1. Introduction
In the context of the shortage of traditional energy, the emergence of new energy batteries has brought a new development direction for global industry. China has always attached importance to the research of new energy battery materials, and listed it as the key project of the 863 Plan in the 1980s. The development of new energy can reduce China’s dependence on traditional energy, balance the continuous increase in the proportion of fossil energy consumption, and facilitate the optimization and transformation of China's energy consumption structure. As an energy storage device, the energy storage and conversion performance of the new energy battery is closely related to the material of the new energy battery. It can be said that the new energy battery material directly affects the development and application of the new energy battery. At present, the typical key battery materials for new energy at home and abroad mainly include lithium-ion battery materials, fuel cell materials, etc. Among them, the core technologies in the field of lithium-ion battery are mostly mastered by the European Union, the United States, Japan, South Korea and other countries and regions; Fuel cells are mainly concentrated in the United States, Canada, Japan, Belgium, etc [1].
With the rapid development of the new energy automobile industry and the smart phone industry, China's investment in research and development of new energy materials such as lithium-ion batteries has always maintained a high intensity, providing strong support for China's high-tech industries such as lithium ion battery materials and fuel cell materials to break through technical barriers and achieve rapid development. After many years of efforts, China's new energy battery material industry has made remarkable development, the technical level is increasing, and the industrial scale is expanding. But on the whole, compared with developed countries, China’s new energy material field still has a large gap compared with the advanced level of foreign countries. The proportion of high-end products of some new materials in new energy batteries is relatively low, the technical content is not high, and the added value of products is low, High-precision automation equipment for high-end materials and batteries still needs to be imported in large quantities, the competitiveness of the new energy material industry still needs to be strengthened, the independent innovation system with enterprises as the main body needs to be improved, and some key core materials are controlled by others, and the high dependence of high-end materials on foreign countries needs to be solved. In recent years, with the research and development of nanomaterials and technologies, the research of nanomaterials is gradually combined with the research of new energy batteries, making the development of new energy batteries have further development. For example, the surface of the battery electrode material will increase, the current density will decrease, and the polarization will decrease, resulting in an increase in capacitance, thus having better electrochemical activity. In particular, carbon nanotubes have made major breakthroughs in their research as new lithium storage materials, electrochemical energy storage materials and high-performance composites.
Based on the development status of nanomaterials, this paper first explores the structure, properties and some preparation methods of nanomaterials. Then the different applications of various new nanomaterials in new energy batteries are described. Finally, the future development prospect of this industry is prospected.
2. Nanomaterials
2.1. Nanomaterials
Nanomaterial is a new material in this century. Nanometer, which means that the unit component of this material is measured on the scale of nanometer. Compared with previous materials, nano-particles are prepared on a smaller scale and synthesized into nanomaterials, which also makes nanomaterials have some physical and chemical properties that traditional materials do not have. After research, nano-materials show many different properties from ordinary materials that people have studied and understood in the past, which also makes its application range very wide, involving medical, electronic, chemical, mechanical, etc. Nanomaterials have gradually become the key research materials around the world, and gradually push it to the process of industrialization and commercialization.
Due to the special particle size of nanomaterials, nanomaterials have special surface effect, interface effect, small size effect and quantum effect [2]. Surface effect means that with the reduction of particle size of materials, the volume decreases and the specific surface area increases rapidly. At this time, the influence of material surface properties on the reaction cannot be ignored in the process of applying materials; Interface effect refers to the change in the arrangement of atoms and molecules at the nanometer level, which will bring different effects and properties to the material, which is reflected in the interface effect, such as the lamellar structure of graphene as we know; Different from surface effect, small size effect refers to the change of physical properties such as magnetic, optical and thermal properties of materials caused by particle size reduction [2]. Based on these special properties, we can develop more application directions.
The range of nanomaterials is very wide, which can be further classified according to the different sizes of nanomaterials at the nanometer scale, specifically nanoparticles, nanosolids and nano-assembly systems. Nanoparticles have various forms, including but not limited to spherical and rod-shaped, while nanosolids are the general name of one-dimensional, two-dimensional or three-dimensional nanomaterials formed by the polymerization of nanoparticles, which can be the aggregation of nanoparticles in the same state or the combination of different forms. Nano assembly system is a synthetic material system with different nanostructures [3].
