1. Introduction
With the progress and development of human society, the energy industry has ushered in a new revolution. Human beings are not only considering energy reserves, but also began to pursue mobile, convenient, and green energy equipment. In many energy materials, lithium-ion batteries stand out with their high voltage, high energy density, and low pollution. Under the unremitting efforts of scientific researchers at home and abroad, lithium-ion batteries have begun to change from theory to reality into our lives. Lithium battery has accelerated the rise in the field of new energy vehicles and landscape energy storage, communication energy storage, and home energy storage fields. We ushered in the growth window period. In 2022, the total output value of the lithium-ion battery industry was 45 billion U.S. dollars in the world, an increase of 9% year-on-year. The average annual compound annual growth rate of 2015-2019 reached 19%. It is expected that the global market will be 14.6% of global area coverage report in 2026. The total amount reached a total of 92 billion US dollars, which was doubled in 2022. Therefore, it can be seen that lithium-ion batteries have a pivotal position and increasingly hot development trend in the energy industry.
Several scientists are working on nanomaterials, lithium-sulfur, lithium-air, and nanomaterials in two different types of batteries nowadays. Li et al. reported new separators for the lithium-ion batteries, the lithium-sulfur batteries etc. Whereas Ping et al. studied the classification of nanomaterials. The “shuttle effect” in this type of batteries does exist, though. When used to lithium-sulfur batteries, three-dimensional porous carbon nanocomposites can provide a stable conductive network, which facilitates electron transport and rapid response of lithium. Reviewing lithium-air batteries was Zhang et al. Researchers have also looked into graphene's use in lithium batteries. Yet, there are still certain issues with nanomaterials that we need to address in the future, such as their high cost and irreversible parasitic reactions.
The main purpose of this article is to analyse the application of nanomaterials in lithium-sulfur and lithium-air batteries. The classification, advantages and disadvantages of nanomaterials and their applications are firstly introduced, and then the lithium-sulfur and lithium-air batteries are described in principle, and the roles played by different nanomaterials in these two batteries are listed.
2. Nanomaterials
2.1. Nanomaterials
Nanomaterials is the abbreviation of nanoscale structured materials. After a substance reaches the nanoscale, approximately in the range space of 0.1-100 nm, the properties of the substance will be mutated and special properties will appear [1]. The properties of the nanomaterials have changed considerably because the nanoparticle size has approached the coherence length of the electrons, so the self-organization due to the strong coherence has changed them [2].
2.2. Advantages and disadvantages
Nanomaterials can be divided into different types according to their origin, size, shape, structure, type, composition, and so on [3]. They are mainly classified as nanometers and other sizes. According to the nanoscale, which is 1 to 100 nm, carbon nanomaterials are divided into zero-dimensional, one-dimensional, two-dimensional, and three-dimensional nanostructures. In which zero-dimensional nanomaterials (0D) are usually defined as nanometers in spatial dimensions such as quantum dots, fullerenes, etc.; one-dimensional nanomaterials (1D) have a non-nanometer scale, including carbon nanotubes and nanowires, etc.; two-dimensional materials (2D) have only one dimension of nanometer, represented by graphene; and three-dimensional (3D) diamond. From its source of production, it can be divided into three categories, incidental nanomaterials, engineered nanomaterials, and naturally produced nanomaterials; its materials can also be subdivided into carbon-based nanomaterials, inorganic-based nanomaterials, organic-based nanomaterials, and bionanomaterials [3].
Nanomaterials have many excellent properties, such as their wide surface area, which allows them to have good mechanical performance, thermal conductivity, optical performance, etc. Especially for some 1D and 2D materials, their mechanical properties and electrical conductance allow them to produce portable, flexible electrodes, extend battery life, and improve battery efficiency [4].
