1. Introduction
Heavy metals have been used for centuries in all fields, including industry and technology, and spread widely in the environment, becoming a ubiquitous contaminant that raises concerns for human health and the environment [1]. Although the term “heavy metals” can be confusing, they are proposed as naturally occurring metals that have an atomic number (Z) greater than 20 and an elemental density greater than 5 g/cm3. [2]. Heavy metals are classified into two categories based on their roles in living organisms: essential heavy metals and non-essential heavy metals. [3] Cadmium (Cd), along with lead (Pb), arsenic (As), mercury (Hg), argentum (Ag), and antimony(Sb), are categorized as non-essential heavy metals that have no benefit to living organisms [3] but are highly toxic to the soil environment even at low concentrations [4] by damaging soil organic matter, pH, clay content [5, 6], microbes [7, 8], and agricultural crops [9].
Despite the soil contamination of Cd, microplastic - including polyethylene (PE), polyvinyl chloride (PVC), and polypropylene (PP), which are among the most common types of microplastics [10] – also pose a serious threat toward terrestrial ecosystems. Plastics-Europe [11] estimates that 367 Mt of plastics were produced worldwide in 2020. Nevertheless, according to Dahlbo, Poliakova [12], plastic waste accumulation is trending upward. As calculated in the previous study [13], household activities are the main source of plastics contamination, accounting for 77%, while 23% of plastics contamination comes from industries. In the same study [13], Julien Boucher and Damien Friot found that plastics undergo degradation cycles for more than a hundred years after entering the environment. As part of this process, plastic particles are micro-degraded by microbial mediated or weathering mechanisms such as hydrolysis and UV radiation, and subsequently form into microparticles known as microplastics, which are capable of contaminating both marine and soil environments [14] through four pathways: ocean pathway, wastewater pathway [15, 16], wind pathway [17], and road runoff pathway [15, 18]. Ultimately, 52% of microplastics ended up in soil ecosystems, a higher ratio compared to 48% of microplastics that went to aquatic ecosystems [13]. Due to the features of microplastics such as the large surface area and small size, they are able to absorb other pollutants such as heavy metals and organic pollutants, facilitating further negative impacts on the soil environment. These include changing soil properties and functions, threatening biodiversity in soil ecosystems [10], affecting the life cycle of soil microbes [19-21], and negatively impacting organisms that live in soil ecosystems [22, 23].
To date, the main strategies to control Cd contamination in soil environments are physical strategy, chemical strategy, and phytoremediation. Physical strategies mainly include electrokinetic technology [24], in which a groove for an electrical solution is inserted between the soil and an electrode, and the heavy metal ions are able to transfer and settle in the solution. However, this technique has the disadvantage of triggering the turbulence of the electrical potential at the soil surface. According to Gao, Peng [25] and Ji and Guo [26], chemical strategies for Cd contamination are soil washing [26] and passivation repair technology [25]. Though they are able to reduce the Cd concentration, they also lead to changes in soil pH. When it comes to phytoremediation, Yan, Wang [27] demonstrated that besides phytostabilization and phytovolatilization, phytoextraction is proven to be the most important technique for remediating heavy metal contamination in soil environments [28, 29] which relies on plants to absorb ions of heavy metal contaminants, then trans-locate and accumulate these contaminants in their above-ground biomass [30, 31].
According to Suman, Uhlik [32], the phytoextraction strategy in remediating heavy metal contamination provides significant advantages over techniques such as physical and chemical strategies. First, phytoextraction is cost-effective, since there is no need to purchase chemical solutions or equipment as is necessary when it comes to traditional methods. Second, phytoextraction is not limited to specific heavy metals, but can pick up multiple heavy metals at once. Moreover, phytoextraction does not require excavation of contaminated soil, which leads to its simple procedure and a minimum of potentially negative impact on the soil ecosystem. Under these circumstances, the public is more prone to accept the strategy of phytoextraction, which also serves as an advantage of phytoextraction.
On the other hand, according to Guo, Huang [33], although the contamination of soil with microplastics is increasingly serious, the study of its effects is still limited due to the complicated structure of soil. Since there are various organic matters and aggregates in the soil environment, which greatly affect the separation of microplastics from soil [34], pretreatments are often carried out to reduce the interference of organic matters. Pretreatments mainly include dissolution with acids, alkalis, enzymes, and oxidation. Although researchers have proposed effective strategies for pretreatments [34-39], these strategies still have potential effects of degrading organic matters, and some are not applicable to temperature sensitive aggregates [40]. In addition, researchers suggest using organisms such as animals and microbes to degrade microplastics in soil [41]. First, insects are proven to be able to chew beeswax and plastic particles [42], including Tenebrio molitor, Zophobas atratus, and the larvae of Galleria mellonella [43]. Second, as for microbe degradation, fungi and bacteria are able to take part in the degradation of microplastics when they form into compounds consisting of various flora [44-46]. In addition, studies [47, 48] demonstrated that fungi are superior to bacteria, due to their hyphae, which attach more tightly to the surface of microplastics and can even make their way to the interior of microplastics.
Based on research, there are studies that proved the interactions between microplastics and heavy metals, also known as “carrier effect” [49]. According to Liu, Dave [50], physical, chemical, and environmental factors such as absorption, speciation, and precipitation, are likely to impact carriage of heavy metals through microplastic particles in soil ecosystems [51]. It is well proven that microplastics (instantly PE) can have carrier effect with heavy metals on the surface of microplastics, including Cd. The results show that in the natural environment, the increase of oxygen-containing functional groups in PE MPs can give PE MPs more electronegativity, so as to enhance its adsorption of ions such as Cu2+ [10].
In addition, previous studies on the impact of the existence of microplastics on the phytoextraction of Cd [49, 52] came to controversial conclusions. According to Manjate, Ramos [52], the presence of PE in diameter between 850 and 1000 µm did not affect the phytoextraction of copper (Cu) and Cd by Phragmites australis. On the other hand, Jia, Wu [49] concluded that the accumulation of Cu and lead (Pb) increased with the presence of microplastics with an average size of 293 µm. Nevertheless, previous studies [53] demonstrated that most microplastic particles ingested by wheat were between 0.2 and 2 µm in size, and there were a minimum number of microplastic particles with a diameter of 5 µm and almost none with 7 µm or larger. Under these circumstances, the microplastic particles used by Jia, Wu [49] and Manjate, Ramos [52] were far above the size that the plants can absorb, which makes us doubt the accuracy of their conclusions, since the microplastics could not enter the plants.
According to CHEN Di, LI Boqun [54], Galinsoga quadriradiata (G. quadriradiata) revealed high sustainability toward Cd and had the greatest effectiveness of transferring Cd from roots to organs above ground comparing with Panicum dichotomiflorum, Setaria geniculate, and Lolium persicum, owing to its traits of fast-growing pace [55] and sustainability toward the toxicity of Cd [56]. Thus, G. quadriradiata is suggested as an effective hyper-accumulator in [54]. However, G. quadriradiata is not proven to be effective in the phytoextraction of PVC or PE, which are both ubiquitous contaminants illustrated above.
In this study, we focus on testing the possibility of phytoextraction of PVC and PE in addition to Cd by G. quadriradiata and finding out the size range of microplastics that can be absorbed by G. quadriradiata. Besides, based on the conclusion in [53], we aimed to test whether the presence of PVC and PE, particles with sizes between 0.3 and 20 µm positively affects Cd absorption by performing carrier effect, and thus increasing the efficiency of phytoextraction by G. quadriradiat. Therefore, we divided G. quadriradiata into nine groups that received nine kinds of different treatments with different concentrations of Cd, PVC, and PE. In the experimental design phase, we proposed several hypotheses:
(1) G. quadriradiata can effectively apply phytoextraction to PVC and PE in addition to Cd.
(2) Microplastics enter the plant through the cracks of new born lateral roots.
(3) The size of microplastics absorbed by G. quadriradiata is between 0.2-2 µm.
(4) With the existence of PVC or PE, the amount of Cd that is absorbed by G. quadriradiata would be increased.
2. Materials and methods
2.1. Materials
PE particles (average size: 2 μm) and PVC particles (average size: 0.8 μm) were purchased from Zhangmutou Huachuang plastic raw material firm (Dongguan, China). CdCl2·2.5H2O was purchased from Shanghai Macklin Biochemical Co., Ltd. G. quadriradiata seeds were purchased from the Seed Bank of Kunming Institute of Botany, Chinese Academy of Sciences.
The soil in this experiment was purchased from Chenghai Perlite Business Department (Kunming, China). The physicochemical properties of the soil were as follows: pH=7.33, organic matter content 279.79 g/kg, total nitrogen (N) content 7.08 g/kg, total phosphorus (P) content 0.7 g/kg, total potassium (K) content 26.7 g/kg, Cd concentration 0.34 mg/kg, cation exchange capacity 46.84 [cmol(+)/kg]. The mechanical composition of the soil was: sand (2-0.05 mm): powder (0.05-0.002 mm): clay (< 0.002 mm) = 52.26 : 41.65 : 6.09.
2.2. Treatments and cultivation
The experimental plants were cultivated in the lab of Kunming Institute of Botany, Chinese Academy of Sciences (Kunming, China). The temperature ranged from 20 ℃ to 25 ℃, and the air humidity ranged from 50% and 65%. We set 9 soil treatments in the experiment: Control, PE, PVC, 20 mg Cd kg-1, 50 mg Cd kg-1, PE + 25 mg Cd kg-1, PE + 50 mg Cd kg-1, PVC + 25 mg Cd kg-1, PVC + 50 mg Cd kg-1. Microplastics were applied at 2% in each treatment. Each treatment was performed in triplicate, thus there were 27 pots in total. They were prepared by mixing 500 g soil with a corresponding amount of CdCl2·2.5H2O, PE powder, or PVC powder evenly and rested for 19 days.
Seeds of G. quadriradiata were planted in petri dishes and buried in agar for germination, and were transplanted into untreated soil for another growth period, 37 days in total. Then the healthy seedlings were planted in the treated soil, one pot each. 14 ml of water was sprayed on each pot twice a day, and an additional light source was placed to promote growth, illuminating from 6 a.m. to 9 p.m. No fertilizer was added to the soil. But in order to avoid pest attack, a minute amount of pesticide (Cypermethrin, Syngenta AG) was used in every pot.
2.3. Sampling
Plant growth indexes including leaf length, leaf width, stem thickness, and plant height were measured and chlorophyll fluorescence imaging was done before the plants were uprooted. Some of the samples present significant difference in plant growth, as is shown in Figure 1. Plant weight (fresh and dry weight, by electronic scale, Ningbo Jiming weighing equipment Co., Ltd), N content, P content, K content, Cd content, and microplastics uptake were determined after uprooting. After 35 days of the soil treatment, G. quadriradiata were uprooted carefully, ensuring to preserve the completeness of their root systems, in order to provide accurate fresh/dry weight data. They were then thoroughly washed in tap water to remove any dust, followed by immediate cutting and weighing. The remaining plants were stored in a refrigerator (4 ℃) awaiting analysis of N, P, K, Cd contents.
|
|
|
Figure 1. Difference in sizes of G. quadriradiata. | ||
2.4. Determination of photosynthesis rate and stomatal conductance
Photosynthesis rate and stomatal conductance were determined by Portable Photosynthesis System GFS-3000 (Heinz Walz GmbH, Germany)(Figure 2). The tests were conducted during day time from 8 a.m. to 7 p.m when plant photosynthesis is active. After pre-heating of 30 min, initial conditions were set to leaf cuvette as follows:
(1) Flow (gas flow through the cuvette): 600 µmol/s;
(2) Imp (impeller): 7 steps;
(3) CO2 abs (CO2 mole fraction in reference cell of analyzer, equal to CO2 concentration at the inlet of cuvette): 500 ppm;
(4) PARtop (photosynthetic active radiation measured with the sensor in upper cuvette half): 1000 µmol/(m2*s);
(5) GH2O (water vapor conductance): 40mmol/(m2*s);
(6) Tcuv (cuvette temperature measured in lower air exit): 23°C.