2.2. Preparation of nanomaterials
With the continuous development of science and technology, nanomaterials are gradually coming into the public's view, and the research on nanomaterials is making continuous progress. More and more nanomaterials are used in various fields of production and life. The research on the preparation methods of nanomaterials can further expand the application scope of nanotechnology and bring about further progress in society.
2.2.1. Evaporation condensation method. The most commonly used method for preparing nanomaterials is evaporation and condensation. Specifically, heating at high temperature and evaporating the raw material of the nanomaterial to be prepared, decomposing to the required particle size, and then reordering the decomposed particles according to the structure and morphology of the target material, and re-condensing according to the design scheme standards, can obtain regular nanomaterials. Among them, the decomposition temperature needs to be carefully controlled. The nature and structure of each material are different, and the corresponding decomposition temperature and other environmental conditions are different. Different decomposition conditions and atomic arrangement combination formula should be designed for different materials.
Nanomaterials prepared by evaporation and condensation can exceed the upper limit size of 100 nm. Czanderna and its collaborators adopted two methods to prepare spectral pure nano-TiO2 in 1957. The main difference between the two methods lies in how to prepare pure Ti(OH)4 gel. The first method uses direct preparation, which uses 90% H2O2 ammonia solution to directly oxidize pure metal Ti to directly obtain the oxidation product Ti(OH)4 gel. The second method uses indirect preparation, which first hydrolyses TiCl4, and then hydrolyzes the liquid Ti(OH)4 in sulfuric acid solution and ammonia solution successively to obtain gelled Ti(OH)4. The final step is to dry and remove impurities, and carry out particle size measurement and structure analysis [4].
2.2.2. Spray method. The spray method belongs to the physical category. The specific method is to atomize the raw material solution through a series of physical means to disperse the atomic or molecular particles of the material, and then dry and sinter. The nanometer particles prepared by the spray method will be more uniform, delicate, and simple, and even can prepare submicron materials, which is an ideal preparation method [5].
3. Application of nanomaterials in new energy batteries
Nowadays, with the rapid development of science and technology, energy and its storage has always been a hot topic in the scientific community. With the development of portable devices, rechargeable new energy batteries, represented by lithium batteries, came into being. In order to expand the application prospects of new energy batteries in the automotive industry and even the aerospace industry, the energy storage, energy density and charging life of batteries need to meet higher requirements, and the application of new materials is particularly important. The emergence of nanomaterials has brought new breakthroughs in the development of new energy batteries. Finding new nanomaterials with specific structural properties can play different roles in the positive and negative electrodes and electrolyte of batteries.
3.1. Nanostructured carbon sulfur cathode
Schematic diagram of conventional lithium-sulphur batteries is shown in Figure 1. Conventional lithium-sulphur batteries are made up of a lithium metal anode, a sulphur composite cathode and an organic liquid electrolyte. However, the development of rechargeable lithium-sulphur batteries faces important problems. During the charging and discharging process, the sulphur forms S8 with a stable structure. S8 undergoes constant morphological and structural changes during the cell reaction, while forming insoluble compounds such as Li2S and Li2S2 with lithium, hindering the further reaction of sulphur within the electrode. Therefore, the practical application of conventional lithium-sulphur batteries is not broad-spectrum.
With the development of research on lithium-sulphur batteries, carbon and sulphur composites as cathodes can effectively solve the above problems. The selection of carbon and sulphur composites focuses on the requirement for high electrical conductivity, sulphurophilicity, bondability, a porous structure with suitable pore size, and structural stability. To solve the problem of low capacity of lithium batteries, Ji, Xiulei's team investigated a carbon and sulphur composite with a nanostructure as a cathode for lithium batteries [6]. The main material used in this study was the mesoporous carbon material CMK- 3. This material has a highly homogeneous and connected porous structure with a large pore volume and can be used as a carrier medium for nanoelectrode materials and battery catalysts, while also possessing the excellent electrical conductivity of carbon materials. These characteristics all point to the great advantages of CMK - 3 as an anode material for batteries. CMK - 3 was prepared specifically by nano-casting using silica SBA - 15 as a hard template. The resulting pores are constructed from carbon fibres, which prevent the collapse of carbon nanopores in two-dimensional ordered hexagonal structures. The prepared CMK - 3 material and sulphur are mixed at a specific gravity of 3: 7 and heated until the sulphur melts. Under capillary action, the liquid sulphur diffuses into the carbon pores, which are then cooled and solidified. The sulphur cools and shrinks and grows uniformly and orderly on the inner walls of CMK - 3, forming a carbon and sulphur composite conductive nanomaterial.
