1. Introduction
With the continuous improvement of the social and economic level of our country, the demand for energy also increases sharply. The extensive use of fossil fuels and other traditional energy sources has caused serious environmental pollution. As one of the renewable and clean energy sources, new energy has consequently attracted wide attention from the society. Electrochemical power has gradually become the focus of research because of its superior conversion and storage technologies. However, due to the difficulty of large-scale transportation and storage that cannot be improved in a short time, individuals’ demand for the development of secondary energy storage batteries is growing urgently. Recently, the most commonly used secondary energy storage batteries contain lead-acid batteries, Ni-MH batteries, sodium sulfur batteries, and lithium-ion batteries. After a period of research, it was found that lithium-ion batteries became the most popular among those due to their advantages of small size, high energy density, security, environmental friendliness, and long lifespan. Lithium-ion batteries are widely used in different important fields, such as renewable energy systems and electric automobiles.
Since the beginning of the 21st century, a large number of the electronic devices used by Chinese residents are equipped with lithium-ion batteries, which are increasingly widely used. Although lithium-ion batteries have great strengths over traditional old batteries, their cycle life, power density, and other electrochemical properties still need to be modified so that they can be applied to larger energy storage occasions. Therefore, how to enhance the behavior of lithium-ion batteries is a central problem in the present industry of battery manufacturing and has become a research hotspot in the current scientific research community. The electrochemical properties of the negative and positive electrode materials is tightly linked to this. Accordingly, the selection and modification of electrode materials are regarded as the primary task for elevating the performance of lithium-ion batteries.
The aim of this paper is to deeply analyze the main electrode materials of lithium-ion batteries. Systematically introduce the latest research achievements and progress of the electrode materials, including their advantages and disadvantages, synthesis methods, and modification studies. Concurrently, briefly predict the future research focus and development trend of lithium-ion batteries.
2. Negative electrode materials for lithium-ion battery
The negative electrode materials used in a lithium-ion battery's construction are crucial to the battery's functionality. They are a crucial component of a lithium-ion battery's structure [1]. Negative electrode materials can be roughly categorized into four groups depending on their basic elements: carbon, silicon, tin, and metal oxidate-based compounds.
2.1. Metal oxidate based negative electrode materials
2.1.1. Lithium titanate (Li4Ti5O12). Lithium titanate is a kind of inorganic substance. It is regarded as a potential anode material for lithium-ion batteries because it is extremely important to improve the function of lithium-ion batteries in various aspects [2]. As shown in Fig. 1, Li4Ti5O12 exhibits a special spinel structure and has the advantages of the simple preparation method, high cycle stability, high safety, and high coulomb efficiency. Therefore, it gets numerous attentions from the public [1, 3].
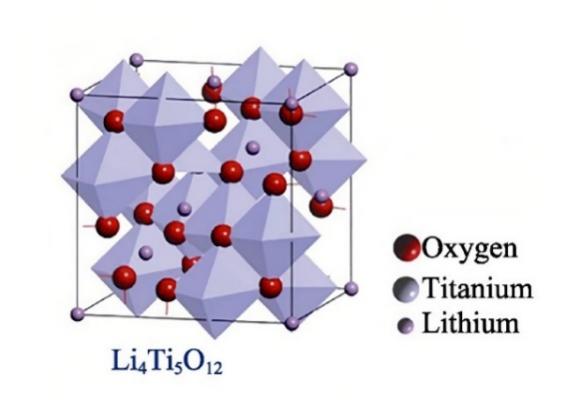
Figure 1. Schematic diagram of lithium titanate structure [2].
Li4Ti5O12 is a composite oxidate composed of lithium, titanium, and oxygen that has certain stability at room temperature. However, the theoretical specific capacity of lithium titanate is only 175 mAh/g due to vacancy restriction in the Li+ diffusion channel [1, 4, 5]. This is the major drawback of lithium titanate as an anode material for lithium-ion battery.
It is generally accepted that electrolyte decomposition occurs at voltages less than 1.2 V. And the lithium-ion embedded phase transition potential of lithium titanate is approximately stable at 1.55 V, so it is believed that there is no SEI film growth in the charging and discharging process [1]. Besides, under this voltage condition, it can be ensured that Li+ will not precipitate lithium dendrites after the battery works for a long time. Thus, the possibility of short circuit has been eliminated, and the security of lithium-ion battery in the charging and discharging processes has been greatly improved [1, 3]. In addition, during the process of Li+ embedding and expelling, the lattice constant basically remains unchanged, and the corresponding cell volume change is only 0.1% [3, 5]. As a consequence, lithium titanate material is regarded as a relatively rare “zero-strain” material [4]. This helps to preserve the integrity of the battery's structure and the materials used for the electrodes. Also, this kind of material has good circularity, with charging and discharging times up to 25,000 [4-6].