However, there are some limitations to the use of nanomaterials, such as the high productivity of some nanomaterials, poor chemical stability due to the high proportion of surface area, and irreversible parasitic reactions occurring in energy storage materials [4]. In addition, nanomaterials are also toxic and are mostly shown to have effects on the health of organisms and the environment, so research into nanomaterial toxicology and recycling needs to be further advanced [3].
2.3. Application in nanomaterials
Nanomaterials have been employed extensively in many different industries and are one of the materials that have developed the most quickly since the turn of the century, as biology, catalysis, environmental preservation, and so on.
2.3.1. Biomaterials. Graphene is a novel substance, and the graphene-new nanomaterials that result from the process offer enormous promise for advancement in the realm of medicine. First, graphene may be utilized as a cell media. Graphene and its related matrices are fantastic. It may be used as a nanoplatform for cell adhesion, proliferation, and differentiation, including embryonic stem cells (ESCS). Simultaneously, various studies have indicated that graphene has the potential to be a good biocompatible scaffold. It has no effect on the proliferation of human mesenchymal stem cells (HMSCs) and hastens their differentiation to osteocytes. Furthermore, the graphene technique can stimulate the adherence of human neural stem cells as well as the development of neurons.
2.3.2. Batteries. The price of electrocatalysts is too expensive, along with anode interference and other issues, in the development of fuel cells. By using careful design, low-cost catalytic materials, such as heteroatom-doped carbon nanomaterials and composites made of stable iron carbide (Fe3C)-based nanoparticles and carbon nanomaterials, can be created that not only increase the surface area of electron-transferred electrocatalysts but also increase their long-term operation stability. It generates fresh concepts for the catalytic use of nanomaterials [5].
2.3.3. Environmental protection. For photocatalytic degradation, photocatalytic nanomaterials are appropriate. The non-toxicity, high stability, and potent oxidation ability of TiO2 are among its photocatalytic degradation's characteristics.
TiO2 is crucial for environmental protection applications as well. Sulfur dioxide, nitrogen oxide, carbon monoxide, and other chemicals in the atmosphere, for instance, will significantly harm human health when it comes to atmospheric treatment. The air purifier's titanium dioxide nano-coating may effectively lower the level of dangerous gases present while also achieving the goal of environmental remediation.
In terms of reducing water pollution, photocatalysis using nanomaterials technology can transform certain contaminants in sewage that are hard to remove or ineffective with current removal techniques into inert substances like water and carbon dioxide. Drinking water may be effectively and safely purified by combining this method with the tap water method.
3. Lithium-sulfur batteries and applications
3.1. Lithium-sulfur batteries
3.1.1. Lithium-sulfur batteries and development. Energy storage systems are being developed with higher energy densities, higher safety standards, and cheaper costs in response to the increasingly stringent demands of the car industry. Theoretically, the sulfur conversion-type reaction provides a battery with four times higher energy density than lithium-ion batteries. Yet, there are still issues with lithium-sulfur batteries, such as their poor safety and limited lifetime in real-world applications. With Danuta's initial proposal for lithium-sulfur batteries in 1960, other research teams investigated various electrolytes, including 1,3-dioxolane (DOL), tetrahydrofuran (THF), and dimethyl sulfoxide (DMSO). It took until 2002 for Linda F. Nazar's team in Canada to advance the process of making lithium-sulfur secondary batteries by adding carbon to sulfur. In order to improve the performance of the batteries and decrease the typical diffusion length of charge carriers. Nazar et al. employed ordered nanostructured mesoporous carbon coated with sulfur nanoparticles. These batteries today have a high specific capacity (1586 mAh/g) and an excellent cycle life thanks in large part to the usage of nanomaterials [6].
3.1.2. Battery principle. Lithium-sulfur batteries mainly use metallic lithium as the negative electrode, sulfur as the positive electrode, and a separator and electrolyte as the main components. This cathodic reaction equation is represented in equation (1). In real life, sulfur reduction at the cathode is a multistep, high-content, soluble polysulfide reaction, as shown in Fig. 1. The S8 open-loop undergoes electrochemical reduction until they all become Li2S and Li2S2 [7].
