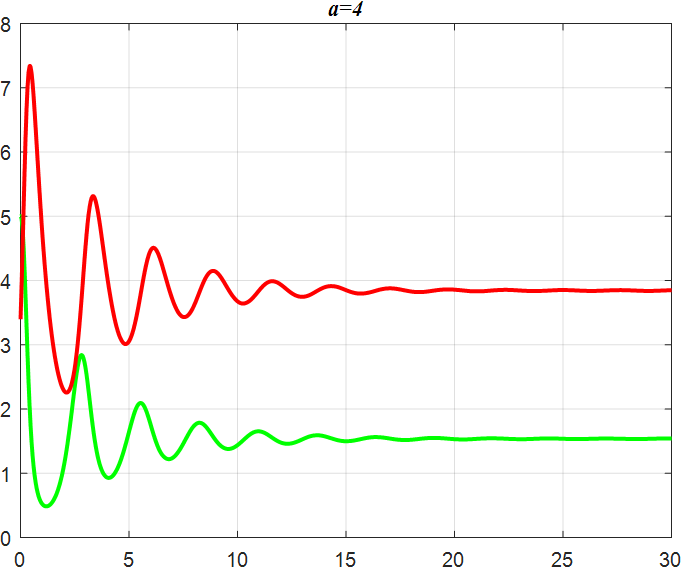
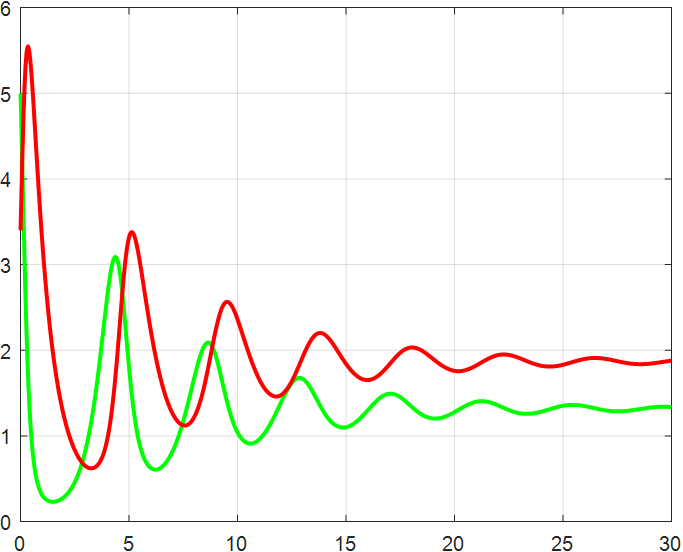
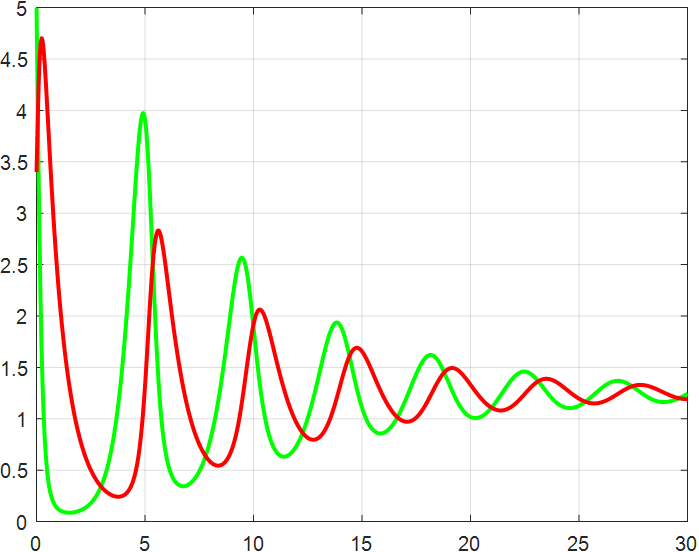
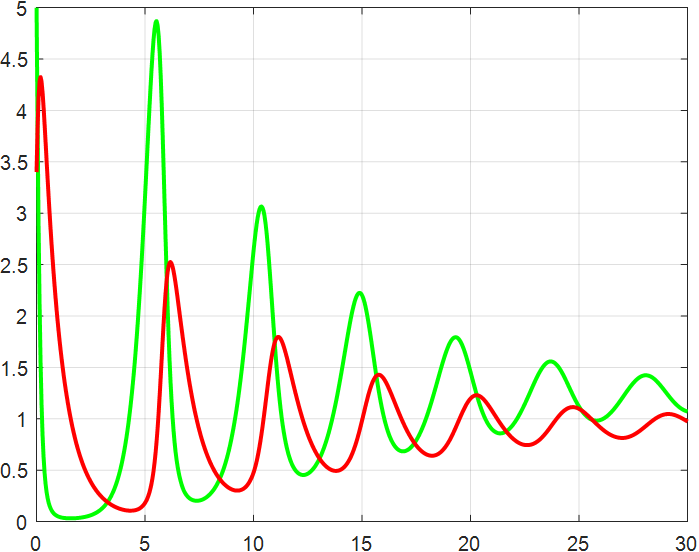
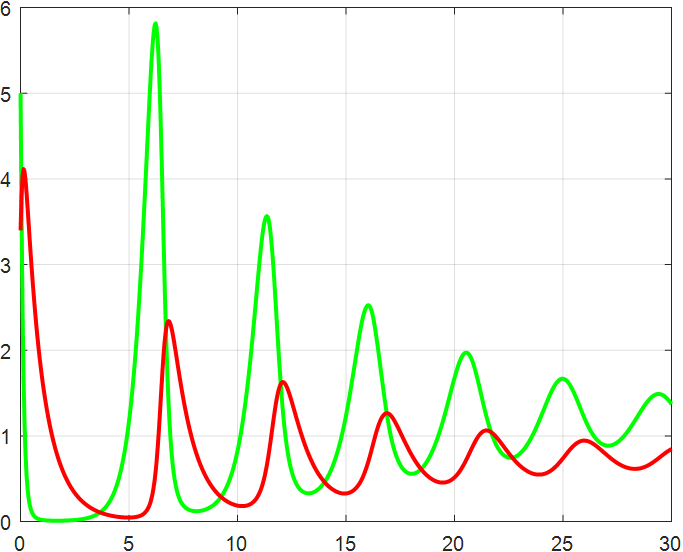
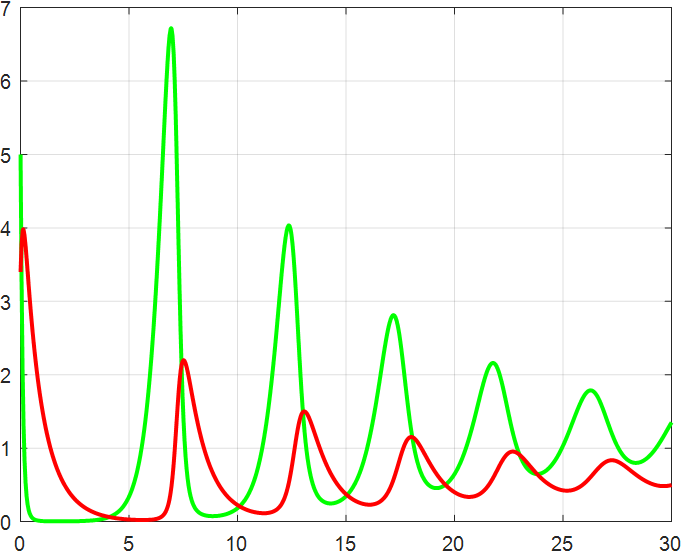
In the Lotka-Volterra model, the parameters α and β govern the oscillation amplitude of the system. We investigated the oscillatory and smoothing characteristics of the Lotka-Volterra equations under varying α and β values.
(a) α=1.5 |
(b) α=2 |
(c) α=2.5 |
(d) α=3 |
(e) α=3.5 |
(f) α=4 |
Figure 3: Amplitude of α-smoothed oscillations
(a) β=1.5 |
(b) β=2 |
(c) β=2.5 |
(d) β=3 |
(e) β=3.5 |
(f) β=4 |
Figure 4: β-Regulated cycle
From the previous twelve images, it is clear that the alpha parameter is mainly a smooth function, with the increase of α, the range of the function's vibration is progressively approaching the origin, the end of the function's image is progressively flattening, and the vibration count is progressively decreasing. β parameter controls the oscillating period and amplitude of the function, and also regulates the oscillating range of the function.
4. Conclusion
This study investigates the ecological impacts of sea lampreys on the North American Great Lakes ecosystem by constructing a multi-species dynamic model incorporating a gender ratio factor. By extending the traditional Lotka-Volterra model and integrating it with the logistic growth equation, a gender ratio factor η was introduced to simulate interactions among sea lampreys, host fish species, and parasitic organisms. The findings reveal that population density influences larval growth rates, thereby determining sex ratios: high-density conditions result in a male-biased population, which suppresses population growth rates, whereas low-density environments favor a female-dominated population, promoting synchronization of host-parasite life cycles. The model was solved using the fourth-order Runge-Kutta method, with parameters optimized via a genetic algorithm. Sensitivity analyses demonstrated that gender ratio imbalances significantly impact ecosystem stability and functionality. This research not only elucidates the critical role of sex ratio dynamics in population regulation but also provides theoretical foundations for fisheries management, emphasizing the necessity of incorporating sex differentiation mechanisms in ecological modeling. The findings contribute to the development of effective control strategies to mitigate the adverse ecological impacts of sea lampreys on aquatic ecosystems.
Acknowledgment
S202410702122 Innovative Training Project: Research on Lightweight Methods for Generative Large Models Based on Knowledge Distillation (Project ID: S202410702122)
References
[1]. Johnson, N. S., Swink, W. D., et al. (2017). Field study suggests that sex determination in sea lamprey is directly influenced by larval growth rate. Proceedings of the Royal Society B: Biological Sciences, 284(1851), 20170262.