Based on the aforementioned nano-pore structure, the lithium ions in the battery reaction can move freely in and out of the conductive carbon pores and react with the insulating sulphur attached to them in the battery. At the same time the complex pores and the porous carbon material prevent the diffusion of large size anions such as sulphides from the reaction, which facilitates the complete reaction of the electrode material to a large extent.
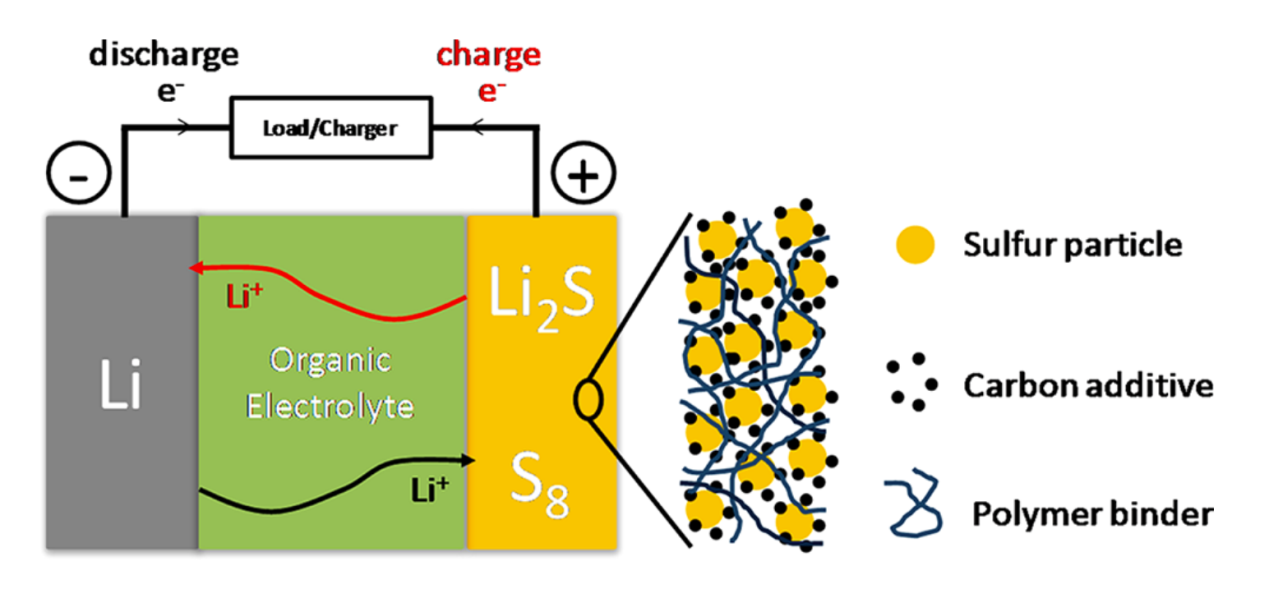
Figure 1. Schematic diagram of lithium sulfur battery [7].
3.2. Nano-Ti material anode
The lamellar graphite material has been an important anode material for lithium batteries for a long time. The layered structure of graphite sheet is very conducive to the insertion and escape of Li and can effectively improve the charge and discharge efficiency of the battery. Over the past few decades, researchers have discovered a large number of carbon nanomaterials, including graphite, graphene and carbon nanotubes, as shown in Figure 2. However, due to the low voltage difference, the electrolyte in the battery is easy to undergo reduction reaction and adhere to the surface of graphite material. This may lead to the deposition of Li and the reduction of battery efficiency, and even have potential safety hazards.
