1. Introduction
GaN is a kind of semiconductor with direct band gaps. Its width of the forbidden band is 3.4 eV, and the band of the alloys formed with AlN and InN can continuously adjust from 0. 65 to 6.2 eV. It covers the spectrum between deep ultraviolet, near-ultraviolet, and visible lights [1].
GaN is unstable at a high temperature and decomposes into Ga and N2 at the melting point. GaN crystal can be melted in 6 GPa and 2200 ℃ environments [2]. But traditional process methods cannot meet the acquirement. Because of this reason, GaN single crystal production is hard and costly.
With the rising demand for all kinds of GaN products and the high preferment GaN products acquiring, GaN is becoming increasingly important to make high current density devices such as lasers and high withstand voltage devices. For example, long-life high-power laser sources acquire a dislocation density that cannot be higher than 105 cm-2. Because of the shortage of heterogeneous epitaxy such as lattice mismatch, high dislocation density due to thermal expansion coefficient mismatch, mosaic crystal structure, biaxial stress and wafer warpage, the preference of products are limited by the quality of the substrate structure. Therefore, cutting on a large and high-quality piece of GaN is a better solution [1]. The main production processes of the single crystal GaN are the ammonothermal method, hydride vapor phase epitaxy and Sodium-flux growth method.
As the crystal growth technology develops, combining two or three methods can be a better choice than using a single method to grow the GaN crystal. And we found that combining ammonothermal and HVPE methods may reduce the cost in the growth period.
2. Hydride Vapor Phase Epitaxy (HVPE)
HVPE is a chemical vapor deposition technology, using hydride (AsH3, PH3, NH3) and chloride (GaCl, GaCl3, InCl) as material. The reaction chamber can be divided into low-temperature areas and material areas. As shown in Figure 1, the reaction is the high purity HCl (850 ℃) and liquid Ga to form GaCl and a small number of by-products, and the N2 or other gas will carry these gases to the high-temperature growing area (~1050 ℃). At the high-temperature area, the GaCl gas and the NH3 gas react to produce GaN and deposit it on the substrate (commonly sapphire, silicon carbide, silicon, gallium arsenide) to form thin films.
Main reaction at low-temperature area:
Ga(l)+HCl(g)⇔GaCl(g)+1/2H2(g).
Main reaction at high-temperature area:
GaCl(g)+NH3(g)⇔GaN(s)+HCl(g)+H2(g) [3].
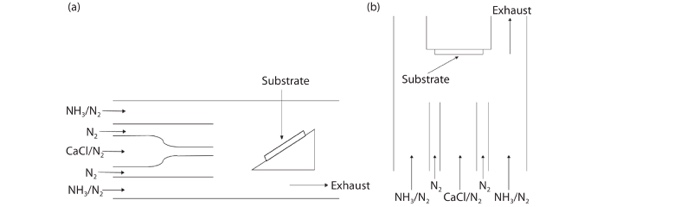
Figure 1. Schematic view of (a) horizontal HVPE reactor and (b) vertical HVPE reactor [3].
The high growth rate of the HVPE (100 μm/h) [4] is an advantage of this method. The purity of the GaN produced by this method is high and the overall impurity concentration can be lower than 1014 cm3 [4]. But the exfoliation technology of the GaN crystal is a problem of the HVPE method. The laser exfoliation technique uses the laser with energy between the band gap of GaN (about 3.4 eV) and band gap of sapphire (about 9.9 eV) to decompose the GaN at the interface of the substrate to separate the GaN crystal and the sapphire substrate. By adjusting the spot and scanning rate, it is possible to separate the 4-inch GaN crystal from the sapphire substrate.[1] Kim with his team realized the separation of the 30*30 mm2, 400-450 μm thick GaN sheets with sapphire [5]. And it is possible to produce GaN crystal at 175 mm diameter using the stitching HVPE method [6].
2.1. Oxide Vapor Phase Epitaxy (OVPE)
The OVPE method is a way to form GaN crystal using Ga2O (g), which is created by H2O (g) and Ga (g) and NH3 (g). This method can prevent NH3 and HCl from forming NH4Cl to increase the purity of the GaN crystal. But because of oxygen impurities, the resistivity of GaN crystal grown by this method is usually lower than that of GaN crystal grown by another method.