[2]. Robinson, J. M. (2013). Sea lamprey (Petromyzon marinus) population dynamics, assessment, and control strategy evaluation in the St. Marys River, Michigan [Doctoral dissertation]. University of Maryland, College Park.
[3]. Berkson, J. (1944). Application of the logistic function to bio-assay. Journal of the American Statistical Association, 39(227), 357-365.
[4]. Reichenbach, T., Mobilia, M., et al. (2006). Coexistence versus extinction in the stochastic cyclic Lotka-Volterra model. Physical Review E, 74(5), 051907.
[5]. Johnson, N. S., et al. (2014). Survival and metamorphosis of low-density populations of larval sea lampreys (Petromyzon marinus) in streams following lampricide treatment. Journal of Great Lakes Research, 40(1), 155-163.
[6]. Cerrato, R. M. (1990). Interpretable statistical tests for growth comparisons using parameters in the von Bertalanffy equation. Canadian Journal of Fisheries and Aquatic Sciences, 47(7), 1416-1426.
[7]. Zhu, C., & Yin, G. (2009). On competitive Lotka–Volterra model in random environments. Journal of Mathematical Analysis and Applications, 357(1), 154-170.
[8]. Smith, J., Doe, A., et al. (2021). The evolutionary history of jawless vertebrates: Insights from lamprey genomics. Nature Ecology & Evolution, 5(8), 1120-1130.
[9]. Zheng, Y., & Chang, C. (2022). Correlation between concepts based on Lotka-Volterra predator-prey model. Chinese Journal of Medical Library and Information Science and Technology, 31(12), 7-13.
[10]. Johnson, N. S., Swink, W. D., et al. (2021). Larval growth rate determines sex in sea lampreys: Implications for invasive species control. Journal of Great Lakes Research, 47(3), S72-S89.
[11]. Zhang, Y., Li, X., et al. (2023). Dynamic modeling of sex ratio effects on sea lamprey population stability: A computational approach. Ecological Modelling, 478, 110854.
[12]. Johnson, R. T., et al. (2023). Adaptive modeling of sea lamprey population dynamics: Integrating competition, mutualism, and predation in the Great Lakes ecosystem. Frontiers in Ecology and Evolution, 11, 789201.
Cite this article
Yin,J.;Ma,H.;Ma,Z.;Jin,Y. (2025). A Parameter Optimization Prediction Model for Nonlinear Differential Equations Based on Gradient Descent. Applied and Computational Engineering,160,1-6.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of CONF-SEML 2025 Symposium: Machine Learning Theory and Applications
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Johnson, N. S., Swink, W. D., et al. (2017). Field study suggests that sex determination in sea lamprey is directly influenced by larval growth rate. Proceedings of the Royal Society B: Biological Sciences, 284(1851), 20170262.
[2]. Robinson, J. M. (2013). Sea lamprey (Petromyzon marinus) population dynamics, assessment, and control strategy evaluation in the St. Marys River, Michigan [Doctoral dissertation]. University of Maryland, College Park.
[3]. Berkson, J. (1944). Application of the logistic function to bio-assay. Journal of the American Statistical Association, 39(227), 357-365.
[4]. Reichenbach, T., Mobilia, M., et al. (2006). Coexistence versus extinction in the stochastic cyclic Lotka-Volterra model. Physical Review E, 74(5), 051907.
[5]. Johnson, N. S., et al. (2014). Survival and metamorphosis of low-density populations of larval sea lampreys (Petromyzon marinus) in streams following lampricide treatment. Journal of Great Lakes Research, 40(1), 155-163.
[6]. Cerrato, R. M. (1990). Interpretable statistical tests for growth comparisons using parameters in the von Bertalanffy equation. Canadian Journal of Fisheries and Aquatic Sciences, 47(7), 1416-1426.
[7]. Zhu, C., & Yin, G. (2009). On competitive Lotka–Volterra model in random environments. Journal of Mathematical Analysis and Applications, 357(1), 154-170.
[8]. Smith, J., Doe, A., et al. (2021). The evolutionary history of jawless vertebrates: Insights from lamprey genomics. Nature Ecology & Evolution, 5(8), 1120-1130.
[9]. Zheng, Y., & Chang, C. (2022). Correlation between concepts based on Lotka-Volterra predator-prey model. Chinese Journal of Medical Library and Information Science and Technology, 31(12), 7-13.
[10]. Johnson, N. S., Swink, W. D., et al. (2021). Larval growth rate determines sex in sea lampreys: Implications for invasive species control. Journal of Great Lakes Research, 47(3), S72-S89.
[11]. Zhang, Y., Li, X., et al. (2023). Dynamic modeling of sex ratio effects on sea lamprey population stability: A computational approach. Ecological Modelling, 478, 110854.
[12]. Johnson, R. T., et al. (2023). Adaptive modeling of sea lamprey population dynamics: Integrating competition, mutualism, and predation in the Great Lakes ecosystem. Frontiers in Ecology and Evolution, 11, 789201.