Without removal from the plant, one leaf of each of the 27 pots of G. quadriradiata was used for testing, with its superior area covering the whole surface of the cuvette. Photosynthesis rate and stomatal conductance measurements were taken every 15 min, 7 times including the initial value, which means 90 min measurement duration for each plant.
|
|
Figure 2. GFS-3000 (Heinz Walz GmbH, Germany). | |
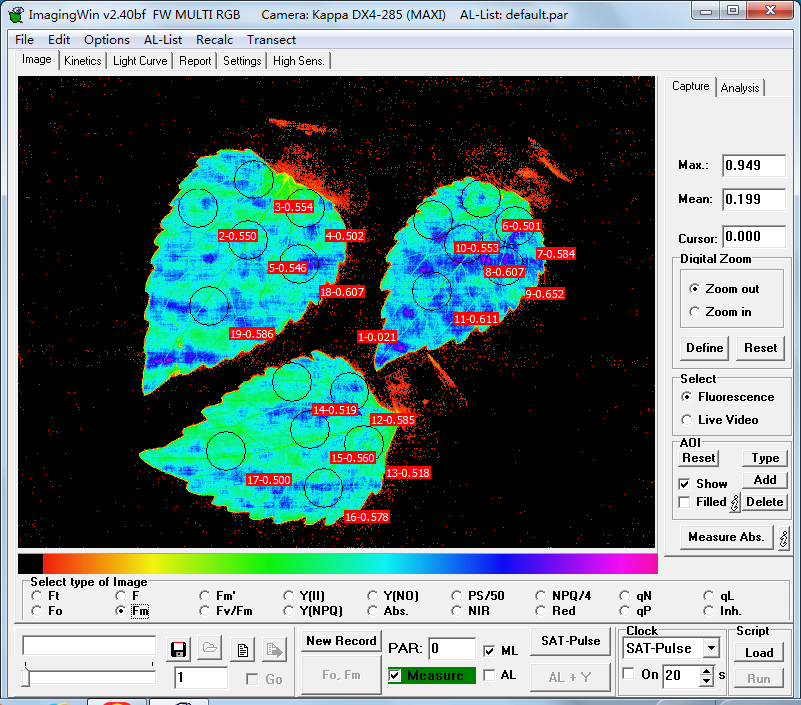
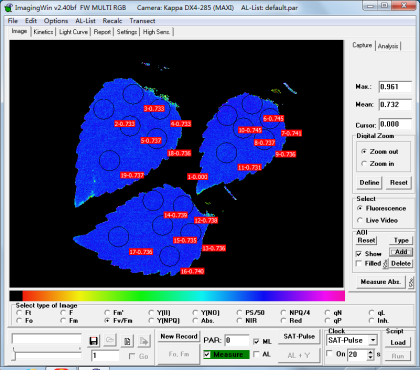
2.5. Chlorophyll fluorescence imaging
After being placed in darkness for 30 min, one leaf at the 2nd node of each pot of G. quadriradiata was amputated and placed inside the observation chamber of IMAGING-PAM (Heinz Walz GmbH, Germany)(Figure 3), and the triplicates of each treatment group were placed together. Six scattered measurement points were selected on each leaf, in similar patterns across all 27 leaves. Maximum fluorescence yield after dark adaptation (Fm) and PSII maximum photosynthetic efficiency (Fv/Fm) were measured and recorded at each selected point on the leaf. After the measurements, all leaves were stored in a freezer at -80 ℃ for later measurements including total weights and contents of N, P, K, and Cd.
|
|
|
Figure 3. IMAGING-PAM (Heinz Walz GmbH, Germany), and the chlorophyll fluorescence images captured by its operating software ImagingWinGigE, including Fm (middle) and Fv/Fm (right). | ||
2.6. Scanning electron microscope (SEM)
G. quadriradiata after uprooting were immediately prepared for SEM observation.3 leaf samples and 3 stem samples were sliced off from each of the top, middle and bottom parts of the plant’s above-ground section. 3 main root samples and 3 lateral root samples were also taken, generating 24 samples in total for each plant. The samples were then freeze-dried (BenchTop Pro with OmnitronicsIM, US)(Figure 4a) and coated with Pt at 20 mA for 60 s, by a sputter coater (Cressington 108 Auto, UK)(Figure 4b), followed by a second round of Pt coating under the same condition. The sample slices were observed under SEM (ZEISS SIGMA 300, Germany)(Figure 4c), and images of microplastic particles found on the surface and inside plant tissues were clearly captured and saved.
(a) |
(b) |
(c) |
Figure 4. (a) Freeze-dryer (BenchTop Pro with OmnitronicsIM, US); (b) sputter coater (Cressington 108 Auto, UK); (c) SEM (ZEISS SIGMA 300, Germany). | ||
2.7. Determination of N, P, K, Cd contents
After G. quadriradiata were weighed and sliced for SEM observations, the remaining were used for assays. Plant nitrogen (N) content was tested according to the procedure of Kjeldahl method (Kjeldahl Nitrogen Determinator-K9840, Hanon Group, Chengdu China); phosphorus (P) content was determined by (Ultraviolet-visible spectrophotometer-UV-1601, Shimadzu Corporation, Japan); potassium (K) content and total cadmium (Cd) was determined by Graphite Furnace Atomic Absorption Spectrometer (ZEEnit700P, Analytik Jena AG, Germany).
2.8. Statistical analyses
Data were statistically analyzed using two-way ANOVA (R version 4.2.1) with an associated Tukey HSD test. The differences among treatments' means were compared using the least significant difference (LSD) at a 5% probability level. Figures (Figure 3) were generated by GraphPad Prism 8.0.2.
3. Results
Table 1. Effect of Cd, PVC, and PE treatments on leaf length, leaf width, stem diameter, height, fresh weight, dry weight, fluorescence chlorophyll (Fv/Fm), fluorescence chlorophyll (Fm), total contents of Nitrogen (N), Potassium (K), Phosphorus (P), and Cd in G. quadriradiata.
Cd25 | Cd50 | No Cd | LSD | PE | PVC | No Plastic | LSD | Cd x Plastic | |
Leaf length (cm) | 6.52 C | 8.32b | 9.50 a | 0.98 | 8.20 a | 8.28 a | 7.86 a | 1.58 | NS |
Leaf width (cm) | 4.77 b | 5.29 b | 6.52 a | 0.86 | 5.69 a | 5.64 a | 5.25 a | 1.13 | NS |
Sten diameter (mm) | 2.95 b | 3.70 a | 3.94 a | 0.49 | 3.55 a | 3.60 a | 3.44 a | 0.65 | NS |
Height (cm) | 36.19 a | 41.84 a | 45.61 a | 10.35 | 43.51 a | 43.38 a | 36.76 a | 10.61 | NS |
Fresh weight (g) | 4.59 C | 7.37 b | 9.74 a | 1.85 | 7.04 a | 7.90 a | 6.77 a | 2.81 | NS |
Dry Weight (g) | 0.32 c | 0.62 b | 0.94 a | 0.19 | 0.62 a | 0.66 a | 0.59 a | 0.32 | NS |
Maximum fluorescence yield (Fm) | 0.60b | 0.61 b | 0.65 a | 0.04 | 0.61 a | 0.65 a | 0.60 a | 0.05 | NS |
PSII maximum photo synthetic efficiency(Fv/FW) | 0.72 6 | 0.71 b | 0.74 a | 0.01 | 0.72 a | 0.73 a | 0.73 a | 0.02 | ** |
Total N (N,%) | 1.83 6 | 2.79 a | 1.61 b | 0.28 | 2.03 a | 2.07 a | 2.13 a | 0.59 | NS |
Total P (P,%) | 0.52 a | 0.17 c | 0.23 b | 0.04 | 0.29 a | 0.31 a | 0.31 a | 0.16 | NS |
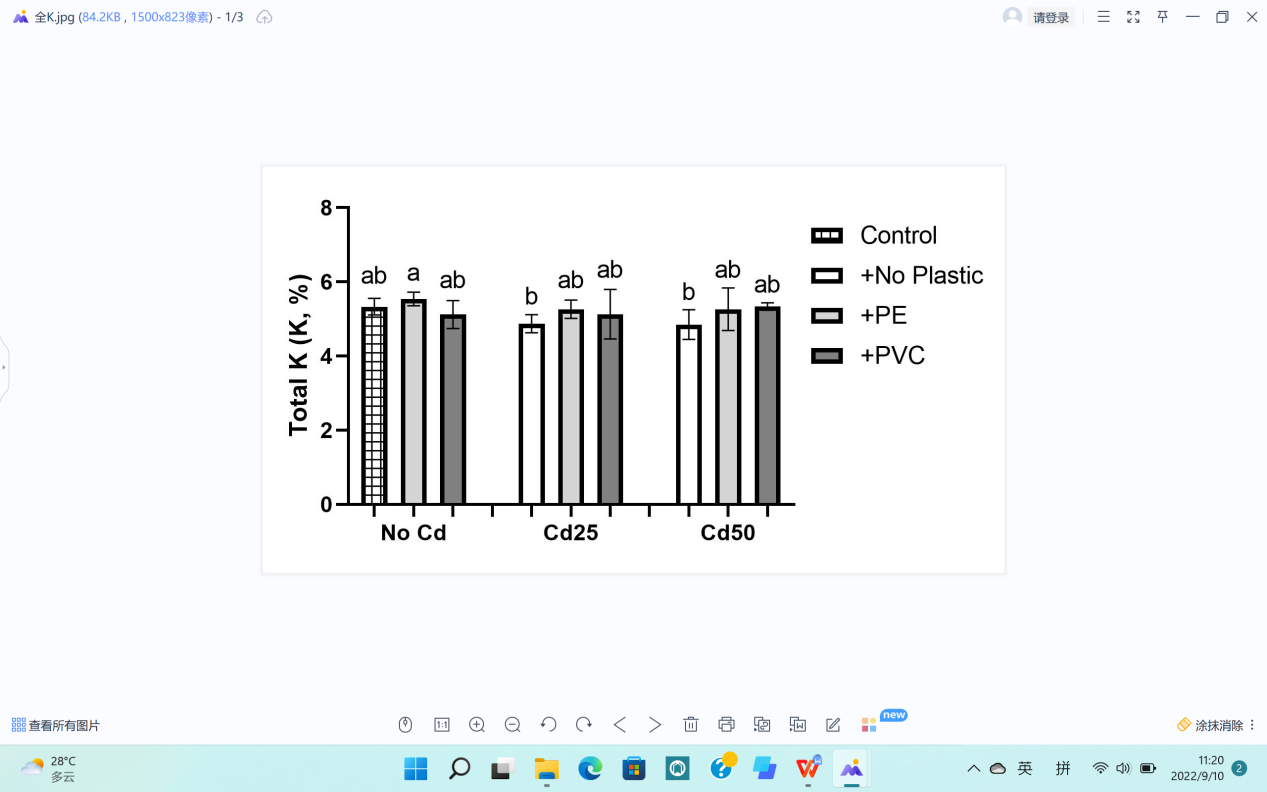
Total K (K,%) | 5.09 a | 5.15 a | 5.33 a | 0.37 | 5.36 a | 5.20 a | 5.02 a | 0.36 | NS |
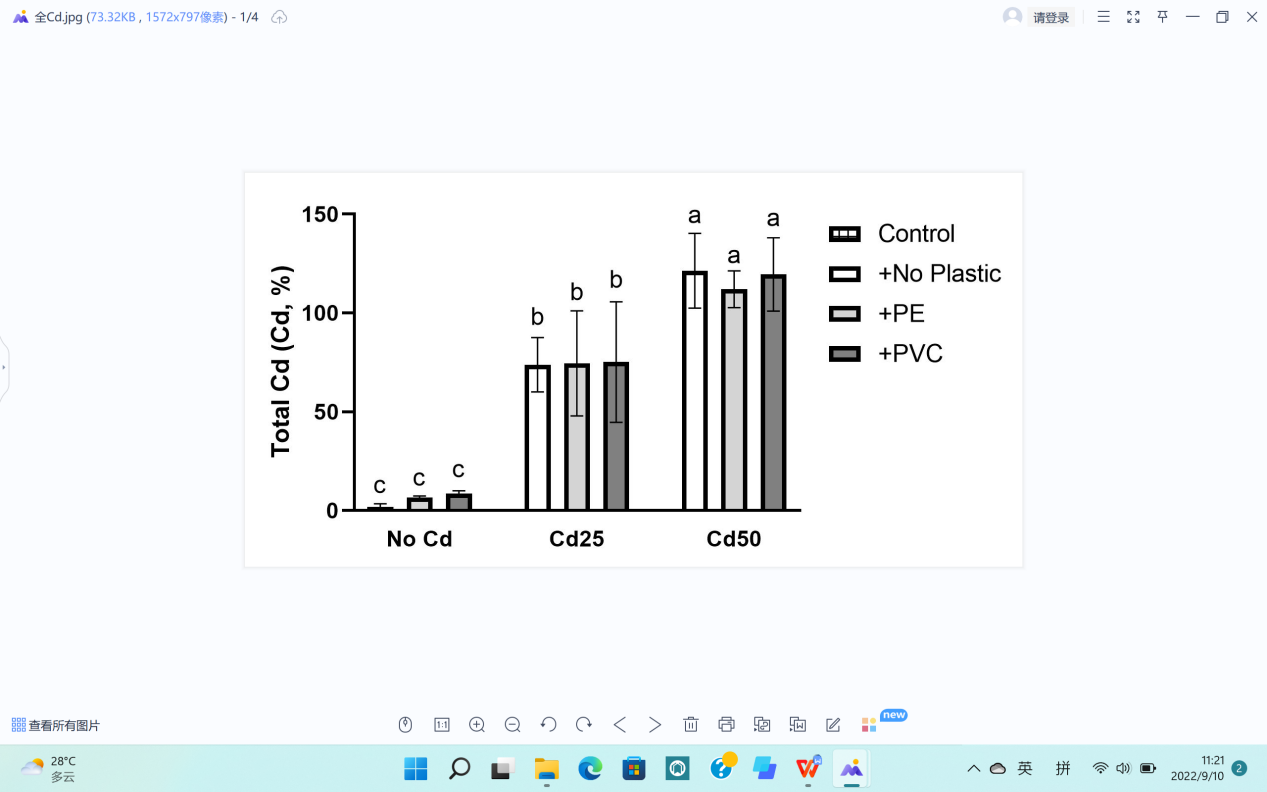
Total Cd (Cd,%) | 74.50 b | 117.66 a | 5.71 c | 14.67 | 64.39 a | 67.76 a | 65.72 a | 49.76 | NS |
*Notes: NS--no significance; **--significance; LSD--least standard difference.
In the six headers in Table 1, each of them includes three treatments, regarding:
• Cd 25: 25 mg of Cd, 25 mg of Cd with 2 % of PVC, and 25 mg of Cd with 2 % of PE.
• Cd50: 50 mg of Cd, 50 mg of Cd with 2 % of PVC, and 50 mg of Cd with 2 % of PE.
• No Cd: control, 2% of PVC, and 2% of PE.
• PE: 2% of PE, 2 % of PE with 25 mg of Cd, and 2% of PE with 50 mg of Cd.
• PVC: 2 % of PVC, 2 % of PVC with 25 mg of Cd, and 2 % of PVC with 50 mg of Cd.
• No plastic: control, 25 mg of Cd, and 50 mg of Cd.