At present, the synthesis methods of Li4Ti5O12 mainly include the solid-phase reaction method, the sol-gel method, the hydrothermal method, and so on. Different preparation methods may cause differences in the appearance and properties of materials [6]. Table 1 briefly summarizes the required conditions, advantages, and disadvantages of the synthesis methods.
Table 1. Comparison of several synthesis methods of Li4Ti5O12 [1-3, 5].
Synthesis Method | Synthesis Conditions | Advantages | Disadvantages |
Solid-phase Reaction | Uniformly mix the titanium source and lithium source by ball milling to obtain powder substance and calcined at 800~1000 ℃ for at least 12h. | (i) Simple synthesis process and conditions, low cost (ii) Easy to mass produce | (i) High energy consumption (ii) Large particle size (micron) (iii) Poor repeatability and uniformity of products |
Sol-gel Reaction | Add Li source and Ti source into the organic chelating agent and heat to obtain the sol. Calcinate at 500~800 ℃ to get the lithium titanate powder. | (i) Low synthesis temperature, short synthesis time, good product uniformity (ii) Large specific surface area, small particle size (nanometer scale) and uniform distribution | (i) Complex synthesis method and high cost (ii) Will produce gas pollution (CO2, etc.) (iii) Not suitable for mass production |
Hydrothermal Reaction | Add Li source and Ti source with water or organic solution as solvent, heat in high pressure reactor and calcinate at 500 ℃ at least. | (i) Can be artificially controlled to obtain a variety of sizes of the materials (ii) The product particles are evenly distributed with good uniformity | (i) Synthetic materials and raw materials are expensive (ii) The washing process is complicated (iii) Difficulty in industrial mass production |
2.1.2. Fe2O3. One of the metal oxides currently most likely to replace the graphite anode is Fe2O3, an oxidation-reduced metal oxide anode material [2, 7]. It can store up to 1004mAh/g of lithium, which is roughly three times as much as a graphite anode [8]. This negative electrode material is widely utilized in gas sensors, information storage, and other industries and has a number of benefits including low cost, abundant reserves, environmental friendliness, and high safety.
2.2. Carbon-based material
2.2.1. Graphene. Many scientific studies have been conducted on graphene due to its benefits of high energy, low consumption, no memory effect, tiny discharge, low internal resistance, high-cost performance, and low pollution. Modern preparation techniques include mechanical stripping, epitaxial growth, vapor deposition, redox, and other techniques. According to studies chemical vapor precipitation is one of the most promising processes with a huge production capacity.
Due to the high product quality and vast production area, chemical vapor precipitation has gained widespread recognition as one of the most promising processes for producing graphene in an industrial setting. The growth matrix is crucial to the growth of graphene. Metal foils or metal films on particular substrates make up the majority of the growth substrates now in use [9]. Many scientific studies have been conducted on graphene due to its benefits of high energy, low consumption, no memory effect, tiny discharge, low internal resistance, high-cost performance, and low pollution. Modern preparation techniques include mechanical stripping, epitaxial growth, vapor deposition, redox, and other techniques. Chemical vapor precipitation is one of the most promising processes with a huge production capacity, according to studies.
Due to the high product quality and vast production area, chemical vapor precipitation has gained widespread recognition as one of the most promising processes for producing graphene in an industrial setting. The growth matrix is crucial to the growth of graphene. Metal foils or metal films on particular substrates make up the majority of the growth substrates now in use [9]. Using a carburizing mechanism, hydrocarbons (such as methane, ethanol, etc.) are injected into the surface of the Cu, Ni-based metal substrate, which is subsequently heated to a high temperature and subjected to a reaction [10]. Several layers or a single layer of graphene are created on the surface of the substrate during this cooling process, allowing for large-area development and transfer to a variety of substrates as well as diffusion growth in two sections [10]. By chemically etching the metal, the graphene and substrate may be more easily separated, making graphene processing easier [10].
The performance of new graphene composite anode materials for lithium storage has been explored, and researchers have developed a way to boost the cycling performance of graphene composites by covalent linkage. The capacity of the nitrogen-doped graphene anode material reached a peak of 828 mAh/g for the first time and could still be maintained at 292 mAh/g after 50 cycles, while the charge retention rate remained at 70% after cycling, according to the researchers' study of the processing performance of doped graphene by chemical vapor deposition. These results can demonstrate that it performs noticeably better than pristine graphene [9].