2Ga(l) + H2O(g)→Ga2O(g) + H2(g) (1)
Ga2O(g) + 2NH3(g)→2GaN(s) + H2O(g) + 2H2(g) (2)
Recently, a team reduced the Ga metal particles by adding N2O to the gas flow. At the same time, adding N2O can reduce the creation of polycrystals, and compared to using the H2O (g), using N2O can increase the speed of crystal growth [7].
3. Ammonothermal
Although HVPE is a great method to produce GaN crystal, there are also some shortages of HVPE, such as high cost and high curvature of crystal. The basic theory of the ammonothermal method of growing GaN crystal is to dissolve all material required for GaN crystal growth into a mineralizer (such as KNH2) to form a supercritical ammonia fluid. The fluid becomes a saturated solution. This saturated solution is then transformed into a sub-stable supersaturated solution by taking appropriate technical measures to crystallize and grow GaN single crystals on seed crystals [8]. The process will heat all material, seed crystals, and mineralizer solution in a high-pressure container. Then it will create a temperature difference between the material area and seed crystal area, and it can increase the speed of convection of the solution to encourage seed crystal growth. When the material has a positive solubility temperature coefficient, the seed crystal should be placed at the top of the container, which is the low-temperature area, and the material should be placed at the bottom of the container, which is the high-temperature area. It is suitable for negative solubility temperature coefficient material by putting the material at a low temperature and putting the seed crystal at a high-temperature area [8].
For example, KNH2.
In an alkaline mineralizer system, firstly, the GaN material reacts with the mineralizer to form the intermediate compound. Then the intermediate compound is transmitted to the crystal seed growth area, which is controlled at a suitable temperature by convection, and then the solution reaches supersaturation. Because growing on the GaN seed crystal does not need nucleation energy and the required energy is lower than spontaneous nucleation, the GaN will grow on the seed crystal first. Therefore, the reaction occurring at the dissolution area and growth area are counter-reactions to each other. The example reaction in the dissolution area with the KNH2 mineralizer is as below:
KNH2+GaN+2NH3→KGa(NH2)4 (3)
The following reaction occurs at the growth area:
KGa(NH2)4→KNH2+GaN+2NH3 (4)
If use NaNH2 as the mineralizer, the intermediate compound(shown in Table 1) will be Na2Ga(NH2)5, and the chemical reaction equation is as below
GaN+2NaNH3+2NH3 ⇔ Na2Ga(NH2)5 (5)
Table 1. Intermediate compounds formed by acid mineralizers [9].
Number of coordinated NH3 ligands | Complex unit | Halide in-vestigated |
1 | 0[Ga(NH3)X3] | Br |
1 | 1∞[Ga(NH3)XX4/2] | F |
2 | 2∞[Ga(NH3)2X2X2/2] | F |
3 | 0[Ga(NH3)2X4]- | F |
5 | 0[Ga(NH3)4X2]+ | Cl |
0[Ga(NH3)5X]2+ | ||
6 | 0[Ga(NH3)6]3+ | Br, I |
In addition to GaN polycrystal as the raw material, Ga metal can also be used as the material.
The chemical reaction equation is as below (take the KNH2 as the example): [7]
KNH2+Ga+NH3→KNH2+GaN+1.5H2 (6) [9].
Andrew P. Purdy and others synthesized GaN crystals using NH4X (X = Cl, Br, I as a mineralizer for acidic mineralizers and studied the growth of GaN crystals at different temperatures [10].) (The figures are shown in Table 2).
Table 2. Temperature for different acidic mineralizers [8].