3.1. Plant growth
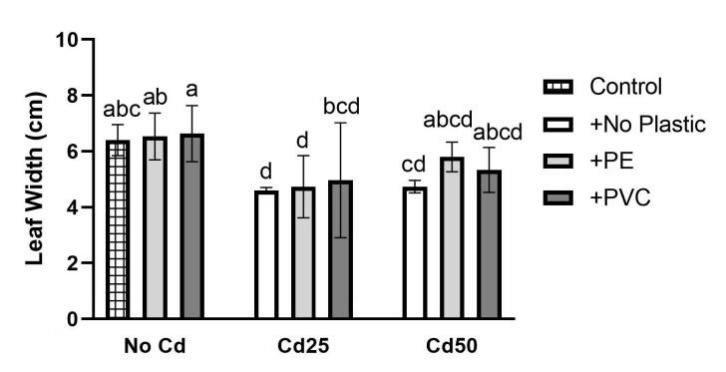
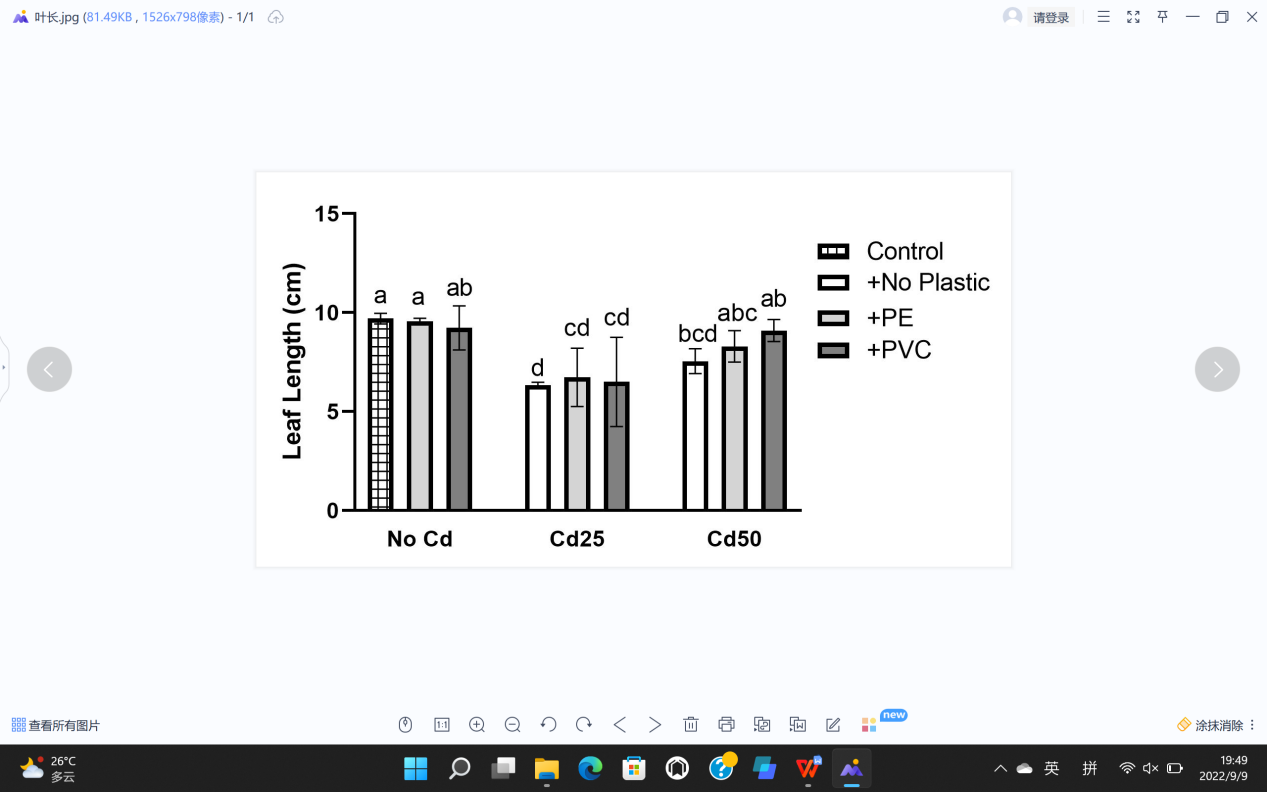
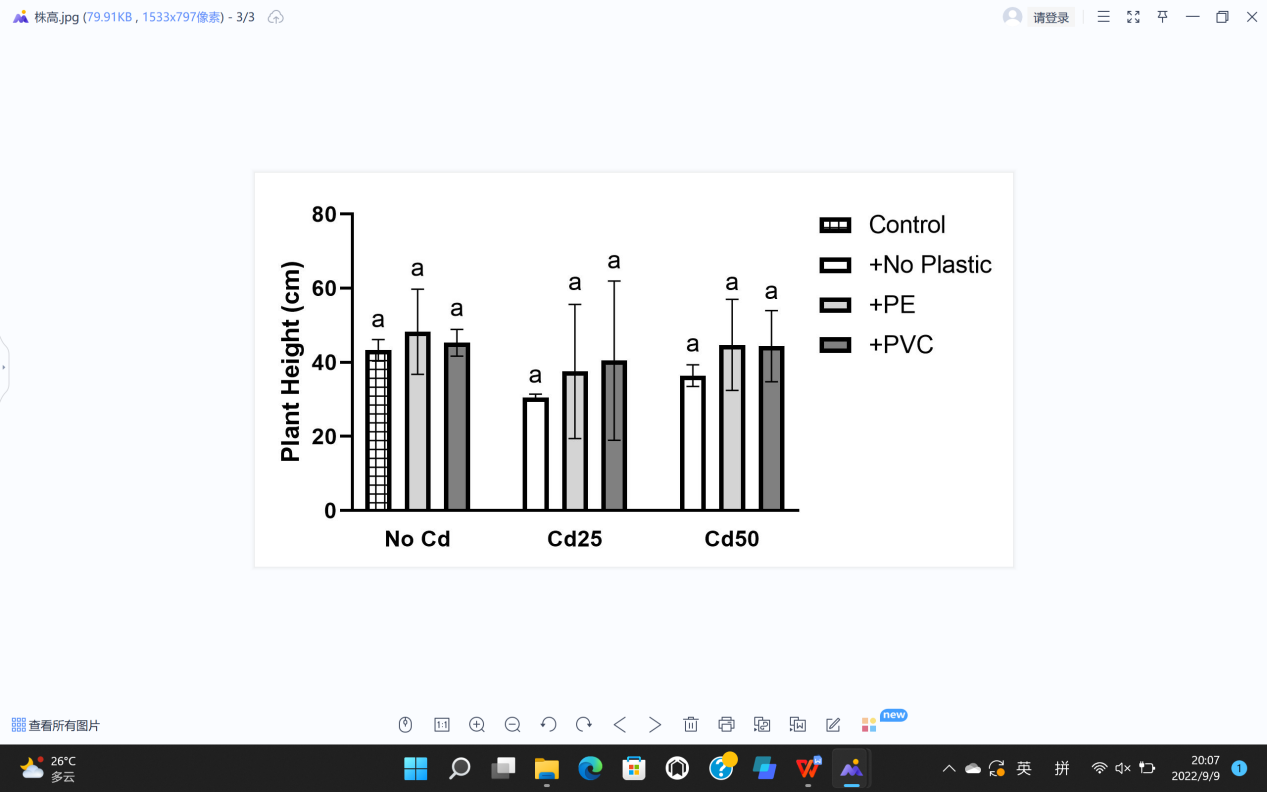
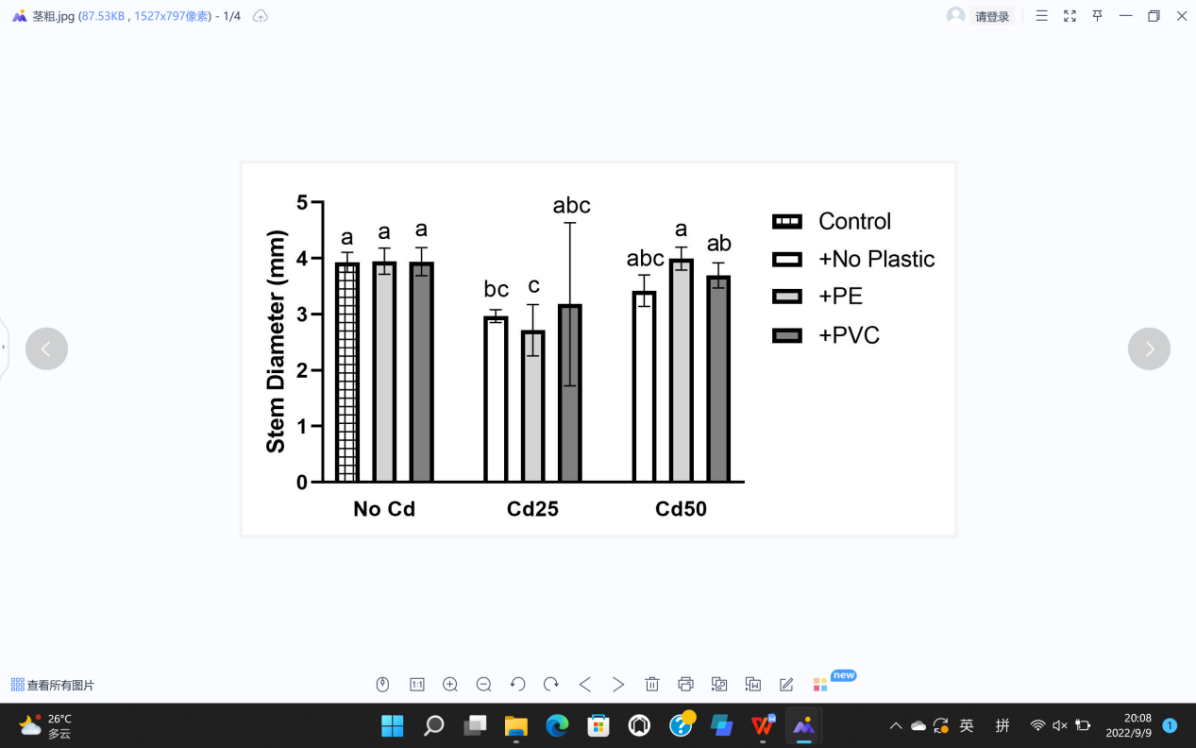
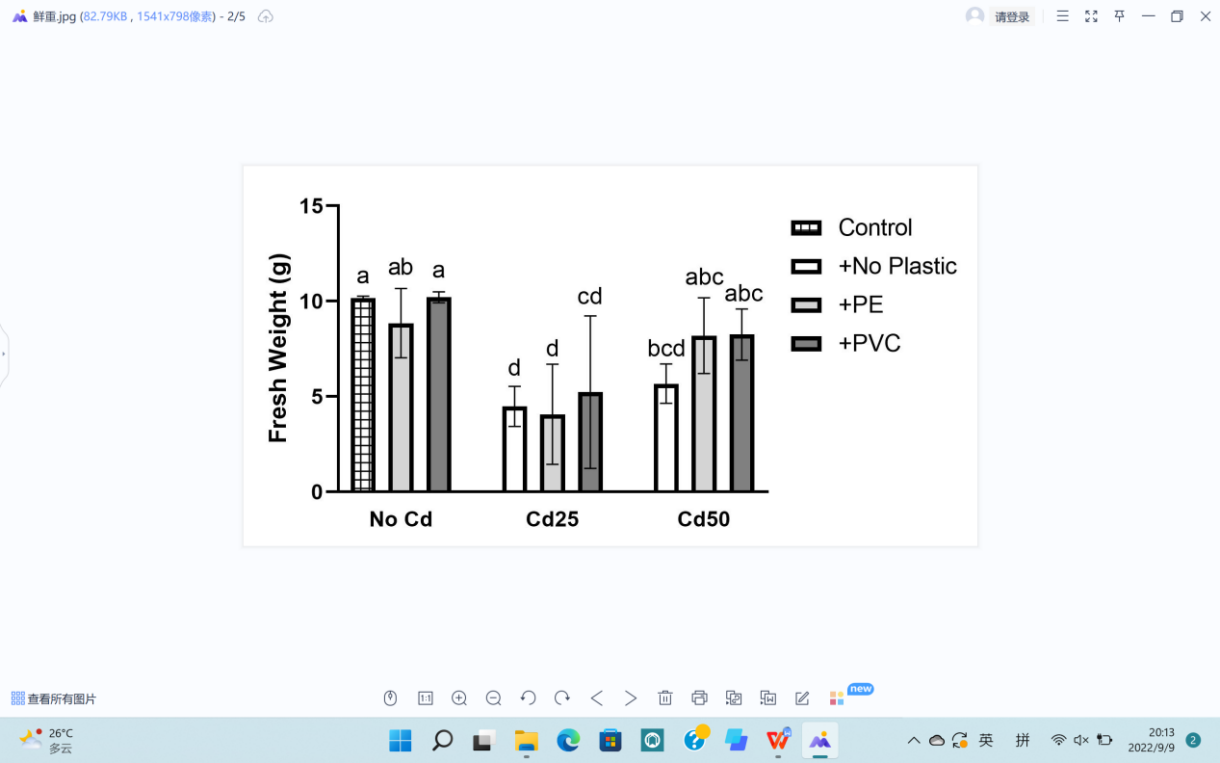
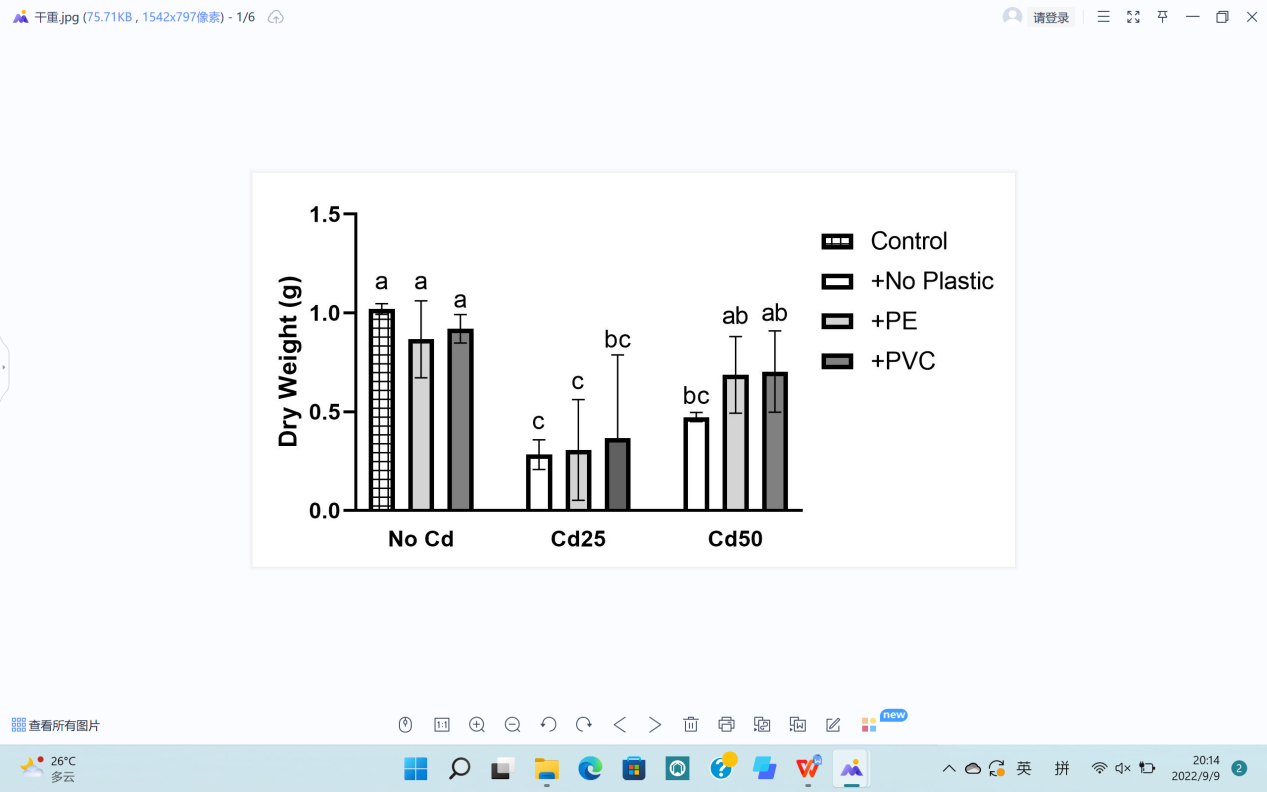
For plant growth, leaf length (L), leaf width (W), stem diameter (S), plant height (H), fresh weight (F), and dry weight (D) were recorded (Figure 5). The soil Cd contamination demonstrated limitation toward the growth of G. quadriradiata in leaf length, leaf width, stem diameter, fresh weight, and dry weight (PL=1.52×10-5; PW=1.39×10-3; PS=1.52×10-3; PF=4.7×10-5; PD=2.18×10-5), but microplastics, including PVC and PE, had no significant effects on G. quadriradiata or carrier effect with Cd (Table 1).