2.2.2. Hard carbon. Because of its high reversible specific capacity, hard carbon has become the most used anode material, stable structure and long cycle life of charge/discharge, low cost, high embedded lithium capacity, good low temperature, porous structure and large distance between layers, suitable specific surface, and ability to provide a significant number of cathode sodium ion storage sites [11, 12].
Methods for preparing carbon include cladding, comminution, pyrolysis, and curing [13]. One of the hard carbon composites that can be created is an asphalt/phenolic resin pyrolytic carbon composite employing coal tar pitch and boron-doped phenolic resin, as well as a high temperature solid phase approach [14]. This composite material can increase the phenolic resin pyrolysis carbon's capacity and first charge efficiency [14]. When the ratio of pyrolysis carbon to asphalt is adjusted to 3:1, the initial charge efficiency is 61%, which is a significant improvement over the reversible capacity and coulombic efficiency of phenolic resin pyrolysis carbon [14]. Tin dioxide can increase the material's capacity but decrease cycle stability if a particular amount is introduced. The other hard carbon composite is a new type of lithium battery cathode paste containing plant hard carbon material. This lithium battery anode slurry containing plant hard carbon material preparation steps include: stirring and mixing conductive agent, plant hard carbon material, sodium carboxymethyl cellulose, to produce sodium carboxymethyl cellulose colloid, then in the planetary mixer in drops of gum liquid and stirring, when the solid content of the material is 70 ~ 55%, to the planetary mixer maximum metric speed mixing and stirring; then add water vacuum mixing, adding butadiene rubber emulsion vacuum mixing [15].The dispersion uniformity of the material prepared by this method and the stability of the lithium battery are significantly improved.
2.3. Silicon-based material
Theoretically, silicon has a specific capacity of 4200 mAh/g, which is nearly ten times greater than that of carbon. Si has a poor intercalation potential for lithium, thus producing lithium dendrites safely is difficult [2]. However, during the charging and discharging process, the volume of silicon-based materials changes significantly, leading to the collapse of the electrode structure. This attenuation of silicon's specific capacity has a significant impact on the cycling of the battery [5]. Hence, a number of modification studies must to be conducted before silicon-based materials may reach the manufacturing stage.
2.4. Tin-based material
Although tin-based materials have relatively abundant reserves and a high specific capacity (993 mAh/g), this material cannot yet be used in commercial mass production since it is susceptible to volume deformation caused by the discharge and charging procedures [2].
3. Positive electrode material
The specific capacity of positive for lithium-ion battery is far less than that of the negative electrode material. It illustrates that enhancement of the positive electrode material is the primary task to the goal of the capacity improvement of the lithium-ion battery. The key to achieving this goal is to improve the defect of low specific capacity of the positive electrode material.
3.1. LiCoO2
3.1.1. Advantages and disadvantages of LiCoO2. LiCoO2 has stable electrochemical performance and simple production process, which promotes its early commercialization. Because of its excellent charging and discharging efficiency and steady charge and discharge voltage energy, LiCoO2 finds significant use in tiny consumer goods batteries [16].
The major flaw in LiCoO2 is its poor thermal resistance, and when the charging voltage increases, its own safety performance frequently degrades [17]. Nevertheless, LiCoO2 cannot be effectively employed in high-power and large-capacity batteries due to its high pollution, toxicity, and lack of raw materials as well as its market price [18].
3.1.2. The modification method of LiCoO2. To start, LiCoO2 ultrafine powder can be made using a multiplicity redox technique [4]. With a fairly homogenous composition and strong sintering activity, the specific surface area can reach 30-50 m2/g and the primary particle size is approximately 30nm [19]. The process uses cheaper metal cobalt or cobaltate as the cobalt source, which significantly lowers the cost of producing LiCoO2 [19]. It also has the advantages of a faster sintering time and lower temperature. Moreover, the technology for producing lithium nickel cobalt manganese oxide by coprecipitation and subsequently sintering with lithium carbide at high temperatures is becoming more sophisticated. It can significantly lower the cost of employing LiCoO2 and increase safety [17].
At higher voltages, safety performance of LiCoO2 suffers. The surface structure's degradation at high voltage circumstances is the primary contributing component to the outcome. The performance of LiCoO2 at high voltage can be enhanced by using phosphate's high chemical stability and better lithium-ion electrical resistance [20, 21]. Moreover, it prevents the solubility of transition metal ions [20, 21]. Wang and other researchers, for instance, used an AlPO4 and Li3PO4 composite surface coating to improve the cycle stability and high-rate performance of LiCoO2 at 4.6V at ambient temperature [22].
