1. Introduction
Solid dosage is one of the most common dosage forms in the pharmaceutical market, and there are plenty of industrial manufacturing methods associated with that. Dry granulation, also known as DG, is a major granule-forming method and is playing an important role in increasing tablet producing efficiency. Roll compaction, also known as RC, is one process in DG that has been deeply researched for nearly three decades. In this process, drug powder and API substances are filled into a pair of rolling compactors, and comparatively loose ribbons of medicine substances are then formed. These ribbons are rather soft and will be broken again into tablet-sized granules and compacted again into tablet form. Some benefits can be found compared to another widely used tablet manufacturing method, such as high producing stability and low raw material wasting. These advantages make DG widely welcomed, but certain disadvantages still exist during the process of research and industrial application, these can be the major challenges of the development of RC, and researchers are still working on them to make RC a better processing method. What challenges RC is facing and what is the reason? In what aspects improvements have already been done? Can we improve it more? This research solves these questions by reviewing research that had already been done to find out in what aspects RC can still be improved to make it more effective, acceptable, and competitive.
2. Challenges
Compared to wet granulation, the number and variety of progress DG obtain is comparatively low, and certain issues still exist after modification [1]. Most of the modifications to RC in the 2000s to 2010s focused on removing fine particles to increase processing flowability, such as adding a sorting chamber or wind-blowing system or trying to reduce fines by improving feeder. These modifications are sometimes inadequate because they leave some old issues unsolved: According to Shanmugam et al., recycling fine particles for granule formation brings a negative influence on granule quality, because the physical property of recycling material is different from the ribbon: a ribbon that is heterogeneous in physical property has a higher risk of segregation [1]. What’s more, the fine particle in the fraction device that is produced by the mill is not the only part of the raw material that is not properly used, the compression system, or the roller, will produce a certain amount of raw material uncompressed into the ribbon as well, and this phenomenon is inevitable. This means unless there is an evolutionary invention that modifies the structure of the roller, this issue will never be solved. According to Clarke et. al., there was still over a third of the material has not been compacted into granules even though the force between rollers is so great that these materials are already over-compacted and cannot be used for secondary compression in tablet forming: according to figure 1 and 2, there is always a 40%-45% fine particles present no matter how roller gap changes [2].
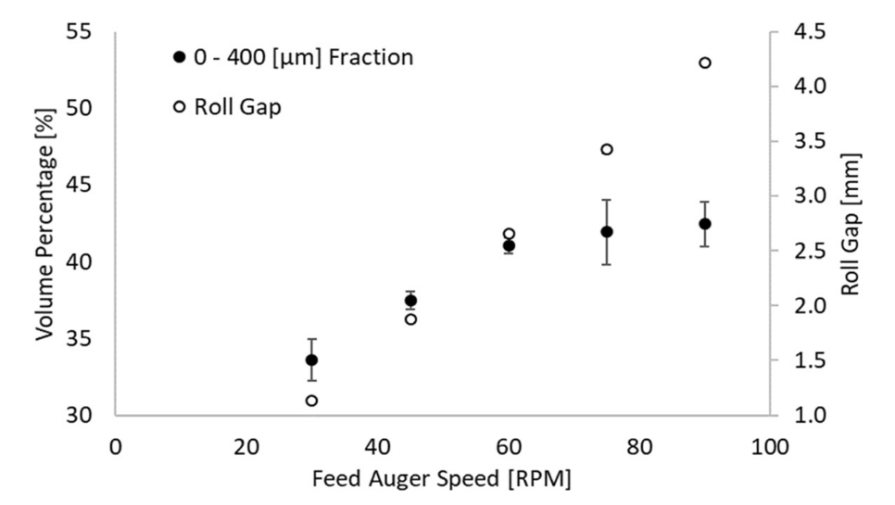
Figure 1. Adapted from Clarke et al., the relationship between roll gap (pression force) and fraction volume [2].