From Table 1, we can see the plants in pots containing 25 mg Cd (with or without PE) have the minimum leaf width, leaf length, stem diameter, dry weight, or fresh weight.; also, the differences between treatments with 2% of PE or PVC and the group with no microplastics of all data are less than LSD, so microplastics contents did not show significant negative effects in plant growth.
As is shown in Table 1 and calculations from ANOVA, the P values of plant height with Cd and PVC/PE are 0.186 and 0.318 which means neither Cd nor microplastics have any effect on the height of G. quadriradiata. In addition, there was no significance of carrier effect between Cd and microplastics in all parameters of plant growth.
(a) Leaf width. |
(b) Leaf length. |
(c) Plant height. |
(d) Stem diameter. |
(e) Fresh weight. |
(f) Dry weight. |
Figure 5. Effects of Cd, PVC, and PE treatments on plant growth. | |
3.2. Plant physiology
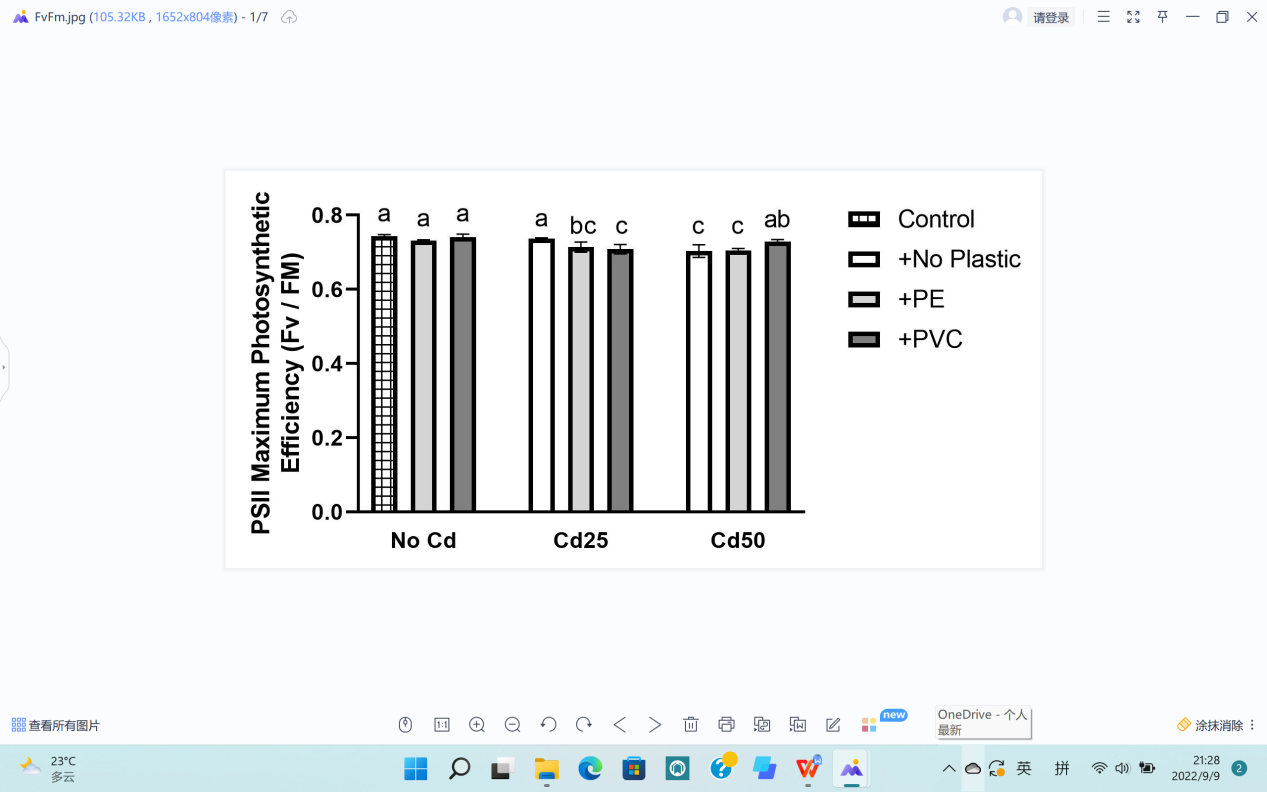
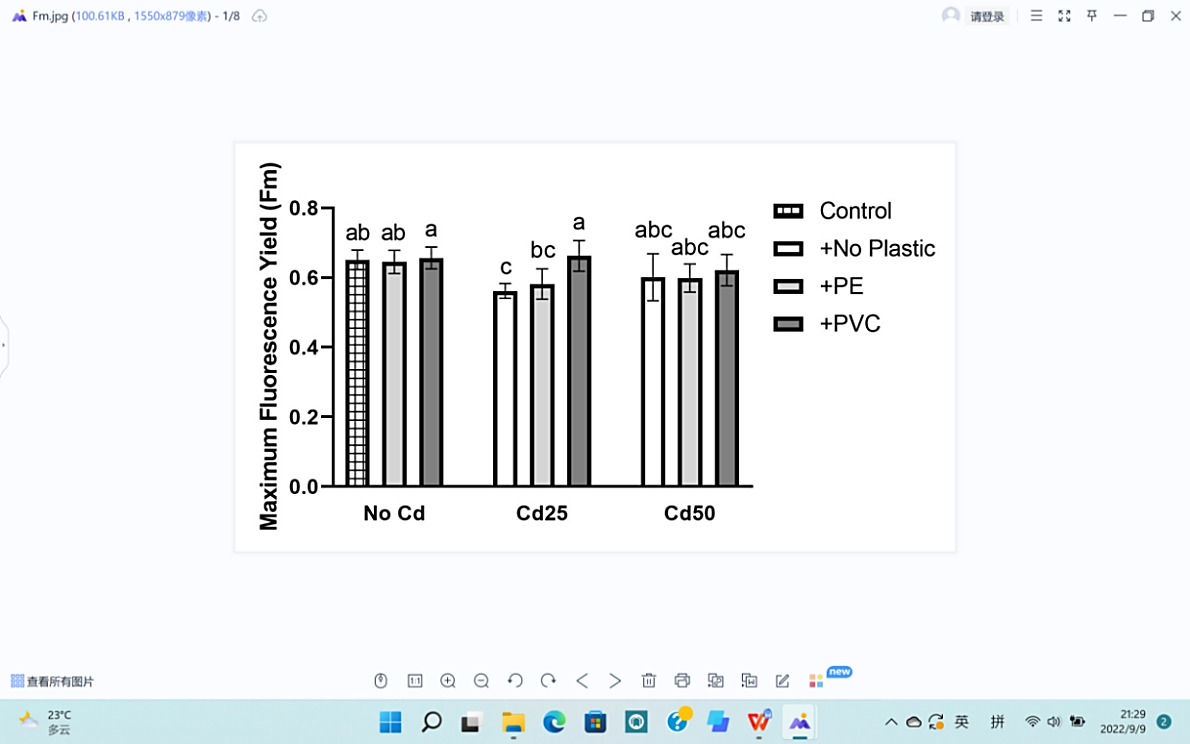
Chlorophyll fluorescence (Fm) and (Fv/Fm) were both tested. The minimum values of the two data appeared in response to 50 mg Cd and 25 mg of Cd, emphasizing the negative effects of Cd in plant physiology (Figure 6).
Soil Cd contents negatively affected both groups of data by showing differences slightly more than LSD comparing to the control group with P values of 0.0421 and 1.01×10-3 respectively. The existence of Cd also affects the color of the leaves, offering obvious differences among different treatments, as shown in Figure 6 (C, D, E). On the other hand, the differences between PE or PVC and the control were below LSD, indicating non-significant effects of microplastics on chlorophyll (Table 1).
In addition, the interaction between Cd and microplastics had a significant effect on PSII maximum photosynthetic efficiency (Fv/Fm), but maximum fluorescence yield (Fm) still showed no significant difference (Table 1).