The cathode-electrolyte reaction is hampered by AlPO4 with high stability (Fig. 2). Li3PO4 can be used as the migration channel of lithium.
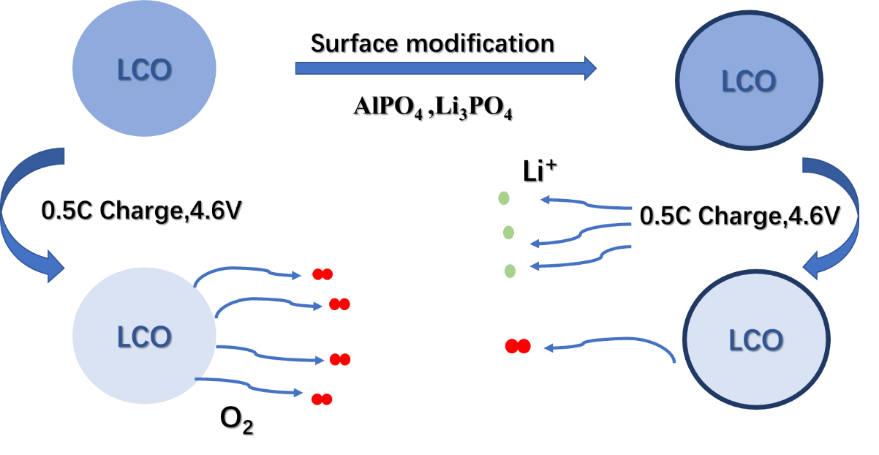
Figure 2. Schematic diagram of surface mechanism of AlPO4 composite coating [23].
3.2. LiMn2O4
3.2.1. Advantages and disadvantages of LiMn2O4. Due to its high discharge voltage, safety, non- toxicity, and low production cost, LiMn2O4 is one of the most prosperous cathode materials to replace LiCoO2. The oxygen atoms are grouped in a face-centered cubic structure in the spinel structure of LiMn2O4, which belongs to the Fd3m space group. The manganese atoms alternately occupy the octahedral spaces created by the oxygen atoms. Mn2O4 is one of them; it has a three-dimensional network structure that aids in the flow of lithium ions and allows for their interior reversible embedding and de-embedding. The high multiplicity performance and stability of the material can be explained by the fact that this technique does not lead to the internal collapse of the crystal structure [24].
However, during charging and discharging, LiMn2O4 suffers from substantial capacity loss, which can be attributable to two factors. First, Mn3+ in the electrode material undergoes disproportionation in the electrolyte, and the resultant Mn2+ then dissolves in the solution leading to the loss of manganese element. Second, because of the Jahn-Teller effect, the unfilled electrons of Mn inhomogeneously occupy the split orbits under the action of the octahedral field, causing the oxygen octahedra to deviate from spherical symmetry [25].
3.2.2. The modification method of LiMn2O4. During the discharge process of the battery with LiMn2O4 as positive electrode, LiMn2O4 is inclined to change to tetragonal structure when lithium is excessively embedded in it [17]. The fatal defect seriously affected the cycle performance of the battery. To improve the cycle stability, researchers have proposed that appropriate metal ions or other anions can be added to LiMn2O4 [17].
The path and the mode of lithium-ion dissociation are determined by the crystal structure. The key to improve the performance of positive electrode materials for lithium-ion batteries. Making LiMn2O4 into cascade structure with large capacity will change the way lithium-ion deblocking and embedding, which can bring the higher storage capacity and cost performance [26].
Through heating high-temperature solid-state synthesis, Chen Meng et al. studied the relationship between the lattice structure and electrochemical performance of the spinel cathode materials, with different amounts of nickel doped LiNixMn2-xO4 (x=0, 0.05, 0.1, 0.2, 0.3) by them [27]. They found that the doped Ni replaced the Mn at the 16d position of the octahedron, resulting in the reduction of the material cell parameters and the increase of the average valence of Mn [27]. These two factors have effectively suppressed the Jahn-Teller effect, resulting in more stable crystal structure and materials with higher cycle performance [27]. Moreover, in the process of increasing the amount of Ni doping, which leads to the increase of charge transfer resistance, they found that it negatively affected the material’s electrochemical performance [27]. The doping amount of x=0.1 can make the electrode material have high initial capacity and good cycle performance [27].
Combined with the research results of Li et al., doping modification research can optimize the preparation conditions of layered LiMn2O4 [28]. It can also reduce the impact of defects such as the high first irreversible capacity of undoped LiMn2O4 and the fast capacity decay rate in the cycle [28]. After their doping modification research on different metal elements, it is found that Co and Al are the elements with better doping effect [28].