Temperature/℃ | 350-375 | 375-400 | 425-450 | 450-475 | 475-500 | 500-525 | 525-550 |
NH4Cl | h&c:10 | - | - | h:20 | - | h:40 | h:70 |
NH4Br | - | c:20 | c:70 | - | - | h:80 | h:100 |
NH4I | - | c:25 | c:95 | - | - | c:95 | h:100 |
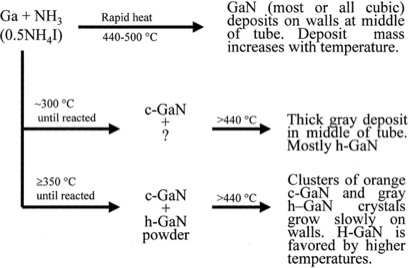
Figure 2. GaN deposit situation in different temperatures [10].
In this studying, the researchers found that,
(1) The lattice deformation of the GaN crystal becomes more severe with the increase of acidity.
(2) The length of the GaN crystal’s vector A increased with the temperature (shown in Figure 2), but the length of the vector C of the GaN crystal was almost unchanged, probably due to the impurity increase along the a-axis.
(3) Whether the seed is polished greatly influences the crystalline quality of the grown GaN crystals. The crystalline quality of the crystals is very poor when the seed GaN crystal is directly grown by the HVPE method without any polishing. But when the crystals are chemically and mechanically polished, the crystalline quality can be significantly improved [8].
Note: P refers to the physical phase, h refers to the hexagonal phase, c refers to the cubic phase; h&c refers to the simultaneous generation of both hexagonal and cubic phase GaN; h refers to the generation of only hexagonal phase GaN, c refers to the generation of only cubic phase GaN, - refers to the generation of no GaN [8].
Dirk Ehrentraut and others cultivated GaN crystals and 2-inch GaN crystals using the ammonothermal method at 750 ℃ and 600 MPa [11] (as shown in Figure 3).
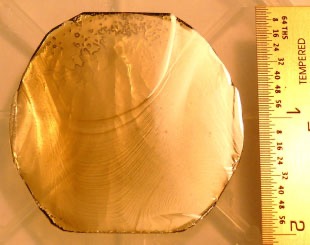
Figure 3. Photographs of SCoRA-grown bulk GaN crystals: 2-in. c-plane with macro-steps [11].
4. Sodium-flux method
The sodium-flux method is a method that adds Na to melted Ga to increase the solubility of N to meet the reaction requirement between Ga and N. Under a certain temperature and pressure, the ion Na will ionize nitrogen at the gas-liquid interface to form N3-. Therefore, the density of the N3- in the melt increases a thousand times compared to the nitrogen gas, and it can exist stably. The N3- is transported to the temperature gradient or concentration gradient downward area. Then when the concentration is above the critical value, GaN will form spontaneous nucleation or liquid phase epitaxy (LPE) growth on GaN seeds [9].
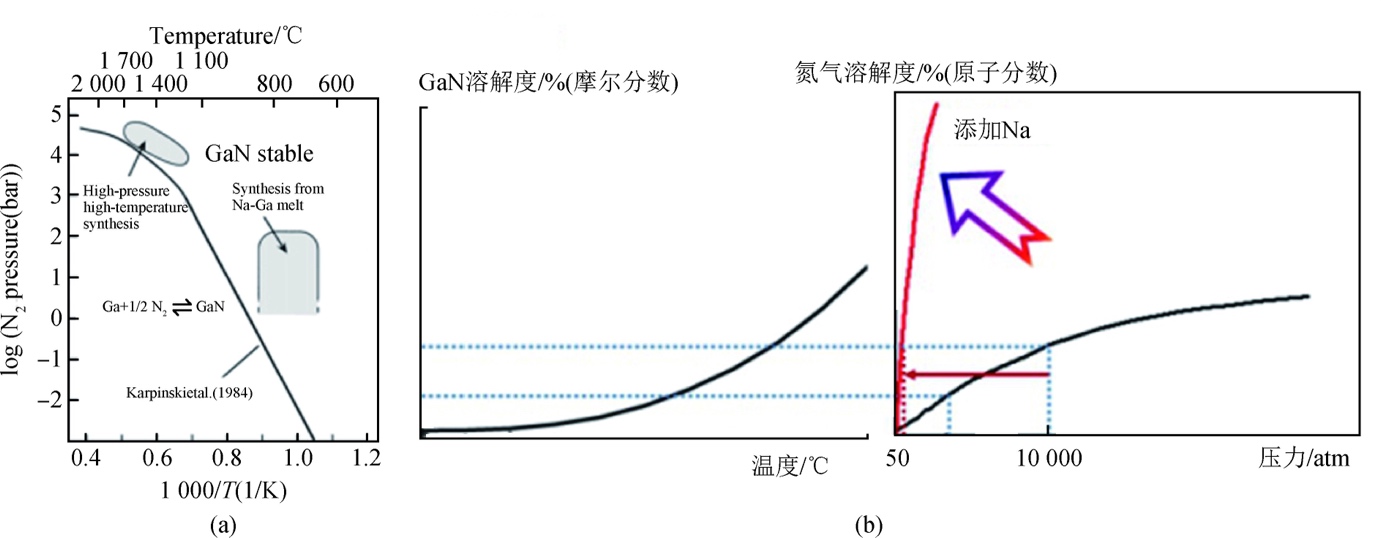
Figure 4. GaN Phase diagram [9].