(a) Fluorescence chlorophyll (Fv/Fm). |
(b) Fluorescence chlorophyll (Fm). | ||
(c) Leaf with dots in Cd contamination, due to chlorophyll deficiency. |
(d) Leaf with natural color in no Cd contamination. |
(e) Leaf in yellow in Cd contamination, due to severe chlorophyll deficiency. | |
Figure 6. Effects of Cd, PVC, and PE treatments on plant physiology. | |||
3.3. Plant elements and Cd contents:
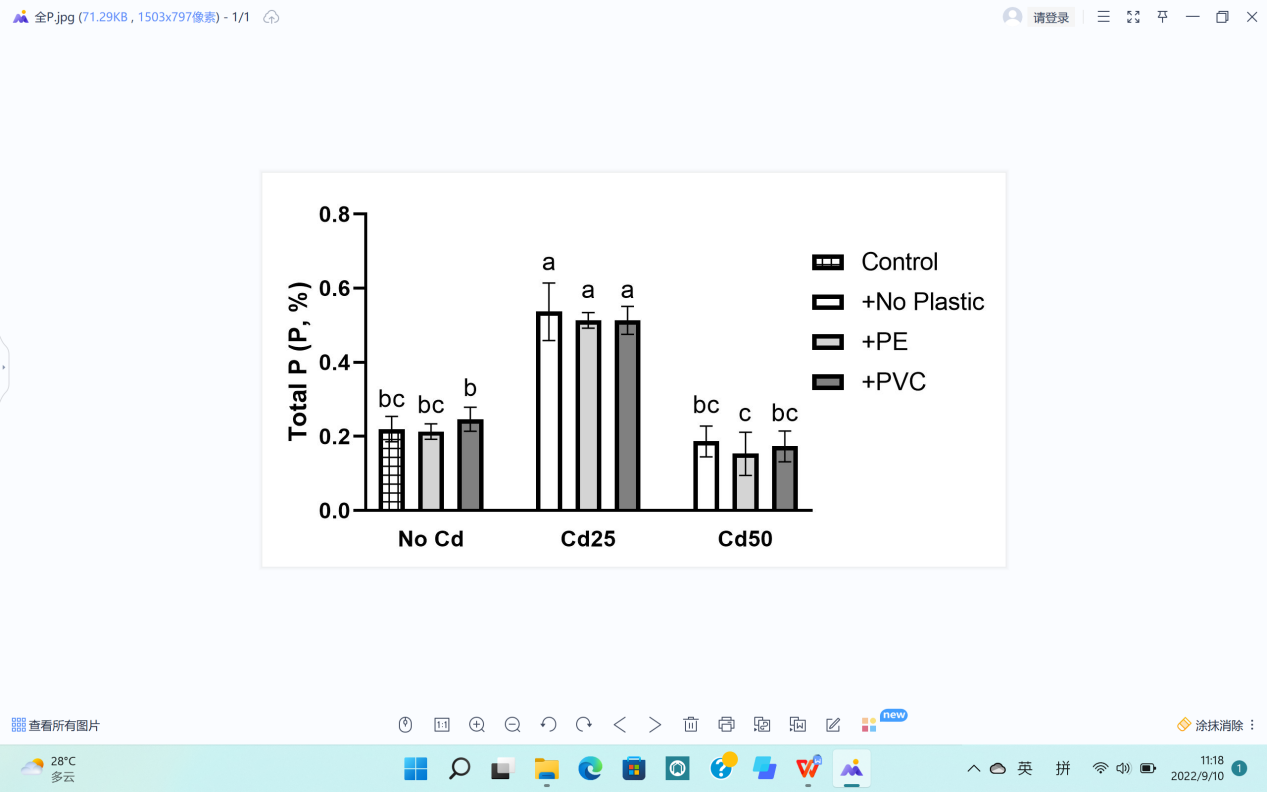
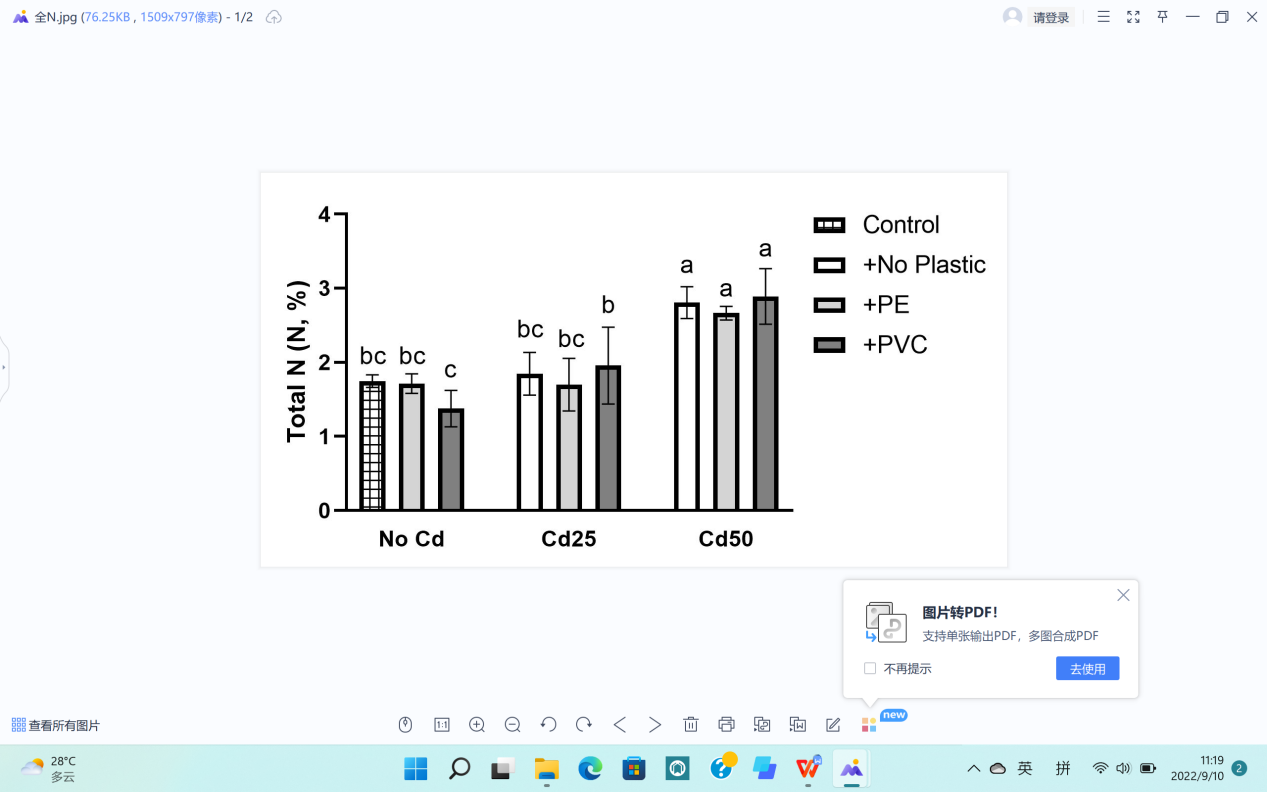
Basic elements in plants include Nitrogen (N), Potassium (K), and Phosphorus (P), which we tested in the current study (Figure 7).
The total contents of P and N in G. quadriradiata are in Figure 7a, b., The maximum values of each data were in either the treatment with 25 mg Cd per pot or the treatment with 50 mg Cd per pot, showing differences higher than LSD (Table 1). Under such circumstances, the calculations led to the conclusion that Cd contamination had significant effects on the levels of basic elements in G. quadriradiata by increasing the values of P and N contents (Pp=1.47×10-14; PN=4.1×10-8).
The level of K in G. quadriradiata showed that there were minimal differences between the control group and groups with either Cd contamination or PE and PVC contamination (Table 1). Therefore, Cd, PVC, and PE contamination had no significant effect on K contents in G. quadriradiata.
The contents of Cd in treatments with either 25 mg Cd per pot or 50 mg Cd per pot increased significantly, which served as evidence to certify the efficiency of phytoextraction that G. quadriradiata effectively absorbed Cd from the soil, shown in Figure 7d.
(a) Total content of P. |
(b) Total content of N. |
(c) Total content of K. |
(d) Total content of Cd. |
Figure 7. Effects of Cd, PVC, and PE treatments on plant elements and Cd contents. | |
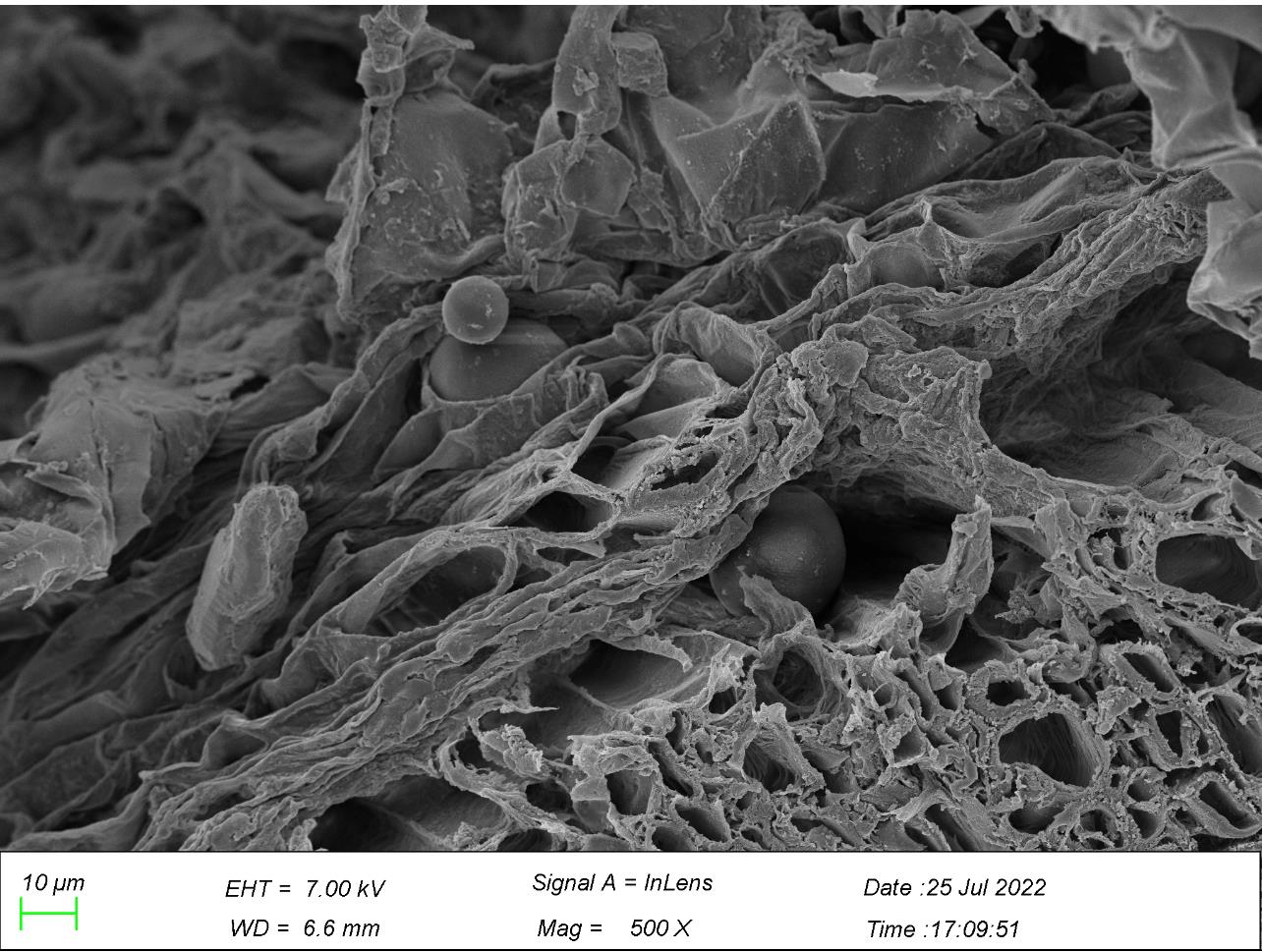
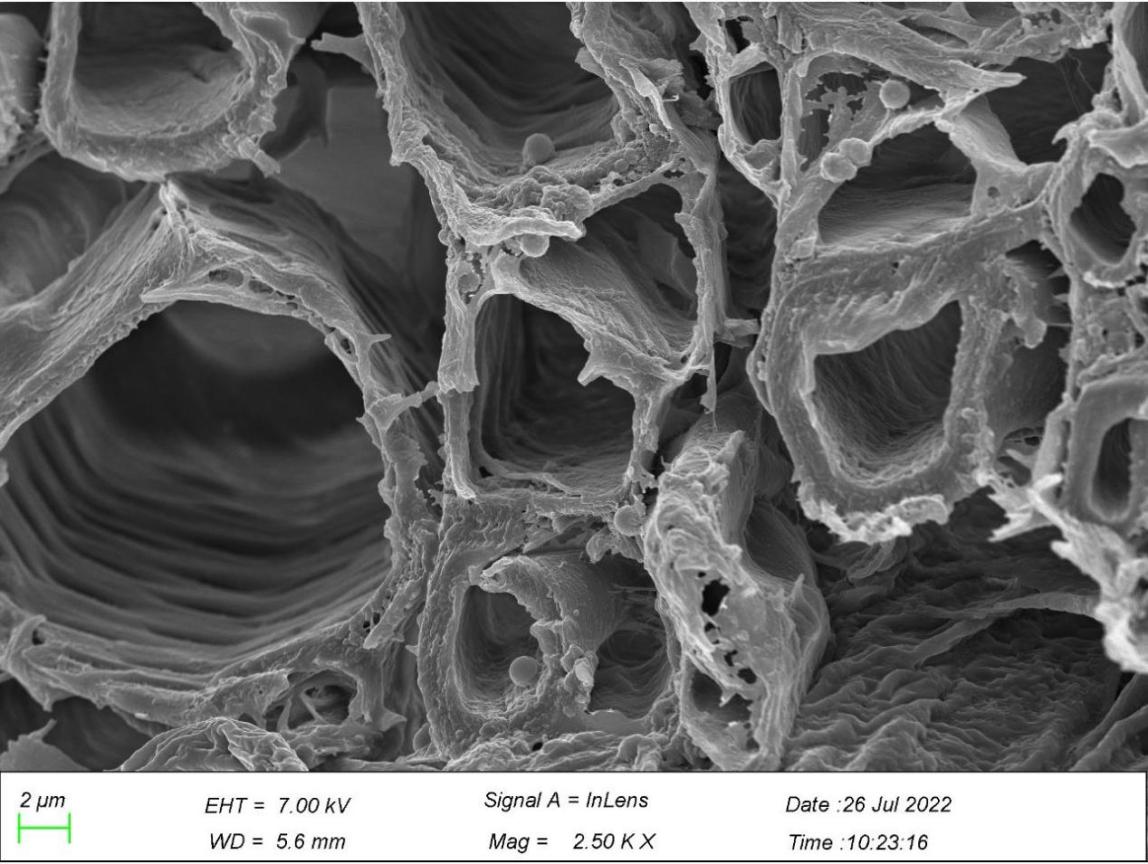
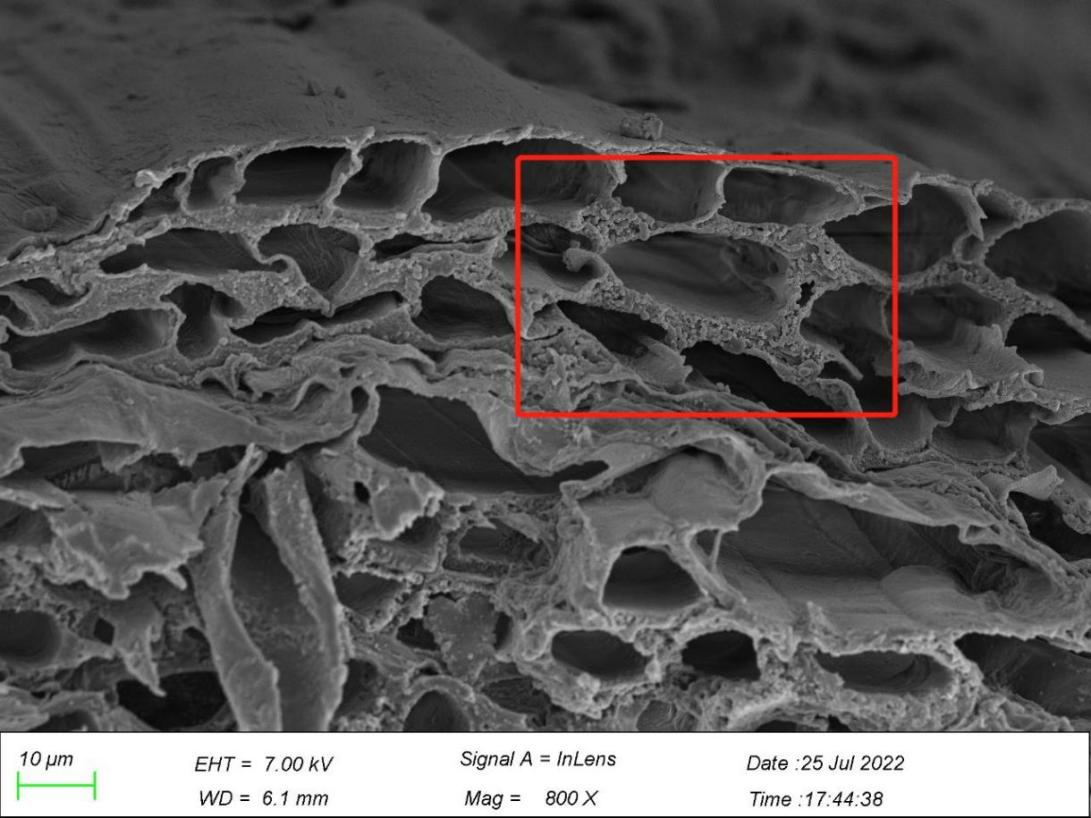
3.4. Roots observation from scanning electron microscope
(a) |
|
(b) |
|
(c) |
|
Figure 8. Particles of microplastics in roots. *Note: Arrows marked in the figures point to particles of microplastics. | |
In Figure 8, microplastics mostly existed in the intercellular spaces of the main roots and lateral roots. Figure 8a shows Microplastics appeared in the layer of epidermis of the root, one of which was embraced by the surface of the root, and another stuck in the space between the surface layer and the layer nearby, with their diameter ranging from 13.3 to 28.3 µm. There are numerous microplastics in the interspace between the xylem and its walls in the central root of G. quadriradiata in the size between 1 and 1.7 µm, with the majority of microplastics sized in 1.3 µm. Moreover, there are also microplastics, varying between 0.5-1.2 µm in diameter, in the walls of xylem and intersects as well in the lateral roots. Thus, the roots of G. quadriradiata contain microplastic particles ranging from 0.5 to 28.3 µm, with a maximum number of them in 1.2-1.3 µm.