3.3. LiFePO4
3.3.1. Advantages and disadvantages of LiFePO4. LiFePO4, one of the most common cathode materials used for lithium batteries, offers the benefits of low cost, environmental friendliness, high safety and long cycle life (>2000 cycles). Lithium iron phosphate materials are the olivine structure, which are part of the orthogonal crystalline system. In the crystal, the oxygen atoms are located in a lightly deformed hexagonal close-packed structure, and the iron and lithium atoms are placed in the middle of the hexagon structure of the oxygen atoms, forming iron oxide and lithium oxide ortho-octahedral structure [29]. The phosphorus atoms and oxygen atoms form a tetrahedral structure where the solid phosphorus-oxygen covalent bonds keep the lithium iron phosphate and lithium phosphate structures intact in atmospheres up to 350 degrees Celsius. It explains the high safety and long cycle life of lithium iron phosphate batteries.
However, there are limits to the structure of lithium iron phosphate as well. The ortho-octahedral FeO6 is connected to the adjacent FeO6 co-vertex in the b-c plane, and this discontinuous octahedral network restricts the electron transport in the Fe-O-Fe path, resulting in a poorly conductive material (~10-9 S·cm-1) [2]. And unlike LiCoO2 and LiMn2O4, the transport of lithium ions in LiFePO4 is generally considered to be one-dimensional along the [010] direction, which leads to a slow diffusion of lithium ions (~10-14 cm2s-1), limiting its high-magnification performance [30, 31].
Therefore, the focus of current research on the al is to enhance the electronic conductivity and ion diffusion rate of LiFePO4 cathode material. The main methods include nanoparticle size, coating modification and element doping, and so on.
3.3.2. The modification method of LiFePO4. The crystal structure of olivine type LiFePO4 determines the slow transport of lithium ions, and the particle size of LiFePO4 can be reduced, which can effectively reduce the path of Li+ diffusion in the material, and then improve its diffusion ability.
A technique for altering the interface of cathode materials is coating modification, which can improve the material's electrochemical performance, stabilize the structure, and keep the active components from coming into contact with the electrolyte, preventing the dissolution of transition metals. Carbon is the most commonly used coating material, has lower production costs and is suitable for commercial applications. Commonly used carbon-coated materials are porous carbon, graphene, carbon nanotubes, etc. The carbon coated can dramatically improve the high magnification performance of the battery due to good carbon conductivity [32].
The element doping essentially improves the electronic conductivity of the mass phase of the LiFePO4 particles, where the carbon coating mainly improves it in the surfaces of the LiFePO4 particles. Elements doping is the doping of other metal or non-metal ions into the crystal to replace Li or Fe sites of LiFePO4, creating lattice defects, expanding the diffusion channels of Li ions and reducing the activation energy of electron transfer. There is also a distinction between mono-ion doping and multi-ion doping according to the amount of doped elements [33].
4. Prospects of electrode materials for lithium-ion batteries
Lithium-ion battery electrode materials still have a lot of room for growth. Future negative electrode materials are likely to develop in a way that is high efficiency and low price. By applying graphite coating, curing, and carbonization, the electrochemical characteristics of lithium-ion negative electrode materials can be further enhanced. Doping, coating, and other efficient modification techniques can help positive electrode materials perform electrochemically more effectively to a certain extent. Future study will focus on improving the stability and capacity of lithium-ion battery cathode materials using the modification technique of surface doping and coating.
5. Conclusion
Lithium-ion battery is the key to achieve the development and application of new energy, which is of great importance for the improvement of energy utilization rate and environment protection. This essay examines the benefits and drawbacks of positive and negative electrode materials used in lithium-ion batteries as well as their manufacture and modification processes. The best preparation technique for each material improves the cycle and initial charge efficiency of lithium-ion batteries while also streamlining the process and using less energy. However, the existing preparation process needs to be further evaluated and improved upon in order to meet the objectives of lithium-ion battery development in the future. The modification techniques that can somewhat increase the cycle performance and energy density of lithium-ion batteries are summarized, which may have some reference significance for future improvements in lithium-ion battery design. How to use these modifications and preparation methods to produce high-quality electrode materials for lithium-ion batteries at low cost remains the focus of future research, in order to gain the better performance and the lower cost of lithium-ion batteries.
References
[1]. Yao, W.J., Zhuang, W., Ji, X.Y., et al, Solid-state synthesis of Li4Ti5O12 whiskers from TiO2-B[J], Mater Res Bull, 2016(75): 20
[2]. Yu, J.Y., Preparation and doping modification of lithium titanate as anode material for lithium-ion batteries. Dalian Maritime University, 2020. (In Chinese.)