In the experiment (shown in Figure 4), the growth temperature and pressure can be managed to achieve the supersaturation condition and control the growth progress to obtain continuous and effective crystal growth. Compared to the high-pressure capacitive growth conditions (temperature >1500 ℃, pressure >1 GPa), this method can grow the GaN crystal in a relatively mild condition (~800°C, <5 MPa) [9]. Compared to the high-pressure solution method without using Na (∼ 1-2 μm/h) [4], the flux method can effectivity speed up the GaN crystal growth progress (20 μm/h) [4]. But this method has disadvantages either. When the local GaN saturation is high, it is easy to form multinuclear [1] spontaneously.
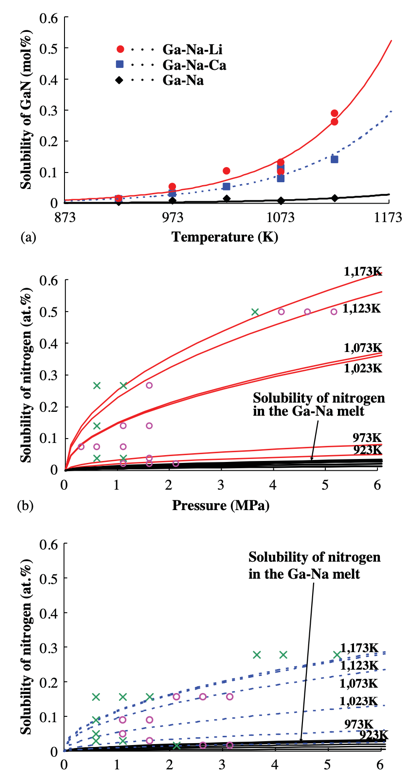
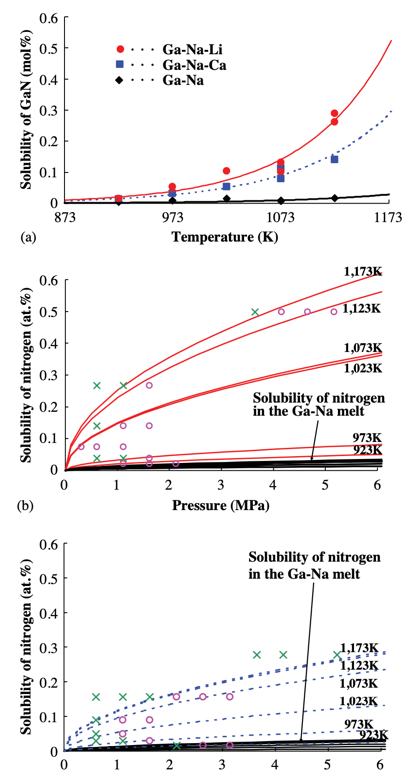
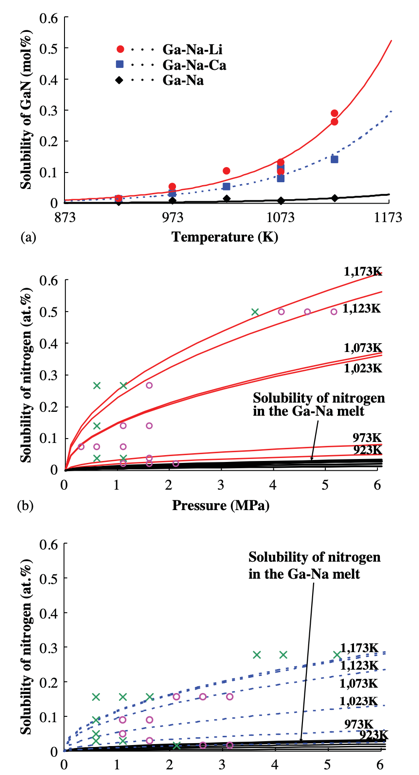
Masanori Morishita et al. studied the effect of Li and Ca on N and GaN generation in the N-GaN system. The data in this experiment demonstrates that adding Li and Ca to the system increases the solubility of N in the system to different degrees and increases the ratio of N to Ga content (shown in Figure 6). Increasing the ratio of N to Ga can reduce the vacancies of N in GaN crystals and improve transparency (shown in Figure 5) [12].
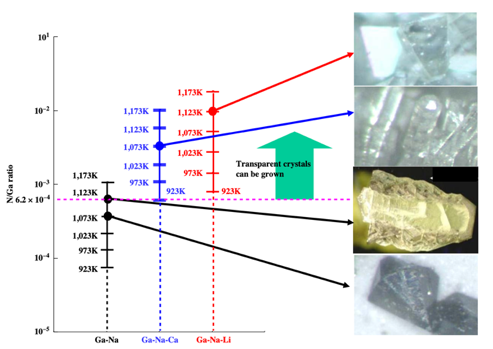
Figure 5. Relationship between the coloration of GaN crystals grown under various conditions and the N/Ga ratio at the onset of the period of growth [12].
|
|
|
(a) | (b) | (c) |
Figure 6. The Ca and Li affect the solubility of N. (a) Solubility curves of solid GaN in Ga–Na, Ga–Na–Li, and Ga–Na–Ca melt. (b) Solubility of gaseous nitrogen in Ga–Na–Li and Ga–Na melt. (c) Solubility of gaseous nitrogen in the case of Ga–Na–Ca and Ga–Na melt [12]. | ||