3.5. Stems observation from scanning electron microscope
(a-1) |
|
(a-2) |
|
(b-1) |
|
(b-2) |
|
Figure 9. Particles of microplastics in stems *Note: Arrows marked in the figures point to particles of microplastics. | |
A great number of microplastics accumulated around the intercellular space and cell walls at the epidermis of stems of G. quadriradiata (Figure 9). Microplastics existed in the interspace between cells from the second layer from the surface of the stem and on cells’ walls at the exterior layer of stem, with their sizes ranged between 0.3-1.3 µm and most of them sized in 0.7 or 1.2 µm. All figures in Figure 9 demonstrated the effectiveness of phytoextraction, that numerous particles of microplastics had been up-taken by G. quadriradiata and transferred from roots to stems.
3.6. Leaves observation from scanning electron microscope
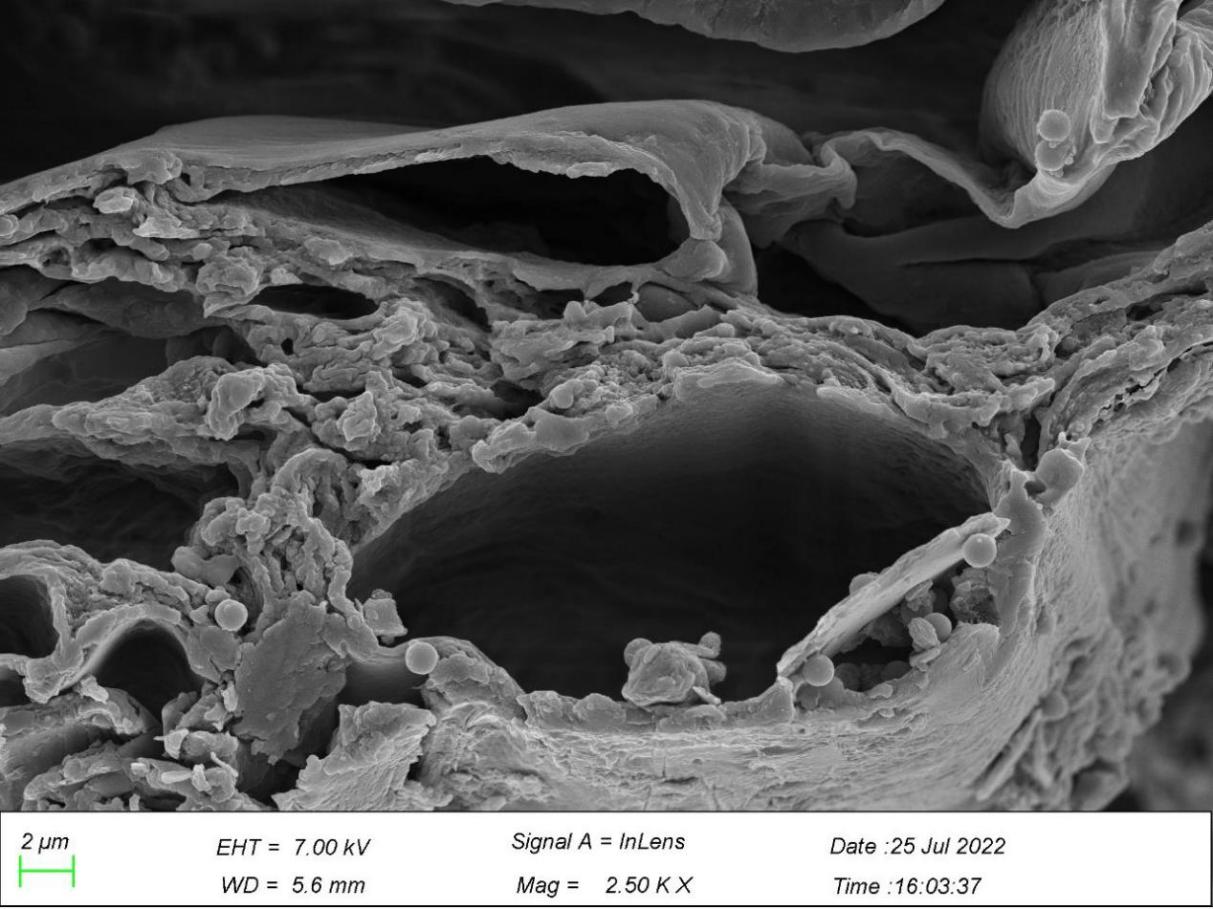
Figure 10. Particles of microplastics in leaves.
*Note: Arrows marked in the figures point to particles of microplastics.
Figure 10 shows the particles of microplastics in leaf veins, cell walls, and the interspaces between cells, sized between 0.3-1.3 µm, and the majority is 1.3 µm in diameter, serving as evidence of the effectiveness of phytoextraction since microplastics were absorbed by G. quadriradiata and transferred to leaf veins.
4. Discussion
4.1. Size range of microplastics to be absorbed and the verification of carrier effect
In this experiment, the particle size range of microplastics absorbed by G. quadriradiata was verified. Scanning electron microscope (SEM) observation results showed that the diameter of microplastic particles absorbed by the root of G. quadriradiata was about 0.5 ~ 28.3 µm, most of which were 1.2 µm. The diameter of microplastic particles in stems and leaves ranged from 0.3 to 1.3 µm, and most of them ranged from 0.7 to 1.2 µm. Compared with the previous research results from Li et al.[53], this experiment selected G. quadriradiata as the experimental subject while Li et al. [53] selected wheat. The adsorption ranges proved by the two experiments were roughly the same. Li et al. [53] showed that the particle size of microplastics adsorbed by plants ranged from 0.2 µm to 2 µm, and the content of 5 µm microplastics entering plants was very low, while 7 and 10 µm sized microplastics were basically not adsorbed by plants, which was consistent with the particle size of most of the microplastics in this experiment. At the same time, the results of this experiment showed that the interaction between microplastics and heavy metals was significant only in the PSII maximum photosynthetic efficiency (Fv/Fm), and no significant carrier effect between the two pollutants were found. The experimental results are different from those previously obtained in Jia et al.[49]. According to the study of Jia et al. [49], the presence of microplastics can promote the adsorption efficiency of heavy metals in plants, which is different from the results of this experiment. At the same time, the experimental results obtained by Manjate et al. [52] are the same as those obtained in this experiment, indicating that the presence of microplastics has no effect on the adsorption efficiency of heavy metals in plants, showing no obvious carrier effect. Although the findings of this experiment are consistent with the research results of Manjate et al. [52] and inconsistent with the experimental results of Jia et al.[49], the particle size selection of microplastics in these two experiments is beyond the range of particle size that can be adsorbed by plants, as verified in this experiment, which makes us question the reliability of the conclusions of the two experiments. In the experiment of Jia et al. [49], the researchers selected PE particles with an average particle size of 293µm as the experimental material, while Manjate et al. [52] selected PE particles with a particle size range of 850-1000µm for the experiment. The particle size range of the microplastics in these two experiments greatly exceeds that of the microplastics that can be adsorbed by plants. Therefore, the author believes that the microplastics particles in these two experiments have not been adsorbed into the body by plants, so the experimental results are of no reference value. In this experiment, the author chose SEM to observe the adsorption of microplastics by plants by observing plant section samples. However, the observed content of microplastics particles in plants was not high. We believes that the selected particle size may lead to limited uptake of microplastics by plants, so it is suggested that future studies can reduce the size of microplastics to nanometers, which may leads to significantly larger amounts of microplastics absorbed. At that time, it will be easier to observe and determine whether the carrier effect between microplastics sized by nanometers and Cd occurs. In addition, if the SEM that are equipped with Energy Dispersive X-ray Spectroscopy can be used, the distribution sites of heavy metals and microplastics in plants can be more accurately located, so as to more accurately judge whether there is a double adsorption between them and whether there is the carrier effect between the two pollutant, thus opening up a new research approach.
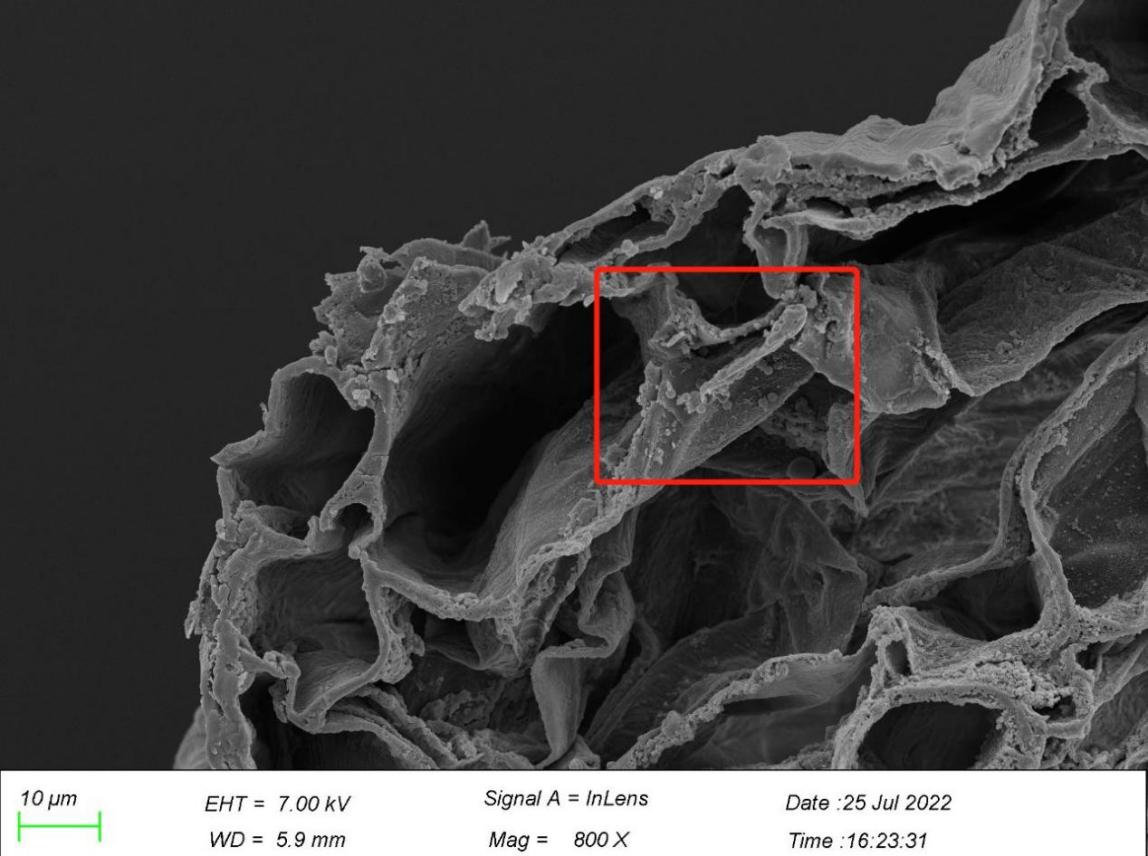
4.2. A new pathway of microplastic uptake
According to the SEM observation of microplastics entering the plant body in the outer epidermis of plant root samples, the observation results (Figure 11) show that there is a microplastic particle sandwiched between the outermost layer and the second outer layer of plant root, and there is another microplastic particle wrapped by the concave surface of the root, which can be used as a schematic diagram of the process node of microplastics entering the plant body. In this regard, we believe that with the growth and expansion of the plant root epidermis, the microplastic particles may be wrapped in the protective tissue by the plant and gradually fixed in the plant tissue. However, this finding is inconsistent with Li et al. [53], who showed that microplastic particles enter plants through cracks between new lateral roots and primary roots, while the results of this experiment tend to support that plants are included by plant protection tissues during the growth and expansion of roots. Therefore, we suggest that further experiments should be carried out to verify the specific way of microplastics entering plants and verify the authenticity of microplastics being wrapped into plants by protective tissues by using more sophisticated instruments such as laser scanning confocal fluorescence microscopy.