[3]. Tan, Y., Xue, B., Research progress of lithium titanate as anode material for lithium-ion batteries. Journal of Inorganic Materials, 2018, 33(05): 475-482.
[4]. Luo, F., Chu, G., Huang, J., et al, Fundamental scientific aspect of lithium batteries (Ⅷ)—Anode electrode materials. Energy Storage Science and Technology, 2014, 3(02): 146-163.
[5]. Wang, C., Liu, Z.H., Sun, Y.H., et al, A review of electric performance and SOH research for Li4Ti5O12 based batteries, Advances in new and renewable energy, 2022, 10(04): 316-324. (In Chinese.)
[6]. Wei, B.X., Zhang, Z.R., Wang, C., Application of Li4Ti5O12 as anode in lithium ion batteries. Marine Electric, 2021, 41(06): 115-120. (In Chinese.)
[7]. Santhoshkumar, P., Prasanna, K., Nirmal, S.I., et al. Time-efficient synthesis of MnO2 encapsulated α-Fe2O3 ellipsoids for lithium ion battery applications[J], Journal of Alloys & Compounds, 2017, 720: 300–3.
[8]. Liu, C.Q., Qiao, X.L., Chi, C.X., Research progress in Fe2O3 anode materials for lithium batteries, China Academic Journal Electronic Publishing House, 2022, 85(11): 1290-1296. (In Chinese.)
[9]. Ren, W.C., Gao, L.B., Ma, L.P., Preparation of graphene by chemical vapor deposition. New carbon materials, 2011, 26(1): 10. (In Chinese.)
[10]. Yuan, X.Y., Progress in preparation of graphene. Journal of Inorganic Materials, 2011, 26(6). (In Chinese.)
[11]. Li, Q.X., Present situation and prospect of hard carbon anode materials for lithium-ion batteries. Journal of Shanghai Electric Power University, 30. 1(2014): 4. (In Chinese.)
[12]. Zhou, R.X., Study on Preparation, Modification and Electrochemical Energy Storage of Hard Carbon, Tian Jing University, 2020 (In Chinese.)
[13]. Yue, M., Wang, F., Yan, H., Composite hard carbon anode material for lithium-ion battery and its preparation method, CN101887966A[P]. 2010. (In Chinese.)
[14]. Wang, D.Y., Preparation and properties of graphite/hard carbon composites for lithium-ion battery anode, Harbin Institute of Technology, 2015. (In Chinese.)
[15]. Wang, J.L., Zheng, S.Q., Ni, J., Preparation method of lithium battery anode paste containing plant hard carbon material, CN114373930A. 2022. (In Chinese.)
[16]. Yue, G.F., Wang, L., Research intelligence analysis of lithium cobaltate industry, Science and technology vision, 2016, 0(5): 123-124. (In Chinese.)
[17]. Cui, B.X., Liu, Y.H., Niu, P.B., New energy materials and their applications in batteries, Information recording materials, 2021, 22(10): 234-236. (In Chinese.)
[18]. Hou, L., Feng, R., Xu, H., Research progress of cobalt doped lithium nickel oxide cathode materials. Inorganic chemical industry, 2009(8): 12-14. (In Chinese.)
[19]. Xi, X.M., Liao, D.Q., Study on the Sintering and Electrochemical Properties of Lithium Cobalt Prepared by Multiphase Oxidation-reduction Method, Mining Engineering, 2013(1): 97-100105 (In Chinese.)
[20]. Wang, K.X., LI, X.H., Chen, J.S., Surface and Interface Engineering of Electrode Materials for Lithium-Ion Batteries, Advanced Materials, 2015, 27(3): 527-545.
[21]. Lee, S.W., Kim, M.S., Jeong, J.H., et al, Li3PO4 surface coating on Ni-rich LiNi0.6Co0.2Mn0.2O2 by a citric acid assisted sol-gel method: Improved thermal stability and high-voltage performance[J], Journal of Power Sources. 2017, 360206-214.
[22]. Wang, X., Wu, Q., Li, S., et al. Lithium-Aluminum-Phosphate coating enables stable 4.6 V cycling performance of LiCoO2 at room temperature and beyond, Energy Storage Materials, 2021(6).
[23]. Li Z., Li A., Zhang H., et al, Multi-scale stabilization of high-voltage LiCoO2 enabled by nanoscale solid electrolyte coating[J], Energy Storage Materials, 2020, 2971-77.
[24]. Liu, Z.L., Zhang, Z.A., Zhu, Y.M., et al, Progress of rare earth materials used as cathode materials for lithium-ion batteries, Battery, 2019, 49(06): 520-523. (In Chinese.)