S8 + 16Li+ 16e- → 8Li2S (1)
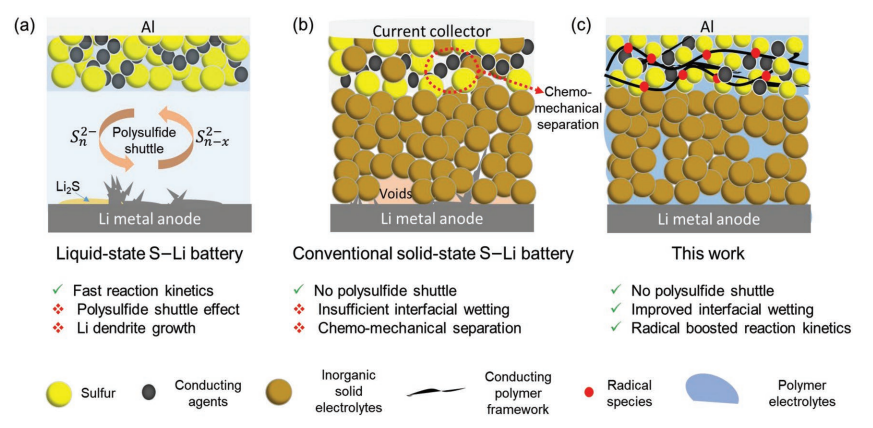
Figure 1. Diagram of the battery operating principle in different phases [7].
Self-discharge refers to a kind of capacitance loss phenomenon that occurs when the battery is at the Open Circuit Voltage (OCV), which will have a serious impact on the storage life of the battery. After the battery is loaded, an SEI film is formed in the electrolyte. However, due to the insufficient separation of the OCV by the SEI film, the electrons continue to pass through the SEI film for a slow reaction, thereby reducing the activity of the electrode. However, it is difficult to form a stable SEI film on the surface of lithium metal electrodes due to the non-planarity. The rough surface can easily lead to the growth of lithium dendrites, resulting in the breakage of some dendrites, which leads to the generation of dead lithium and accelerates the loss of negative electrode materials. Dendrites penetrate the diaphragm, which will cause a short circuit in the battery, release heat, and even cause a fire. The reason for the self-discharge of the cathode is mainly due to the dissolution of transition metal ions and the formation of the SEI film caused by the interaction between the metal and the electrolyte [8]. The dissolution of polysulfides is an important factor leading to their self-discharge in OCV [9].
3.2. Applications of nanomaterials in lithium sulfur batteries
3.2.1. 1D nanomaterials. 1D carbon nanomaterials, due to their low density, excellent electrical conductivity, mechanical performance, ultra-high surface area, and perpendicular ratio, can effectively inhibit the crossing and transfer of polysulfides and reduce the loss of active substances. In order to further improve their positive performance, metal materials can be introduced into carbon nanomaterials. For example, metal electrocatalysts, metal sulfides, metal carbides, etc., can improve the positive sulfide conductance and accelerate the oxidation restoration reaction. And the chemical action of metal oxides and polysulfides can improve battery stability and extend battery life. However, the use of these materials still faces some challenges. For example, the activity point of metal electrocatalysts is insufficient and the load of sulfur is limited; the electrical conductivity of metal oxides is poor; the synthesis process of metal carbides is complex, conditions are harsh, etc. [10].
Furthermore, the combination of MXenes or MOFs with 1D carbon nanomaterials can absorb polysulfides through the Louisiana acid reaction, showing tremendous potential in the preparation and performance improvement of sulfur-positive poles [10].