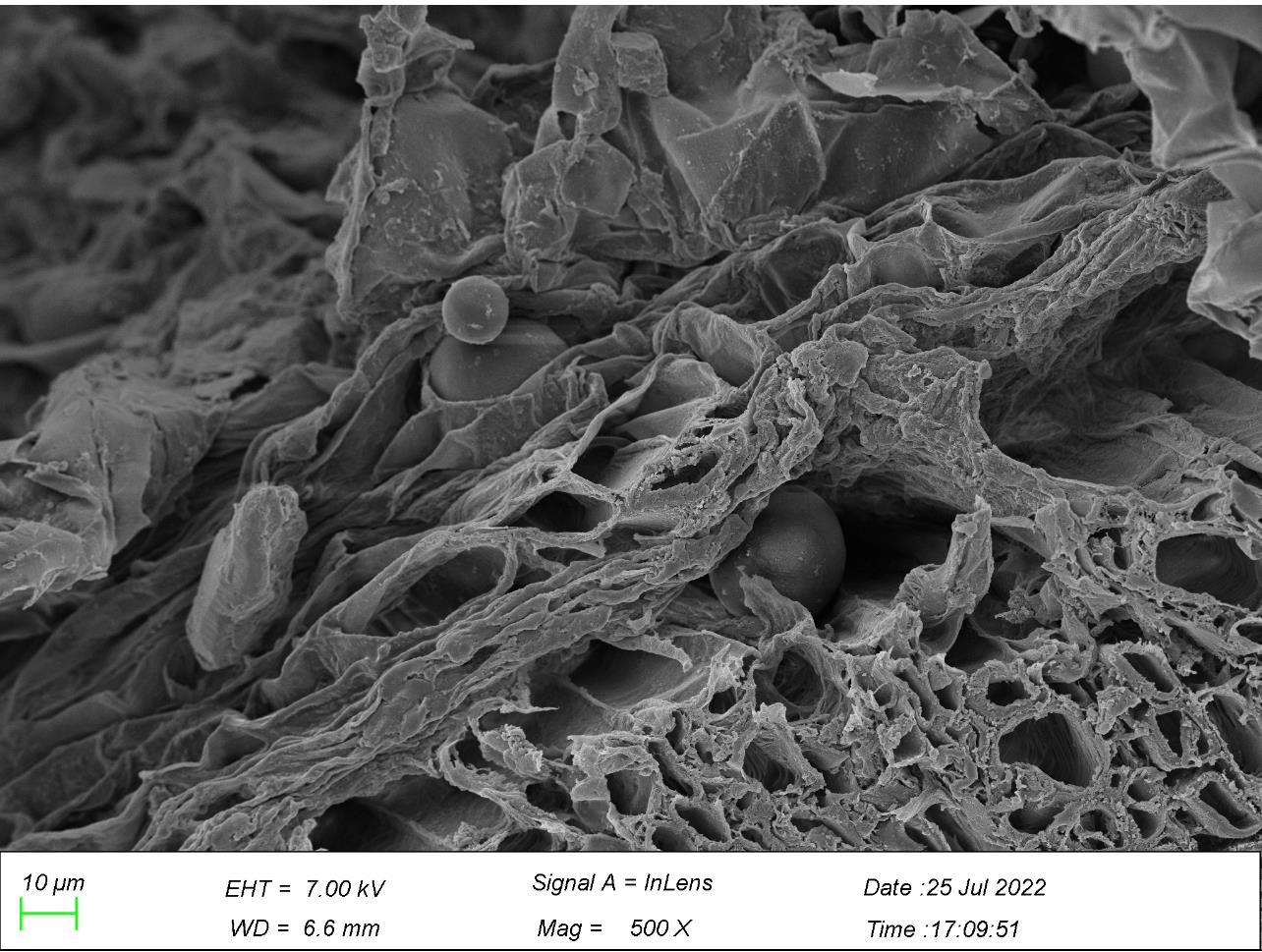
Figure 11. Particles of microplastics in root.
*Note: Arrows marked in the figures point to particles of microplastics.
4.3. Efficient application of phytoextraction and efforts in agricultural development
In previous studies, Chen Di et al.[54] first explored the effective adsorption treatment of invasive plant G. quadriradiat on heavy metal pollution (Cd pollution), and the results showed that the plant has high tolerance to Cd and is capable in phytoextraction of Cd, so it was classified as an effective Cd hyper-accumulator. However, the researchers did not extend the subject of phytoextraction to other pollutants. In this experiment, the author applied G. quadriradiat to the extraction and treatment of microplastics, and the SEM observation results proved that there were a large number of microplastic particles in the plant body, realizing the double adsorption of heavy metals and microplastics by G. quadriradiat, proving that plants with high adsorption capacity for heavy metals may also present phytoextraction to more pollutants. Thus, through the growth process of G. quadriradiat, the plant can achieve effective remediation of two serious pollutants, regarding microplastics and heavy metals, which greatly improves the efficiency of phytoextraction. Therefore, we propose to use this plant as a "cover crop" in farmland, that is, planting G. quadriradiat during the fallow period of farmland to repair heavy metal and microplastic pollution in farmland ecosystems and improve soil quality. This will help agriculture and improve food safety by improving the growing environment for crops.
5. Conclusion
In this study, the existence of Cd and microplastics in G. quadriradiat were tested to shown that while Cd contamination had significant negative effects on G. quadriradiata , microplastic contamination had little effect on the plant. Interestingly, the interaction between Cd and microplastics only exists in specific values in G. quadriradiata, but does not increase the amount of Cd that is absorbed by the G. quadriradiata, with the maximum level of Cd absorption existing in the treatment with 50 mg of Cd and 2% of PVC. In this study, G. quadriradiata has been proved to be an effective hyper-accumulator for both Cd and microplastics. Thus, phytoextraction with G. quadriradiata may offer an effective solution for the remediation of heavy metal or microplastic contamination.
References
[1]. Tchounwou, P.B., et al., Heavy metal toxicity and the environment. Exp Suppl, 2012. 101: p. 133-64.
[2]. Ali, H. and E. Khan, What are heavy metals? Long-standing controversy over the scientific use of the term ‘heavy metals’ – proposal of a comprehensive definition. Toxicological & Environmental Chemistry, 2018. 100(1): p. 6-19.
[3]. Fasani, E., et al., The potential of genetic engineering of plants for the remediation of soils contaminated with heavy metals. Plant Cell Environ, 2018. 41(5): p. 1201-1232.
[4]. Rizwan, M., et al., Cadmium minimization in wheat: A critical review. Ecotoxicol Environ Saf, 2016. 130: p. 43-53.
[5]. Speir, T.W., et al., Is soil acidification the cause of biochemical responses when soils are amended with heavy metal salts? Soil Biology and Biochemistry, 1999. 31(14): p. 1953-1961.
[6]. Khan, S., et al., Soil enzymatic activities and microbial community structure with different application rates of Cd and Pb. J Environ Sci (China), 2007. 19(7): p. 834-40.
[7]. Jaiswal, A., A. Verma, and P. Jaiswal, Detrimental Effects of Heavy Metals in Soil, Plants, and Aquatic Ecosystems and in Humans. J Environ Pathol Toxicol Oncol, 2018. 37(3): p. 183-197.
[8]. Kim, J.J., Y.S. Kim, and V. Kumar, Heavy metal toxicity: An update of chelating therapeutic strategies. J Trace Elem Med Biol, 2019. 54: p. 226-231.
[9]. ZHANG Shengnan. 2021. Inducing effects and physiological mechanism of exogenous plant hormones on the tolerance of rice and rape to Cadmium and Arsenic stress. Chinese Academy of Agricultural Science.
[10]. RAN Fu-lin, ZHAO Bao-wei, DUAN Kai-xiang, HAO Ai-hong (2021) Research status and progress of microplastics in soil ecosystem. Environmental Ecology, 3, 56-62.
[11]. Kimura, M., et al., Ecology of viruses in soils: past, present and future perspectives. Soil Science Plant Nutrition, 2008. 54(1): p. 1-32.
[12]. Dahlbo, H., et al., Recycling potential of post-consumer plastic packaging waste in Finland. Waste Manag, 2018. 71: p. 52-61.
[13]. Abe, K., N. Nomura, and S. Suzuki, Biofilms: hot spots of horizontal gene transfer (HGT) in aquatic environments, with a focus on a new HGT mechanism. FEMS Microbiology Ecology, 2020. 96(5): p. fiaa031.
[14]. Auta, H.S., C.U. Emenike, and S.H. Fauziah, Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environ Int, 2017. 102: p. 165-176.
[15]. Lassen, C., et al. Microplastics: Occurrence, effects and sources of releases to the environment in Denmark. 2015.
[16]. Phillips, P.J., et al., Combined Sewer Overflows: An Environmental Source of Hormones and Wastewater Micropollutants. Environmental Science & Technology, 2012. 46(10): p. 5336-5343.
[17]. Wang, R., et al., Sources, transport and deposition of iron in the global atmosphere. Atmos. Chem. Phys., 2015. 15(11): p. 6247-6270.
[18]. Baensch-Baltruschat, B., et al., Tyre and road wear particles - A calculation of generation, transport and release to water and soil with special regard to German roads. Science of The Total Environment, 2021. 752: p. 141939.
[19]. Wang, J., et al., Effects of plastic film residues on occurrence of phthalates and microbial activity in soils. Chemosphere, 2016. 151: p. 171-7.
[20]. Roy, P.K., et al., Degradable polyethylene: fantasy or reality. Environ Sci Technol, 2011. 45(10): p. 4217-27.
[21]. Teuten, E.L., et al., Transport and release of chemicals from plastics to the environment and to wildlife. Philos Trans R Soc Lond B Biol Sci, 2009. 364(1526): p. 2027-45.
[22]. Wang, J., et al., Microplastics as contaminants in the soil environment: A mini-review. Sci Total Environ, 2019. 691: p. 848-857.
[23]. de Souza Machado, A.A., et al., Microplastics Can Change Soil Properties and Affect Plant Performance. Environ Sci Technol, 2019. 53(10): p. 6044-6052.
[24]. Li, Z., J.-W. Yu, and I. Neretnieks, Removal of Pb(II), Cd(II) and Cr(III) from sand by electromigration. Journal of Hazardous Materials, 1997. 55(1): p. 295-304.
[25]. Gao, X., et al., Effects of magnesium ferrite biochar on the cadmium passivation in acidic soil and bioavailability for packoi (Brassica chinensis L.). J Environ Manage, 2019. 251: p. 109610.
[26]. Ji, G. and F. Guo, Impact of ultrasonic power density on hot water elution of severely biodegraded heavy oil from weathered soils. Chemosphere, 2010. 79(2): p. 210-5.
[27]. Yan, A., et al., Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front Plant Sci, 2020. 11: p. 359.
[28]. Ali, H., E. Khan, and M.A. Sajad, Phytoremediation of heavy metals--concepts and applications. Chemosphere, 2013. 91(7): p. 869-81.
[29]. Sarwar, N., et al., Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere, 2017. 171: p. 710-721.
[30]. Salt, D.E., R.D. Smith, and I. Raskin, PHYTOREMEDIATION. Annu Rev Plant Physiol Plant Mol Biol, 1998. 49: p. 643-668.
[31]. Jacob, J.M., et al., Biological approaches to tackle heavy metal pollution: A survey of literature. J Environ Manage, 2018. 217: p. 56-70.
[32]. Suman, J., et al., Phytoextraction of Heavy Metals: A Promising Tool for Clean-Up of Polluted Environment? Front Plant Sci, 2018. 9: p. 1476.
[33]. Guo, J.J., et al., Source, migration and toxicology of microplastics in soil. Environ Int, 2020. 137: p. 105263.
[34]. CHEN Xian, GU Xuanning, BAO Lijing, MA Xuan, PAN Yanan, MA Shanshan, MU Yinghui, Extraction of Microplastics in Soil Using Floatation Digestion Method. Journal of Jiangsu University of Technology, 2020. 26(04): p. 1-7.
[35]. DENG Yan-hui, WAN Bing-zhou, Tanveer M.ADYEL, LI Dan, Collection and Separation of Microplastic Samples in Natural Environment. The Administration and Technique of Environmental Monitoring, 32, 1-4+9.
[36]. CHEN Yalan, SUN Ke, HAN Lanfang, et al. (2022) Separation, Identification, and Quantification Methods in Soil Microplastics Analysis: A Review. Acta Pedologica Sinica, 59, 364-380.
[37]. TANG Qing-feng, LI Qin-mei, WEI Xiao-xiao, SHAO Peng, GAO Li-juan, CHEN Qi-rong, HU Guang-hui, LIU Wei-li, GAO Xia (2019) Progress on Research of Analysis Techniques for Microplastics in Environmental Samples. Journal of Instrumental Analysis, 38, 1009-1019.
[38]. YU Gou-bin, CHEN Ming-zhou, TAO Ping, Study on the Removal of Organic Matter for Separation and Analysis of Microplastics in Sugarcane Soil. Sugarcane and Canesugar, 2017(02): p. 66-70.
[39]. REN Xin-wei, TANG Jing-chun, YU Chen, et al. Advances in research on the ecological effects of microplastic pollution on soil ecosystems[J]. Journal of Agro-Environment Science, 2018, 37(6): 1045-1058
[40]. Yan Yuchen, Yang Zhongfang, Yu Tao. 2022. Sources, ecological hazards and treatment technologies of microplastics in soil[J]. Geology in China, 49(3): 770-788.
[41]. LIU Xinbei, DONG Xusheng, XIE Zhihong, MA Xuewen, LUO Yongming. Ecological Effects and Biodegradation of Microplastics in Soils[J]. Acta Pedologica Sinica, 2022, 59 (2): 349–363.
[42]. Kannan, M., et al., Insect gut as a bioresource for potential enzymes - an unexploited area for industrial biotechnology. Biocatalysis and Agricultural Biotechnology, 2019. 18: p. 101010.
[43]. GUO Hongqin, LUO Liping, YANG Yuhang, WANG Yumeng, LU Yaoli, ZHAO Xin & HU Xiaomin (2020) Research progress on plastic degradation by worms. Chinese Journal of Applied and Environmental Biology, 26, 1546-1553.