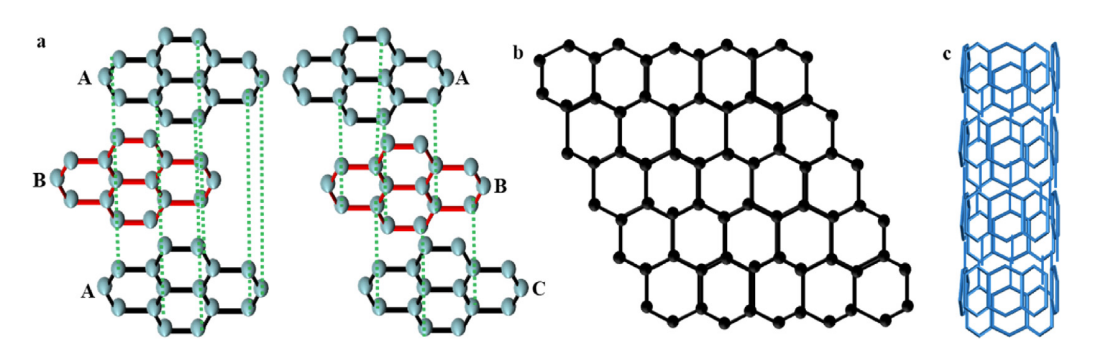
Figure 2. (a) Graphite diagram; (b) Graphene diagram; (c) Carbon nanotube diagram [8].
Therefore, we need a more suitable nano anode material than graphite. It has been found that the defective spinel Li4Ti5O12 has good properties. The material is non-toxic, and the battery reaction occurs at a high voltage, effectively avoiding the deposition of Li. This material can be converted on different components (Li4+xTi5O12, 0<x<3), which means that Li has higher access efficiency. However, due to the fact that the Li storage capacity is far lower than that of graphite and the low battery voltage, the energy density of Li4Ti5O12 is too low to be used as anode material.
Peter Bruce et al. [9] have investigated the discovery of a titanium oxide TiO2 - (B) with a specific crystal shape, consisting of a combination of co-edged and co-angular octahedral TiO6 arrangements. The reversibility of the embedding of TiO2 - (B) nanowires is far superior to that of the nanoparticles alone. This is because the size effect of the individual nanoparticles has a significant impact on the properties and may swell or shrink during charging and discharging, thus affecting the embedding of Li ions. The combination of TiO2 - (B) particles is prepared as nanotubes or nanowires arranged in the negative electrode of the battery, perpendicular to the diffusion direction of Li, so that Li ions are more easily trapped. The large number of gaps in the internal octahedral structure of TiO2 - (B) provides space for the Li ions to embed and the embedding rate is enhanced. Due to the small diameter of the tubular or wire structure, Li has a high transfer rate, which ensures a constant movement of charge [9, 10].
4. Future development direction
Metal composite nanomaterials and carbon nanomaterials have excellent performance. In the field of new energy battery applications, the research direction is expanding in a diversified way, including the improvement of the morphology and preparation of nanomaterials, and the use of the surface effect, interface effect, small size effect and quantum effect of nanomaterials to expand more applications, such as the combination of different electrode materials and nanomaterials, and the improvement of battery charging speed and energy density. In addition, nanotechnology can also be used in more fields, for example, as a catalyst carrier. The catalyst can provide more binding sites by uniformly covering the nano-materials. Nanomaterials can also be used as additives to change the apparent physical properties of materials, such as reducing the melting point, etc. This method can be used to prepare new materials with excellent properties. In the field of semiconductors, the application of nanotechnology can make non-luminescent materials glow at nanometer scale, and can be used as repair materials and targeted drugs in the medical field [2, 11].
5. Conclusion
The application of nanomaterials in the field of new energy batteries is developing at a high speed. Nanomaterials with different morphologies and raw materials are widely used in new energy battery materials, with their own advantages and disadvantages. The development and research of new energy batteries is an important guarantee for the realization of the concept of sustainable development, and the research of new energy battery materials is an important link in the development of new energy. In the development process of new energy, the application and research of new energy batteries has always been the focus of new energy development, which is of great significance to the improvement of energy utilization and environmental protection. With the development of nano-materials and technology research, its application in new energy batteries will surely achieve a higher and newer breakthrough, which will play an important role in achieving the two major long-term goals of “carbon peak” and “carbon neutral” in 2030 and 2060 respectively, and in optimizing and transforming China's energy consumption structure.