3.2.2. 2D nanomaterials. 2D carbon nanomaterials not only have high conductivity and a high surface area ratio, but also their unique sheet-like structures make them highly loaded with sulfur and absorb polysulfide, thus inhibiting the shuttle effect of polystyrene. 2D carbon nanomaterials are represented by graphene-based materials. Their high conductivity and chemical stability make them ideal electrode materials. But due to its internal porosity and the potential loss of active substances, layer-interconducting differences, and stacked graphene layer leading to low-load sulfur problems. These factors still hinder its application and commercial development [11].
The limitations of graphene-based materials can be overcome by changing the structure and functionalization of graphene. The graphene structure consists mainly of an in-plane configuration, a sandwich configuration, and a core-shell configuration. In addition, a number of composite structures, such as the porous microsphere graphene construction, graphene/sulfur (G-S) hybrid fiber materials, etc., have been designed to show good electrochemical properties. The functionalization of the graphene is mainly achieved by branching the functional clusters. Usually branching oxygen-containing functional blocks such as aluminum bases, alumina bases, etc. to enhance its polarity, thereby enhancing its bond and strength with the sulfur. Furthermore, single atomic dopants in graphene, such as nitrogen, aluminum, and so on, can enhance their polarity [12].
3.2.3. 3D nanomaterials. Although 1D and 2D nanomaterials have many excellent properties, it is generally not possible to achieve high sulfur loads. 3D carbon nanomaterials are typically combined with 1D and 2D carbon nanomaterials, through the combination of different materials, preparing different porous spacing carbon nanometers with a high load of sulfur, inhibiting polysulfide cross-linking and dissolving purposes [11]. For example, Li Min's team embedded carbon nanotubes/sulfur compounds with graphene-made clamp structures into foam tubes. Tested, this 3D nanomaterial effectively reduced the loss of active substances and achieved higher electron movement speeds and cycle stability [13]. In addition, Li Nuo's group designed and synthesized the 3D conductive carbon nanotubes/MXene framework and used a CMP diaphragm. Because the carbon nanotubes avoided the stacking of MXene, it effectively suppressed the shuttle effect of polysulfide, with high stability and high sulfur utilization, showing excellent electrochemical performance [14].
4. Lithium-air battery
4.1. Lithium-air battery and principle
Lithium-air batteries are batteries that have better energy density than other lithium-ion batteries using oxygen in air as the cathode reactant [15]. Previous the batteries have problems, such as the accumulation of solid reaction products in the positive electrode. According to the electrolyte system, lithium-air batteries are primarily categorized into organic electrolyte bodies (non-aqueous electrolyte system), aqueous electrolyte system, mixed electrolyte system, and all-solid electrolyte system.
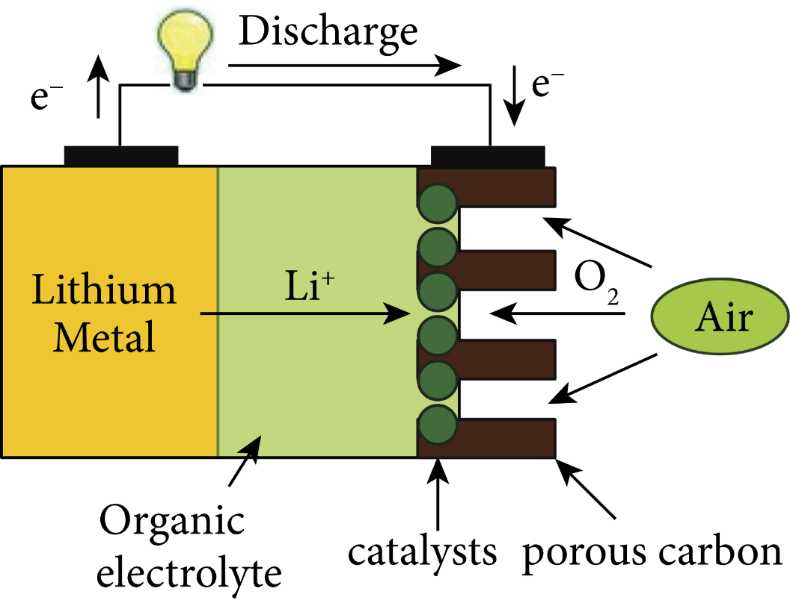
Figure 2. Water-based electrolyte lithium-air batteries [16].