[44]. Krueger, M.C., H. Harms, and D. Schlosser, Prospects for microbiological solutions to environmental pollution with plastics. Applied Microbiology and Biotechnology, 2015. 99(21): p. 8857-8874.
[45]. Yuan, J., et al., Microbial degradation and other environmental aspects of microplastics/plastics. Science of The Total Environment, 2020. 715: p. 136968.
[46]. Yoshida, S., et al., A bacterium that degrades and assimilates poly(ethylene terephthalate). Science, 2016. 351(6278): p. 1196-1199.
[47]. Muhonja, C.N., et al., Biodegradability of polyethylene by bacteria and fungi from Dandora dumpsite Nairobi-Kenya. PLoS One, 2018. 13(7): p. e0198446.
[48]. Sánchez, C., Fungal potential for the degradation of petroleum-based polymers: An overview of macro- and microplastics biodegradation. Biotechnology Advances, 2020. 40: p. 107501.
[49]. Jia, H., et al., Impact of microplastics on bioaccumulation of heavy metals in rape (Brassica napus L.). Chemosphere, 2022. 288(Pt 2): p. 132576.
[50]. Liu, G., et al., Influence of Microplastics on the Mobility, Bioavailability, and Toxicity of Heavy Metals: A Review. Bull Environ Contam Toxicol, 2021. 107(4): p. 710-721.
[51]. Kumar, R., et al., Coupled effects of microplastics and heavy metals on plants: Uptake, bioaccumulation, and environmental health perspectives. Science of The Total Environment, 2022. 836: p. 155619.
[52]. Manjate, E., S. Ramos, and C.M. Almeida, Potential interferences of microplastics in the phytoremediation of Cd and Cu by the salt marsh plant Phragmites australis. Journal of Environmental Chemical Engineering, 2020. 8: p. 103658.
[53]. Li, L., et al., Effective uptake of submicrometre plastics by crop plants via a crack-entry mode. 2020.
[54]. CHEN Di, LI Bo-qun, YANG Yong-ping, HE Zhao-rong, LI Xiong (2021) Cadmium Accumulation Characteristics of Four Herbs. School of Life Science, 42, 960-966.
[55]. TAO Bo, DONG Yu-mei, TANG Dong-sheng, Study on Seed Maturation and Germination of Invasive Weed Galinsoga parviflora and G. ciliata. Hubei Agricultural Sciences, 2013. 52(02): p. 331-333.
[56]. Lin, L., et al., Screening of a new cadmium hyperaccumulator, Galinsoga parviflora, from winter farmland weeds using the artificially high soil cadmium concentration method. Environ Toxicol Chem, 2014. 33(11): p. 2422-8.
Cite this article
Han,X.;Li,X. (2023). Analysis of double phytoextraction of Cadmium and microplastics by Galinsoga Quadriradiata in soil — An exploration for a comprehensive treatment method for the environment. Applied and Computational Engineering,24,7-24.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2023 International Conference on Functional Materials and Civil Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Tchounwou, P.B., et al., Heavy metal toxicity and the environment. Exp Suppl, 2012. 101: p. 133-64.
[2]. Ali, H. and E. Khan, What are heavy metals? Long-standing controversy over the scientific use of the term ‘heavy metals’ – proposal of a comprehensive definition. Toxicological & Environmental Chemistry, 2018. 100(1): p. 6-19.
[3]. Fasani, E., et al., The potential of genetic engineering of plants for the remediation of soils contaminated with heavy metals. Plant Cell Environ, 2018. 41(5): p. 1201-1232.
[4]. Rizwan, M., et al., Cadmium minimization in wheat: A critical review. Ecotoxicol Environ Saf, 2016. 130: p. 43-53.
[5]. Speir, T.W., et al., Is soil acidification the cause of biochemical responses when soils are amended with heavy metal salts? Soil Biology and Biochemistry, 1999. 31(14): p. 1953-1961.
[6]. Khan, S., et al., Soil enzymatic activities and microbial community structure with different application rates of Cd and Pb. J Environ Sci (China), 2007. 19(7): p. 834-40.
[7]. Jaiswal, A., A. Verma, and P. Jaiswal, Detrimental Effects of Heavy Metals in Soil, Plants, and Aquatic Ecosystems and in Humans. J Environ Pathol Toxicol Oncol, 2018. 37(3): p. 183-197.
[8]. Kim, J.J., Y.S. Kim, and V. Kumar, Heavy metal toxicity: An update of chelating therapeutic strategies. J Trace Elem Med Biol, 2019. 54: p. 226-231.
[9]. ZHANG Shengnan. 2021. Inducing effects and physiological mechanism of exogenous plant hormones on the tolerance of rice and rape to Cadmium and Arsenic stress. Chinese Academy of Agricultural Science.
[10]. RAN Fu-lin, ZHAO Bao-wei, DUAN Kai-xiang, HAO Ai-hong (2021) Research status and progress of microplastics in soil ecosystem. Environmental Ecology, 3, 56-62.
[11]. Kimura, M., et al., Ecology of viruses in soils: past, present and future perspectives. Soil Science Plant Nutrition, 2008. 54(1): p. 1-32.
[12]. Dahlbo, H., et al., Recycling potential of post-consumer plastic packaging waste in Finland. Waste Manag, 2018. 71: p. 52-61.
[13]. Abe, K., N. Nomura, and S. Suzuki, Biofilms: hot spots of horizontal gene transfer (HGT) in aquatic environments, with a focus on a new HGT mechanism. FEMS Microbiology Ecology, 2020. 96(5): p. fiaa031.
[14]. Auta, H.S., C.U. Emenike, and S.H. Fauziah, Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environ Int, 2017. 102: p. 165-176.
[15]. Lassen, C., et al. Microplastics: Occurrence, effects and sources of releases to the environment in Denmark. 2015.
[16]. Phillips, P.J., et al., Combined Sewer Overflows: An Environmental Source of Hormones and Wastewater Micropollutants. Environmental Science & Technology, 2012. 46(10): p. 5336-5343.
[17]. Wang, R., et al., Sources, transport and deposition of iron in the global atmosphere. Atmos. Chem. Phys., 2015. 15(11): p. 6247-6270.
[18]. Baensch-Baltruschat, B., et al., Tyre and road wear particles - A calculation of generation, transport and release to water and soil with special regard to German roads. Science of The Total Environment, 2021. 752: p. 141939.
[19]. Wang, J., et al., Effects of plastic film residues on occurrence of phthalates and microbial activity in soils. Chemosphere, 2016. 151: p. 171-7.
[20]. Roy, P.K., et al., Degradable polyethylene: fantasy or reality. Environ Sci Technol, 2011. 45(10): p. 4217-27.
[21]. Teuten, E.L., et al., Transport and release of chemicals from plastics to the environment and to wildlife. Philos Trans R Soc Lond B Biol Sci, 2009. 364(1526): p. 2027-45.
[22]. Wang, J., et al., Microplastics as contaminants in the soil environment: A mini-review. Sci Total Environ, 2019. 691: p. 848-857.
[23]. de Souza Machado, A.A., et al., Microplastics Can Change Soil Properties and Affect Plant Performance. Environ Sci Technol, 2019. 53(10): p. 6044-6052.
[24]. Li, Z., J.-W. Yu, and I. Neretnieks, Removal of Pb(II), Cd(II) and Cr(III) from sand by electromigration. Journal of Hazardous Materials, 1997. 55(1): p. 295-304.
[25]. Gao, X., et al., Effects of magnesium ferrite biochar on the cadmium passivation in acidic soil and bioavailability for packoi (Brassica chinensis L.). J Environ Manage, 2019. 251: p. 109610.
[26]. Ji, G. and F. Guo, Impact of ultrasonic power density on hot water elution of severely biodegraded heavy oil from weathered soils. Chemosphere, 2010. 79(2): p. 210-5.
[27]. Yan, A., et al., Phytoremediation: A Promising Approach for Revegetation of Heavy Metal-Polluted Land. Front Plant Sci, 2020. 11: p. 359.
[28]. Ali, H., E. Khan, and M.A. Sajad, Phytoremediation of heavy metals--concepts and applications. Chemosphere, 2013. 91(7): p. 869-81.
[29]. Sarwar, N., et al., Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere, 2017. 171: p. 710-721.
[30]. Salt, D.E., R.D. Smith, and I. Raskin, PHYTOREMEDIATION. Annu Rev Plant Physiol Plant Mol Biol, 1998. 49: p. 643-668.
[31]. Jacob, J.M., et al., Biological approaches to tackle heavy metal pollution: A survey of literature. J Environ Manage, 2018. 217: p. 56-70.
[32]. Suman, J., et al., Phytoextraction of Heavy Metals: A Promising Tool for Clean-Up of Polluted Environment? Front Plant Sci, 2018. 9: p. 1476.
[33]. Guo, J.J., et al., Source, migration and toxicology of microplastics in soil. Environ Int, 2020. 137: p. 105263.
[34]. CHEN Xian, GU Xuanning, BAO Lijing, MA Xuan, PAN Yanan, MA Shanshan, MU Yinghui, Extraction of Microplastics in Soil Using Floatation Digestion Method. Journal of Jiangsu University of Technology, 2020. 26(04): p. 1-7.
[35]. DENG Yan-hui, WAN Bing-zhou, Tanveer M.ADYEL, LI Dan, Collection and Separation of Microplastic Samples in Natural Environment. The Administration and Technique of Environmental Monitoring, 32, 1-4+9.
[36]. CHEN Yalan, SUN Ke, HAN Lanfang, et al. (2022) Separation, Identification, and Quantification Methods in Soil Microplastics Analysis: A Review. Acta Pedologica Sinica, 59, 364-380.
[37]. TANG Qing-feng, LI Qin-mei, WEI Xiao-xiao, SHAO Peng, GAO Li-juan, CHEN Qi-rong, HU Guang-hui, LIU Wei-li, GAO Xia (2019) Progress on Research of Analysis Techniques for Microplastics in Environmental Samples. Journal of Instrumental Analysis, 38, 1009-1019.
[38]. YU Gou-bin, CHEN Ming-zhou, TAO Ping, Study on the Removal of Organic Matter for Separation and Analysis of Microplastics in Sugarcane Soil. Sugarcane and Canesugar, 2017(02): p. 66-70.
[39]. REN Xin-wei, TANG Jing-chun, YU Chen, et al. Advances in research on the ecological effects of microplastic pollution on soil ecosystems[J]. Journal of Agro-Environment Science, 2018, 37(6): 1045-1058
[40]. Yan Yuchen, Yang Zhongfang, Yu Tao. 2022. Sources, ecological hazards and treatment technologies of microplastics in soil[J]. Geology in China, 49(3): 770-788.
[41]. LIU Xinbei, DONG Xusheng, XIE Zhihong, MA Xuewen, LUO Yongming. Ecological Effects and Biodegradation of Microplastics in Soils[J]. Acta Pedologica Sinica, 2022, 59 (2): 349–363.
[42]. Kannan, M., et al., Insect gut as a bioresource for potential enzymes - an unexploited area for industrial biotechnology. Biocatalysis and Agricultural Biotechnology, 2019. 18: p. 101010.
[43]. GUO Hongqin, LUO Liping, YANG Yuhang, WANG Yumeng, LU Yaoli, ZHAO Xin & HU Xiaomin (2020) Research progress on plastic degradation by worms. Chinese Journal of Applied and Environmental Biology, 26, 1546-1553.
[44]. Krueger, M.C., H. Harms, and D. Schlosser, Prospects for microbiological solutions to environmental pollution with plastics. Applied Microbiology and Biotechnology, 2015. 99(21): p. 8857-8874.
[45]. Yuan, J., et al., Microbial degradation and other environmental aspects of microplastics/plastics. Science of The Total Environment, 2020. 715: p. 136968.
[46]. Yoshida, S., et al., A bacterium that degrades and assimilates poly(ethylene terephthalate). Science, 2016. 351(6278): p. 1196-1199.
[47]. Muhonja, C.N., et al., Biodegradability of polyethylene by bacteria and fungi from Dandora dumpsite Nairobi-Kenya. PLoS One, 2018. 13(7): p. e0198446.
[48]. Sánchez, C., Fungal potential for the degradation of petroleum-based polymers: An overview of macro- and microplastics biodegradation. Biotechnology Advances, 2020. 40: p. 107501.
[49]. Jia, H., et al., Impact of microplastics on bioaccumulation of heavy metals in rape (Brassica napus L.). Chemosphere, 2022. 288(Pt 2): p. 132576.
[50]. Liu, G., et al., Influence of Microplastics on the Mobility, Bioavailability, and Toxicity of Heavy Metals: A Review. Bull Environ Contam Toxicol, 2021. 107(4): p. 710-721.
[51]. Kumar, R., et al., Coupled effects of microplastics and heavy metals on plants: Uptake, bioaccumulation, and environmental health perspectives. Science of The Total Environment, 2022. 836: p. 155619.
[52]. Manjate, E., S. Ramos, and C.M. Almeida, Potential interferences of microplastics in the phytoremediation of Cd and Cu by the salt marsh plant Phragmites australis. Journal of Environmental Chemical Engineering, 2020. 8: p. 103658.
[53]. Li, L., et al., Effective uptake of submicrometre plastics by crop plants via a crack-entry mode. 2020.
[54]. CHEN Di, LI Bo-qun, YANG Yong-ping, HE Zhao-rong, LI Xiong (2021) Cadmium Accumulation Characteristics of Four Herbs. School of Life Science, 42, 960-966.
[55]. TAO Bo, DONG Yu-mei, TANG Dong-sheng, Study on Seed Maturation and Germination of Invasive Weed Galinsoga parviflora and G. ciliata. Hubei Agricultural Sciences, 2013. 52(02): p. 331-333.
[56]. Lin, L., et al., Screening of a new cadmium hyperaccumulator, Galinsoga parviflora, from winter farmland weeds using the artificially high soil cadmium concentration method. Environ Toxicol Chem, 2014. 33(11): p. 2422-8.