5. Conclusion
In the commercial view, the ammonothermal method is the most promising; the ammonothermal method can improve transparency by adding different elements to reduce N vacancy. Compared to the HVPE method, the ammonothermal method can reduce process costs and require mild production conditions. Compared to the flux method, it can speed up the growth progress and has a low possibility of forming polycrystals. Growing GaN crystal on a GaN substrate can avoid the difficulty of stripping in the HVPE method. And its dislocation density is also the lowest among the three methods. The large GaN crystal block can be grown to cut into small blocks.
References
[1]. Ren, G., Wang, J., Liu, Z., et al. (2019) Advances in single crystal growth of gallium nitride. Journal of Artificial Crystals, 48(09):1588-1598.
[2]. Utsumi, W., Saitoh, H., Kaneko, H., et al. (2003) Congruent melting of gallium nitride at 6 GPa and its application to single-crystal growth. Nature Mater, 2: 735–738.
[3]. Jun, H., Hongyuan W, Shaoyan Y, et al. (2019) Hydride vapor phase epitaxy for gallium nitride substrate. Journal of Semiconductors, 40(10): 101801
[4]. Boćkowski, M., I. Grzegory, (2022) Recent Progress in Crystal Growth of Bulk GaN. Acta Physica Polonica, A (141): 3.
[5]. Kim, H., Oh, J.E., Kang, T.W. (2001) Preparation of large area free-standing GaN substrates by HVPE using mechanical polishing liftoff method. Materials Letters, 4: 276-280.
[6]. Yoshida, T., Imanishi, M., Kitamura, T., et al. (2017), Development of GaN substrate with a large diameter and small orientation deviation. Phys. Status Solidi B, 254: 1600671.