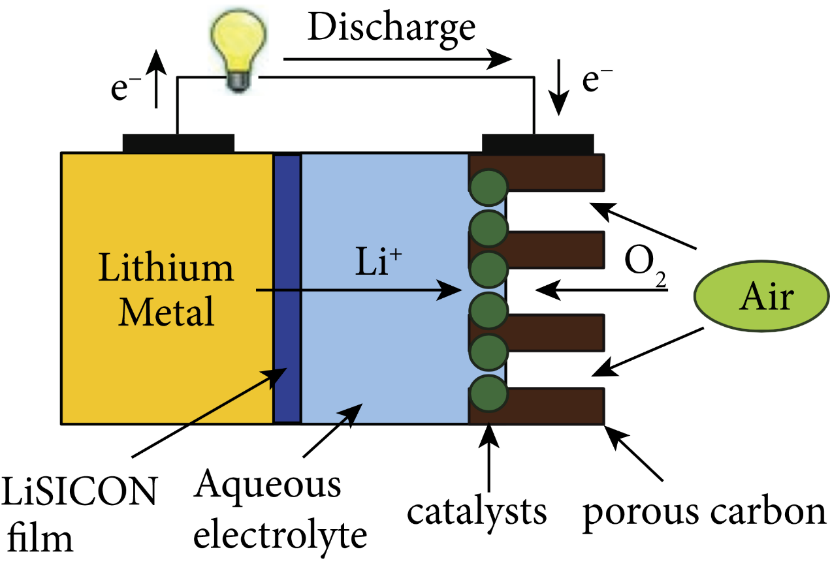
Figure 3. Organic electrolyte lithium air batteries [16].
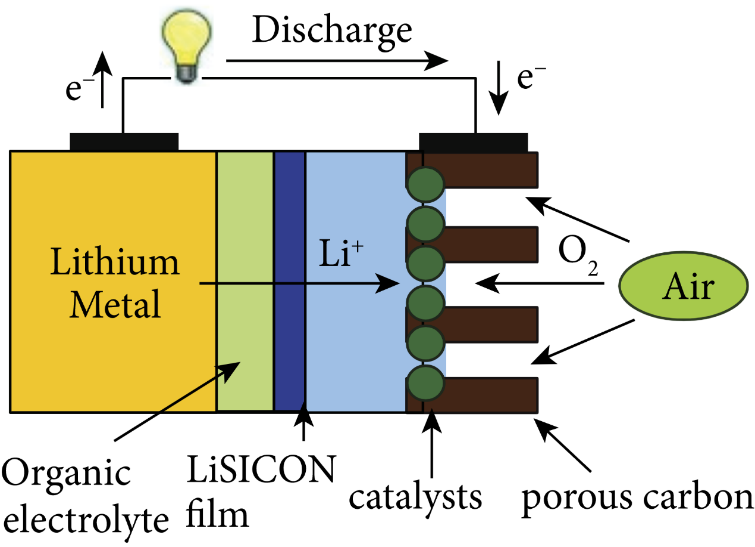
Figure 4. Water-organic dual liquid-based lithium-air batteries [16].