[25]. Guo, G.H., Chen, S., Liu, F.F., et al, Spinel type LiMn2O4 Progress in doping research, New Chemical Materials, 2013, 41(10): 169-171. (In Chinese.)
[26]. Sun, X.M., Preparation and performance study of LiMn2O4 lithium-ion battery with large capacity and laminated structure, Henan Science and Technology, 2014(11): 1. (In Chinese)
[27]. Chen, M., Yang, C., Xiao, B., Effect of Nickel Doping on the Structure and Electrochemical Properties of Spinel LiMn2O4, Rare metals, 2006, 30(4): 453-456. (In Chinese.)
[28]. Xu, M., Li, X., Zhang, Y., Preparation and Modification of Layered Lithium Manganate. Power supply technology, 2003, 27(4): 366-369. (In Chinese.)
[29]. Liu, X.Y., Li, X.Y., Qu, S.W., Research status of lithium iron phosphate cathode materials, Non-ferrous metal materials and engineering, 2021, 42(03): 41-47. (In Chinese.)
[30]. Yu, F., Zhang, L.L., LI, Y.C., et al. Mechanism studies of LiFePO4 cathode material: lithiation/delithiation process, electrochemical modification and synthetic reaction, RSC Adv, 2014, 4, 54576-54602.
[31]. Chen, S., Lv, D., Chen, J, Zhang, Y., Shi, F., Review on Defects and Modification Methods of LiFePO4 Cathode Material for Lithium-Ion Batteries. American Chemical Society, Energy & Fuels, 2022 36 (3), 1232-1251
[32]. Li, G.F., Li, Z.M., Ni, T., et al, Research Progress of Cathode Materials Modified by Surface Coating for Lithium-ion Batteries, Journal of Materials Engineering, 2018, 46(9): 23-30.
[33]. Chen J.M., Preparation and doping modification of cathode materials for lithium ion batteries, Jiangsu University of Science and Technology, 2022.
Cite this article
He,H.;Huang,J.;Wang,J.;Xu,X. (2023). Research status and prospect of electrode materials for lithium-ion battery. Applied and Computational Engineering,23,1-9.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2023 International Conference on Functional Materials and Civil Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Yao, W.J., Zhuang, W., Ji, X.Y., et al, Solid-state synthesis of Li4Ti5O12 whiskers from TiO2-B[J], Mater Res Bull, 2016(75): 20
[2]. Yu, J.Y., Preparation and doping modification of lithium titanate as anode material for lithium-ion batteries. Dalian Maritime University, 2020. (In Chinese.)
[3]. Tan, Y., Xue, B., Research progress of lithium titanate as anode material for lithium-ion batteries. Journal of Inorganic Materials, 2018, 33(05): 475-482.
[4]. Luo, F., Chu, G., Huang, J., et al, Fundamental scientific aspect of lithium batteries (Ⅷ)—Anode electrode materials. Energy Storage Science and Technology, 2014, 3(02): 146-163.
[5]. Wang, C., Liu, Z.H., Sun, Y.H., et al, A review of electric performance and SOH research for Li4Ti5O12 based batteries, Advances in new and renewable energy, 2022, 10(04): 316-324. (In Chinese.)
[6]. Wei, B.X., Zhang, Z.R., Wang, C., Application of Li4Ti5O12 as anode in lithium ion batteries. Marine Electric, 2021, 41(06): 115-120. (In Chinese.)
[7]. Santhoshkumar, P., Prasanna, K., Nirmal, S.I., et al. Time-efficient synthesis of MnO2 encapsulated α-Fe2O3 ellipsoids for lithium ion battery applications[J], Journal of Alloys & Compounds, 2017, 720: 300–3.
[8]. Liu, C.Q., Qiao, X.L., Chi, C.X., Research progress in Fe2O3 anode materials for lithium batteries, China Academic Journal Electronic Publishing House, 2022, 85(11): 1290-1296. (In Chinese.)
[9]. Ren, W.C., Gao, L.B., Ma, L.P., Preparation of graphene by chemical vapor deposition. New carbon materials, 2011, 26(1): 10. (In Chinese.)
[10]. Yuan, X.Y., Progress in preparation of graphene. Journal of Inorganic Materials, 2011, 26(6). (In Chinese.)
[11]. Li, Q.X., Present situation and prospect of hard carbon anode materials for lithium-ion batteries. Journal of Shanghai Electric Power University, 30. 1(2014): 4. (In Chinese.)