The mass of un-compacted matter also increases significantly as the feeding auger and roller speed increase to enlarge producing rate, and these fragments cannot be used in tablet forming. The underlying reason is that the decreasing dwelling time of substances in the roller makes the compression force less likely to be applied to substances, and they are less likely to be fully compressed (fig. 3). The only method to reduce this issue in industrial production is modifying these two factors according to statistical data to magnify the production rate while reducing fragment amounts as much as possible.
.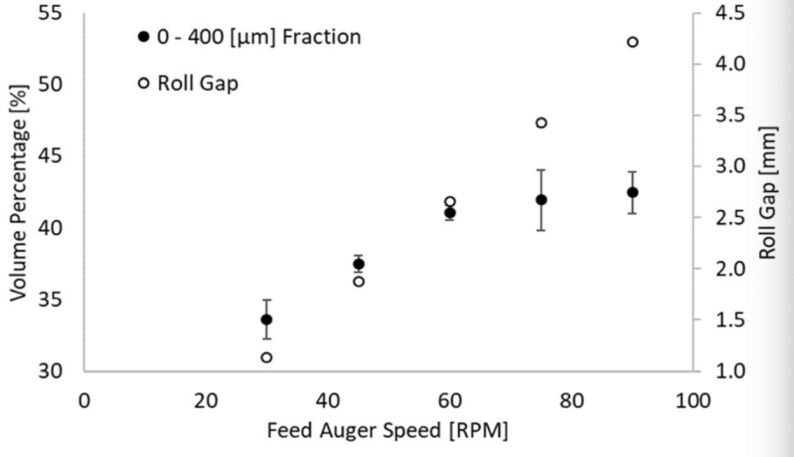
Figure 2. Adapted from Clarke et al., the relationship between feed auger speed and fraction percentage [2].
According to Taipale et. al., the outcomes of DG and high-shear wet granulation, HSWG, are compared when working on the same type of drug that certain ingredients are modified to fit both two manufacturing methods. According to the data collected, a greater compression force is required in the DG process when forming tablets, and the tablet’s breaking force that processed with DG is lower: this means tablets are more fragile. Researchers believe granules may have lost part of their compact property before the tableting process, which means the ribbon is overly compacted in the RC process and make the structure unstable. Although the same type of tablet can be produced with a greater flow and efficiency, it also gains certain short come in physical properties [3]. Another issue that hinders the application of RC is the splitting and sticking of the ribbon. According to Mahmah et. al.’s research, two splitting patterns are most common among all situations: transversally (through the ribbon thickness) or longitudinally (through the ribbon width) [4]. Transversal splitting is always associated with the lack of lubrication, but longitudinal splitting is far more complex: it is associated with the uneven distribution of ribbon density and the yield strength of the ribbon. Physical analysis is also involved in the research, and the conclusion is complex: increasing yield strength enlarges the elastic recovery of the ribbon and makes it more likely to break, since the ribbons are more intended to recover to their original state without bending. The most applicable method to control the yield strength is manipulating the pressure between the rollers: the lighter the force becomes, the less yield strength ribbons get, but a weak yield strength also leads to the breaking of ribbon in certain cases. The relationship between the maximum pressure and roller speed on material with high moisture is reported by Wu et. al., and another limit of RC is also revealed: RC has a low tolerance to water, and too much moisture in the material will lead to the failure of ribbon formation. Wu’s research focuses on the effect of moisture of MCC material (microcrystalline cellulose) on the RC behavior and ribbon shape [5]. According to figure 3, when the mass of water in the material exceeds the 10% of the total mass, maximum pressure increases dramatically when the speed of roller decreases, and the distribution of pressure on ribbon is uneven: center part bares much more pressure than edge part, and when moisture approaches 9.72% the powerful pressure may increase the temperature and change the color of material at center part. Such unevenness has been reported in other studies under drier conditions, which is similar but has a smaller impact. When the mass of water exceeds 10% of the total ribbon mass, the ribbon tends to split into two halves. It is believed that due to the uneven feeder pattern, more water is gathered at the center part of the ribbon and decreasing the overall tensile strength. It is well-known that DG needs very little water to process normally, however, too much water will also hinder the process, which means DG is still not able to process the raw material with high moisture requirement and cannot replace other processing methods so far.