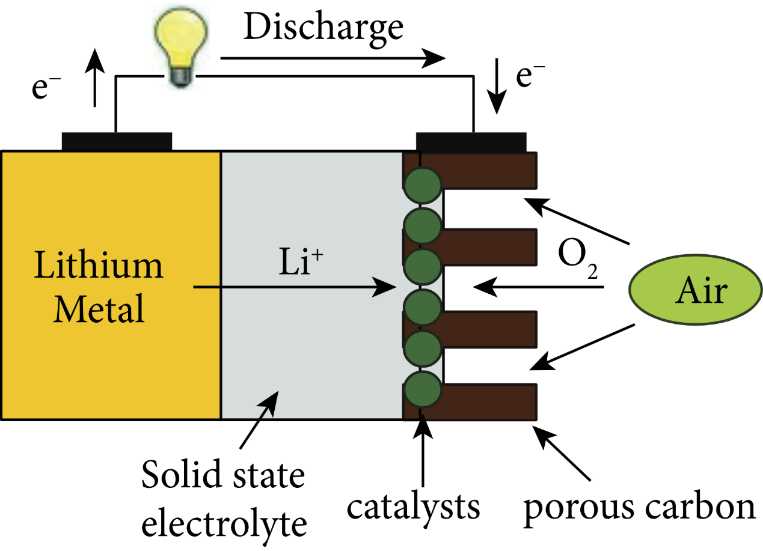
Figure 5. Solid-state lithium-air batteries [16].
The oxygen performs an oxygen reduction reaction at the air electrode to generate hydroxide, as indicated in the Fig. 2, Fig. 3, Fig. 4, and Fig. 5, where the electrolyte is an alkaline aqueous solution. The equation of the discharge reaction is shown in equation (2). During the discharge process, Li, H2O and O2 are consumed and a protective film is generated on the Li surface to prevent the rapid electrochemical reaction. The self-discharge rate of Li is high in the open circuit or low power state and is accompanied by the corrosion reaction of Li (equation 3). In the aqueous electrolyte, the metal Li reacts with water very easily, so there is a high requirement for the water resistance of lithium-ion diaphragm, and there is no commercially available product. Considering the practicality and safety, water-based batteries are not the first choice for final practical applications.
4Li + O2 + 2H2O→ 4LiOH (2)
Li + H2O→ LiOH + 1/2H2 (3)
Lithium-air batteries with non-aqueous electrolyte systems use organic electrolytes containing soluble lithium salts and work based on the generation and decomposition of Li2O2 (equations (2) and (4)). According to the calculation of equation (1)-(3), this battery has a calculated energy density of 5200 Wh/kg, but the actual energy density is 11430 Wh/kg because of the need to exclude oxygen, which comes from the external environment. Although solid-state lithium-air batteries avoid the production of lithium branches and have superior cycle performance, their development is constrained by their low conductivity, capacity, and energy density.
2Li + O2 → Li2O2 (4)
4.2. Application
4.2.1. Application of diamond-like carbon nanomaterials in lithium-air batteries. Thin membrane of this type of batteries have been presented for the first time, and nanostructured diamond-like carbon membranes have been manufactured using RF (radio frequency) sputtering technique and utilized as anode materials for the batteries. The discharge plateau of this nanostructured, diamond-like carbon membrane air electrode is observed at a current density of 220 mA per gram, indicating a reversible capacity of over 2000 mA per gram. SEM, Raman spectroscopy, X-ray diffraction, as well as blue battery tests were applied to analyze the diamond-like carbon membrane's physical and electrochemical characteristics. Based on this, the use of transfer electron and chosen area region-based electron diffraction technology were used to preliminary expose the electrochemical reaction mechanism of the diamond-like carbon membrane played as a positive electrode material for lithium-air batteries. The research results show that the diamond-like carbon film with a high degree of sp3 hybridization as the anode material for the batteries has good cycling performance and a relatively low voltage difference between charge and discharge plateaus.