[7]. Shimizu, A., Kitamoto, A., Kamiyama, M., et al. (2022) Effect of additional N2O gas on the suppression of polycrystal formation and high-rate GaN crystal growth by OVPE method. Journal of Crystal Growth, 581: 126495.
[8]. He, X., Zang, C., Zhou, H., et al. (2013) Advances in ammonia-thermal growth of gallium nitride crystals. Journal of Artificial Crystallography, 42(7): 1293-1298.
[9]. Ren, G., Liu, Z., Li, T., et al. (2020) Liquid-phase growth of gallium nitride single crystals. Journal of Artificial Crystallography, 49(11): 2024.
[10]. Purdy, Andrew, P. (1999) Ammonothermal Synthesis of Cubic Gallium Nitride. Chemistry of Materials, 11: 1648-1651
[11]. Ehrentraut, D., Pakalapati, R.T., Kamber, D.S., et al. (2013) High-quality, low-cost ammonothermal bulk GaN substrates. Japanese Journal of Applied Physics, 52(8S): 08JA01.
[12]. Masanori, M., Fumio, K., Minoru, K., et al. (2005) Promoted nitrogen dissolution due to the addition of Li or Ca to Ga-Na melt; some effects of additives on the growth of GaN single crystals using the sodium flux method. Journal of Crystal Growth, 284: 91-99.
Cite this article
Zhao,Y. (2023). Research on the status of GaN single crystal growth. Applied and Computational Engineering,26,28-34.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2023 International Conference on Functional Materials and Civil Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Ren, G., Wang, J., Liu, Z., et al. (2019) Advances in single crystal growth of gallium nitride. Journal of Artificial Crystals, 48(09):1588-1598.
[2]. Utsumi, W., Saitoh, H., Kaneko, H., et al. (2003) Congruent melting of gallium nitride at 6 GPa and its application to single-crystal growth. Nature Mater, 2: 735–738.
[3]. Jun, H., Hongyuan W, Shaoyan Y, et al. (2019) Hydride vapor phase epitaxy for gallium nitride substrate. Journal of Semiconductors, 40(10): 101801
[4]. Boćkowski, M., I. Grzegory, (2022) Recent Progress in Crystal Growth of Bulk GaN. Acta Physica Polonica, A (141): 3.
[5]. Kim, H., Oh, J.E., Kang, T.W. (2001) Preparation of large area free-standing GaN substrates by HVPE using mechanical polishing liftoff method. Materials Letters, 4: 276-280.
[6]. Yoshida, T., Imanishi, M., Kitamura, T., et al. (2017), Development of GaN substrate with a large diameter and small orientation deviation. Phys. Status Solidi B, 254: 1600671.
[7]. Shimizu, A., Kitamoto, A., Kamiyama, M., et al. (2022) Effect of additional N2O gas on the suppression of polycrystal formation and high-rate GaN crystal growth by OVPE method. Journal of Crystal Growth, 581: 126495.
[8]. He, X., Zang, C., Zhou, H., et al. (2013) Advances in ammonia-thermal growth of gallium nitride crystals. Journal of Artificial Crystallography, 42(7): 1293-1298.
[9]. Ren, G., Liu, Z., Li, T., et al. (2020) Liquid-phase growth of gallium nitride single crystals. Journal of Artificial Crystallography, 49(11): 2024.
[10]. Purdy, Andrew, P. (1999) Ammonothermal Synthesis of Cubic Gallium Nitride. Chemistry of Materials, 11: 1648-1651
[11]. Ehrentraut, D., Pakalapati, R.T., Kamber, D.S., et al. (2013) High-quality, low-cost ammonothermal bulk GaN substrates. Japanese Journal of Applied Physics, 52(8S): 08JA01.
[12]. Masanori, M., Fumio, K., Minoru, K., et al. (2005) Promoted nitrogen dissolution due to the addition of Li or Ca to Ga-Na melt; some effects of additives on the growth of GaN single crystals using the sodium flux method. Journal of Crystal Growth, 284: 91-99.