References
[1]. Huang, X.J., Zhao, W.W., Shao, Z.G., et al. (2020) Development Strategies for New Energy Materials in China. Engineering Science., 22: 60-67.
[2]. Gu, HC. (1999) Nano materials: new materials in the 21st century. World science., 4: 22-23. (In Chinese)
[3]. Li, Q.Q., Fan, S.S., Han, W.Q., et al. (1998) Mossbauer study of catalytically grown carbon nanotube. Chinese physics letters, 15: 68-69.
[4]. Birringer, R.H., Gleiter, H.P., et al. (1984) Phys Lett., 102(A):365~369.
[5]. Zhang, T.H., Yang, W.L., Yu, R.H., et al. (2022) Research status and development direction of nanomaterials preparation. Mass standardization, 368: 124-126. (In Chinese)
[6]. Ji, XL., Lee, K.T., Nazar, L.F. (2009) A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nature Materials, 8: 500-506.
[7]. Manthiram, A., Fu, Y.Z., Su, Y.S. (2013) Challenges and Prospects of Lithium-Sulfur Batteries. Accounts of chemical research, 46: 1125-1134.
[8]. Li, L., Zhang, D., Deng, J.P., et al. (2021) Carbon-based materials for fast charging lithium-ion batteries. CARBON., 183: 721-734.
[9]. Bruce, P.G., Scrosati, B., Tarascon, J.M. (2008) Nanomaterials for rechargeable lithium batteries. Angewandte chemie international edition, 47: 2930-2946.
[10]. Ji, LW., Lin, Z., Alcoutlabi, M., et al. (2001) Recent developments in nanostructured anode materials for rechargeable lithium-ion batteries. Energy and environmental science, 4: 2682-2699.
[11]. Collier, C.P., Saykally, R.J., Shiang, J.J., et al. (1997) Reversible tuning of silver quantum dot monolayers through the metal-insulator transition. Science, 277: 1978-1981.
Cite this article
Wu,T. (2023). Application of nanomaterials in new energy batteries. Applied and Computational Engineering,23,10-15.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2023 International Conference on Functional Materials and Civil Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Huang, X.J., Zhao, W.W., Shao, Z.G., et al. (2020) Development Strategies for New Energy Materials in China. Engineering Science., 22: 60-67.
[2]. Gu, HC. (1999) Nano materials: new materials in the 21st century. World science., 4: 22-23. (In Chinese)
[3]. Li, Q.Q., Fan, S.S., Han, W.Q., et al. (1998) Mossbauer study of catalytically grown carbon nanotube. Chinese physics letters, 15: 68-69.
[4]. Birringer, R.H., Gleiter, H.P., et al. (1984) Phys Lett., 102(A):365~369.
[5]. Zhang, T.H., Yang, W.L., Yu, R.H., et al. (2022) Research status and development direction of nanomaterials preparation. Mass standardization, 368: 124-126. (In Chinese)
[6]. Ji, XL., Lee, K.T., Nazar, L.F. (2009) A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nature Materials, 8: 500-506.
[7]. Manthiram, A., Fu, Y.Z., Su, Y.S. (2013) Challenges and Prospects of Lithium-Sulfur Batteries. Accounts of chemical research, 46: 1125-1134.
[8]. Li, L., Zhang, D., Deng, J.P., et al. (2021) Carbon-based materials for fast charging lithium-ion batteries. CARBON., 183: 721-734.
[9]. Bruce, P.G., Scrosati, B., Tarascon, J.M. (2008) Nanomaterials for rechargeable lithium batteries. Angewandte chemie international edition, 47: 2930-2946.
[10]. Ji, LW., Lin, Z., Alcoutlabi, M., et al. (2001) Recent developments in nanostructured anode materials for rechargeable lithium-ion batteries. Energy and environmental science, 4: 2682-2699.
[11]. Collier, C.P., Saykally, R.J., Shiang, J.J., et al. (1997) Reversible tuning of silver quantum dot monolayers through the metal-insulator transition. Science, 277: 1978-1981.