4.2.2. Application of nanofibers in lithium-air batteries. Cobalt is generated via electrospinning and pyrolysis to create composite materials made of multi-level porous carbon nanofibers in three dimensions. The sample created after adding polymethyl methacrylate (PMMA) was a multi-level porous nano cobalt-modified carbon nanofiber, according to the findings of the microstructure test of the generated nanofibers and the electrochemical test. It was used as a lithium-air battery cathode that could cycle 45 times and had an initial discharge capacity of 4583.9 mAh per gram relating to the overall mass of the cathode. The results showed that the three-dimensional network structure of porous nanofibers has a higher specific surface area, and it is conducive to the mass transfer of the electrode and provides reaction and storage space for reactants and reaction products, increasing the stability of the electrode structure. At the same time, the self-supporting structure avoids the side reactions of binders and other reactants, accelerates the electron transfer rate, improves the durability of the catalyst, achieves high specific capacity and long cycle.
5. Conclusion
Lithium-sulfur and lithium-air batteries have low pollution and superior energy density, making them one of the most promising battery energies; their development has profoundly impacted the development of the entire energy storage field. This article first introduces the definition, classification, and superiority of nanomaterials, then discusses the outline and principles of the two batteries separately, as well as the application and research progress of different nanomaterials in both batteries. Carbon nanomaterial, with its superior mechanical performance and conductivity, can greatly improve the performance of the electrodes of a lithium-air battery or a lithium-sulfur battery. However, the current research on the nanomaterials applied to lithium-ion batteries is still in the development stage. In the future, researchers should focus on developing more economically efficient nanomaterials.
References
[1]. Yong X., et al. Information Integration Technology of Disaster Prevention and Mitigation System Based on Nanometer Material. Ferroelectrics, 2021, 580(1): 55-70.
[2]. Bao F.L. Design of Sports Field Based on Nanometer Materials. Applied Mechanics and Materials, 2013, 2488(340): 366-369.
[3]. Nanotechnology: Trends and Future Applications [M]. Springer Nature, 2021.
[4]. Pomerantseva E., Bonaccorso F., Feng X., et al. Energy storage: The future enabled by nanomaterials. Science, 2019, 366(6468): eaan8285.
[5]. Zhou M., Wang H L, Guo S. Towards high-efficiency nanoelectron catalysts for oxygen reduction through engineering advanced carbon nanomaterials. Chemical Society Reviews, 2016, 45(5): 1273-1307.
[6]. Ji X., Lee K.T., Nazar L.F., A highly ordered nano-structured carbon-sulphur cathode for lithium-sulphur batteries. Nature Materials, 2009, 8(6): 500-506.
[7]. Zheng D., Zhang X., Wang J., et al. Reduction mechanism of sulfur in lithium sulfur battery: from elemental sulfur to polysulfide. Journal of Power Sources, 2016, 301: 312-316.
[8]. Wei Z., Ren Y., Sokolowski J., et al. Mechanistic understanding of the role separators playing in advanced lithium sulfur batteries. InfoMat, 2020, 2(3): 483-508.
[9]. Tian J., Xiong R., Shen W., et al. Electrode ageing estimation and open circuit voltage reconstruction for lithium-ion batteries. Energy Storage Materials, 2021, 37: 283-295.
[10]. Luo J., Guan K., Lei W., et al. One dimensional carbon-based composites as cathodes for lithium-sulfur battery. Journal of Materials Science & Technology, 2022.
[11]. Knoop J.E., Ahn S. Recent advances in nanomaterials for high-performance Li–S batteries. Journal of Energy Chemistry, 2020, 47: 86-106.
[12]. Xu H., Kong Z., Siegenthaler J., et al. Review on recent advances in two‐dimensional nanomaterials‐based cathodes for lithium-sulfur batteries. EcoMat, 2023: e12286.
[13]. Li M., Zhou X., Ma X., et al. Development of sulfonated-carbon nanotubes/graphene three-dimensional conductive spongy framework with ion-selective effect as cathode in high-performance lithium-sulfur batteries. Chemical Engineering Journal, 2021, 409: 128164.
[14]. Li N., Cao W., Liu Y., et al. Impeding polysulfide shuttling with a three-dimensional conductive carbon nanotubes/MXene framework modified separator for highly efficient lithium-sulfur batteries. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2019, 573: 128-136.