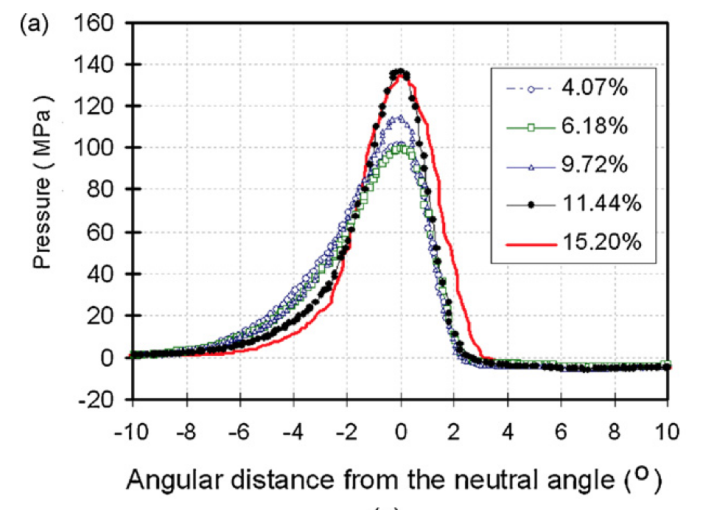
Figure 3. Adapted from Wu et. al., horizontal pressure distribution on the ribbon when rotation speed equals 1 rpm [5].
3. Improvement
The overall benefits of RC and DG have been concluded in multiple articles and textbooks [6]. Compared to other widely adapted methods such as wet granulation or direct compression, RC requires less water use, brings less pollution, improves the uniformity of granules, and is easier to be monitored [7][8]. These benefits make RC an outstanding manufacturing method and all risks that take place are worth to be solved and making RC a better one. Articles in recent years also state the progress that RC newly obtains, and these improvements stabilize the advantages, and somehow reduce the existing issues.
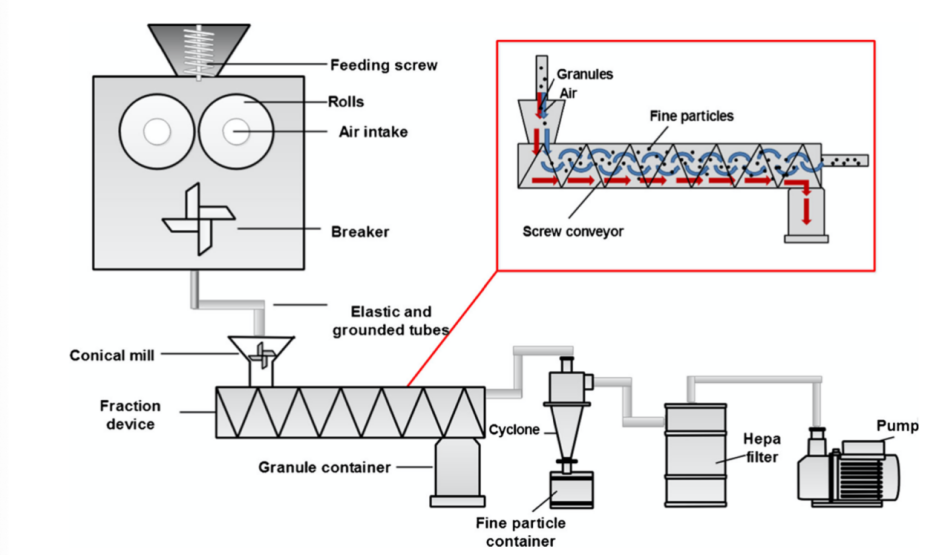
Figure 4. Adapted from Saarinen et al., the structure of GARC [9].