[12]. Zhou, R.X., Study on Preparation, Modification and Electrochemical Energy Storage of Hard Carbon, Tian Jing University, 2020 (In Chinese.)
[13]. Yue, M., Wang, F., Yan, H., Composite hard carbon anode material for lithium-ion battery and its preparation method, CN101887966A[P]. 2010. (In Chinese.)
[14]. Wang, D.Y., Preparation and properties of graphite/hard carbon composites for lithium-ion battery anode, Harbin Institute of Technology, 2015. (In Chinese.)
[15]. Wang, J.L., Zheng, S.Q., Ni, J., Preparation method of lithium battery anode paste containing plant hard carbon material, CN114373930A. 2022. (In Chinese.)
[16]. Yue, G.F., Wang, L., Research intelligence analysis of lithium cobaltate industry, Science and technology vision, 2016, 0(5): 123-124. (In Chinese.)
[17]. Cui, B.X., Liu, Y.H., Niu, P.B., New energy materials and their applications in batteries, Information recording materials, 2021, 22(10): 234-236. (In Chinese.)
[18]. Hou, L., Feng, R., Xu, H., Research progress of cobalt doped lithium nickel oxide cathode materials. Inorganic chemical industry, 2009(8): 12-14. (In Chinese.)
[19]. Xi, X.M., Liao, D.Q., Study on the Sintering and Electrochemical Properties of Lithium Cobalt Prepared by Multiphase Oxidation-reduction Method, Mining Engineering, 2013(1): 97-100105 (In Chinese.)
[20]. Wang, K.X., LI, X.H., Chen, J.S., Surface and Interface Engineering of Electrode Materials for Lithium-Ion Batteries, Advanced Materials, 2015, 27(3): 527-545.
[21]. Lee, S.W., Kim, M.S., Jeong, J.H., et al, Li3PO4 surface coating on Ni-rich LiNi0.6Co0.2Mn0.2O2 by a citric acid assisted sol-gel method: Improved thermal stability and high-voltage performance[J], Journal of Power Sources. 2017, 360206-214.
[22]. Wang, X., Wu, Q., Li, S., et al. Lithium-Aluminum-Phosphate coating enables stable 4.6 V cycling performance of LiCoO2 at room temperature and beyond, Energy Storage Materials, 2021(6).
[23]. Li Z., Li A., Zhang H., et al, Multi-scale stabilization of high-voltage LiCoO2 enabled by nanoscale solid electrolyte coating[J], Energy Storage Materials, 2020, 2971-77.
[24]. Liu, Z.L., Zhang, Z.A., Zhu, Y.M., et al, Progress of rare earth materials used as cathode materials for lithium-ion batteries, Battery, 2019, 49(06): 520-523. (In Chinese.)
[25]. Guo, G.H., Chen, S., Liu, F.F., et al, Spinel type LiMn2O4 Progress in doping research, New Chemical Materials, 2013, 41(10): 169-171. (In Chinese.)
[26]. Sun, X.M., Preparation and performance study of LiMn2O4 lithium-ion battery with large capacity and laminated structure, Henan Science and Technology, 2014(11): 1. (In Chinese)
[27]. Chen, M., Yang, C., Xiao, B., Effect of Nickel Doping on the Structure and Electrochemical Properties of Spinel LiMn2O4, Rare metals, 2006, 30(4): 453-456. (In Chinese.)
[28]. Xu, M., Li, X., Zhang, Y., Preparation and Modification of Layered Lithium Manganate. Power supply technology, 2003, 27(4): 366-369. (In Chinese.)
[29]. Liu, X.Y., Li, X.Y., Qu, S.W., Research status of lithium iron phosphate cathode materials, Non-ferrous metal materials and engineering, 2021, 42(03): 41-47. (In Chinese.)
[30]. Yu, F., Zhang, L.L., LI, Y.C., et al. Mechanism studies of LiFePO4 cathode material: lithiation/delithiation process, electrochemical modification and synthetic reaction, RSC Adv, 2014, 4, 54576-54602.
[31]. Chen, S., Lv, D., Chen, J, Zhang, Y., Shi, F., Review on Defects and Modification Methods of LiFePO4 Cathode Material for Lithium-Ion Batteries. American Chemical Society, Energy & Fuels, 2022 36 (3), 1232-1251
[32]. Li, G.F., Li, Z.M., Ni, T., et al, Research Progress of Cathode Materials Modified by Surface Coating for Lithium-ion Batteries, Journal of Materials Engineering, 2018, 46(9): 23-30.
[33]. Chen J.M., Preparation and doping modification of cathode materials for lithium ion batteries, Jiangsu University of Science and Technology, 2022.