[15]. Gao Z.Y., et al. Recent Progress in Developing a LiOH-based Reversible Nonaqueous Lithium-Air Battery. Advanced materials (Deerfield Beach, Fla.), 2022: e2201384-e2201384.
[16]. Suryatna A., et al. A Review of High-Energy Density Lithium-Air Battery Technology: Investigating the Effect of Oxides and Nanocatalysts. Journal of Chemistry, 2022.
Cite this article
Chen,X.;Wang,K.;Yin,J.;Yue,K. (2023). Application of nanomaterials in lithium-sulfur batteries and lithium-air batteries. Applied and Computational Engineering,23,30-38.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2023 International Conference on Functional Materials and Civil Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Yong X., et al. Information Integration Technology of Disaster Prevention and Mitigation System Based on Nanometer Material. Ferroelectrics, 2021, 580(1): 55-70.
[2]. Bao F.L. Design of Sports Field Based on Nanometer Materials. Applied Mechanics and Materials, 2013, 2488(340): 366-369.
[3]. Nanotechnology: Trends and Future Applications [M]. Springer Nature, 2021.
[4]. Pomerantseva E., Bonaccorso F., Feng X., et al. Energy storage: The future enabled by nanomaterials. Science, 2019, 366(6468): eaan8285.
[5]. Zhou M., Wang H L, Guo S. Towards high-efficiency nanoelectron catalysts for oxygen reduction through engineering advanced carbon nanomaterials. Chemical Society Reviews, 2016, 45(5): 1273-1307.
[6]. Ji X., Lee K.T., Nazar L.F., A highly ordered nano-structured carbon-sulphur cathode for lithium-sulphur batteries. Nature Materials, 2009, 8(6): 500-506.
[7]. Zheng D., Zhang X., Wang J., et al. Reduction mechanism of sulfur in lithium sulfur battery: from elemental sulfur to polysulfide. Journal of Power Sources, 2016, 301: 312-316.
[8]. Wei Z., Ren Y., Sokolowski J., et al. Mechanistic understanding of the role separators playing in advanced lithium sulfur batteries. InfoMat, 2020, 2(3): 483-508.
[9]. Tian J., Xiong R., Shen W., et al. Electrode ageing estimation and open circuit voltage reconstruction for lithium-ion batteries. Energy Storage Materials, 2021, 37: 283-295.
[10]. Luo J., Guan K., Lei W., et al. One dimensional carbon-based composites as cathodes for lithium-sulfur battery. Journal of Materials Science & Technology, 2022.
[11]. Knoop J.E., Ahn S. Recent advances in nanomaterials for high-performance Li–S batteries. Journal of Energy Chemistry, 2020, 47: 86-106.
[12]. Xu H., Kong Z., Siegenthaler J., et al. Review on recent advances in two‐dimensional nanomaterials‐based cathodes for lithium-sulfur batteries. EcoMat, 2023: e12286.
[13]. Li M., Zhou X., Ma X., et al. Development of sulfonated-carbon nanotubes/graphene three-dimensional conductive spongy framework with ion-selective effect as cathode in high-performance lithium-sulfur batteries. Chemical Engineering Journal, 2021, 409: 128164.
[14]. Li N., Cao W., Liu Y., et al. Impeding polysulfide shuttling with a three-dimensional conductive carbon nanotubes/MXene framework modified separator for highly efficient lithium-sulfur batteries. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2019, 573: 128-136.
[15]. Gao Z.Y., et al. Recent Progress in Developing a LiOH-based Reversible Nonaqueous Lithium-Air Battery. Advanced materials (Deerfield Beach, Fla.), 2022: e2201384-e2201384.
[16]. Suryatna A., et al. A Review of High-Energy Density Lithium-Air Battery Technology: Investigating the Effect of Oxides and Nanocatalysts. Journal of Chemistry, 2022.