One of the most significant issues about RC is the problem of fine particles. According to Bacher et al., the fine particles in RC are particles whose diameter is less than 125 m [9]. Fine particles are not favored in RC because they have strong surface charge density and will stick everywhere either in the machine or on granules and decrease the flowability. To solve this issue, certain sorting methods are invented. According to Saarinen et. al., a modified RC method called Gas-assisted Roller Compaction (GARC) is involved in their research, show as figure 4. In this system, drug ribbon is broken into granules and then transported into a fraction device, which is the “sorting chamber” of granules and fine particles. A high-velocity air flow continuously blows through the system so that the fine particles can be removed and collected. Fine particle is not favored in RC because they have strong surface charge density and will stick everywhere either in the machine or on granules. Compared to conventional roller compaction, this modification brings benefits: 1, the particle size of the granule got stabilized in all three different conical mill sieve sizes according to several mathematical modules, 2, the flowability of the system increases because fewer fine particles are stuck on granules [9]. Pneumatic dry granulation, PDG, created by Atacama LabsOy is another significant invention for DG in recent years [10]. PDG is a fragment-collecting mechanism, and the principle of this modification is rather simple: after the raw material was compressed by the roller, the ribbon and fragments are transported into the classification chamber and separated by an additional pneumatic system by blowing air into the chamber. Fragments can be collected and reloaded into the roller system. According to Shanmugam, this modification increases the amount of drug loading, the stability of production, the purity of tablets, and decreases the raw material waste and the cost [1]. The modification mentioned above improves the benefits within the ribbon production that had already existed to a more extreme level, such as improving the stability and flowability of this process, however, as mentioned before, sorting fine fragments, and reusing them for ribbon forming make tablets and ribbon less stable, so a more comprehensive improvement is highly favored. In 2017, research done by Mingzhe et. al. focuses on the roller and feeder itself: Shown in figure 5, this recent unprecedented modification optimizes the physical structure of medicine ribbon greatly with a special designed feeding guider and make the ribbons less likely to split and produce less fragment in processing [12].
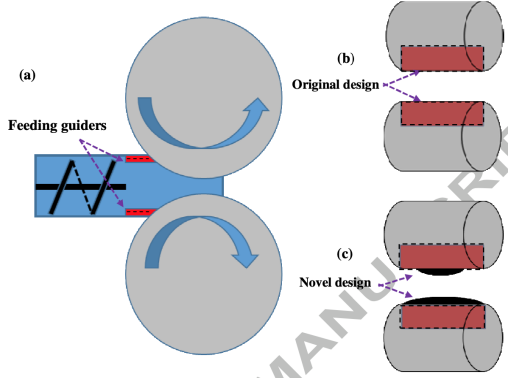
Figure 5. adapted from Mingzhe et al., newly designed feeding guiders [12].
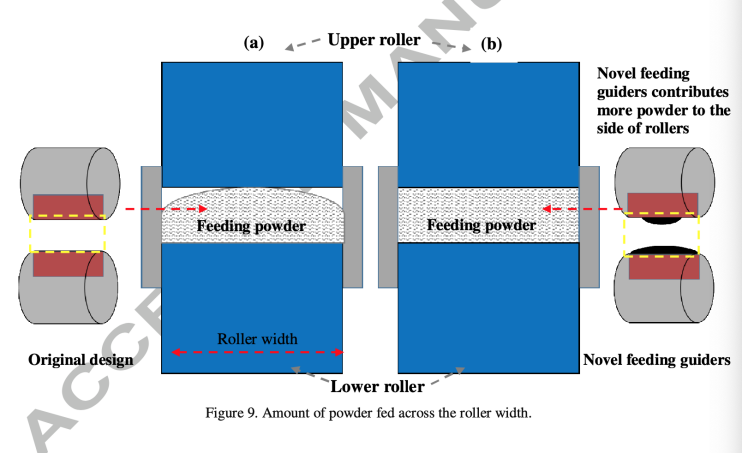
Figure 6. adapted from Mingzhe et al., how feeding guider help fill up the gap between rollers [12].
In this research, as shown in figure 6, the shape of feeding guiders in the front of rollers were modified from flat surface to concave ones with 3mm thickness. Several different designs on guider width were applied and the overall condition of ribbon reaches maximum when the length of upper guider equals 0.5 times of length of the roller and lower guider equals 1 times length of roller, shown in figure 7.
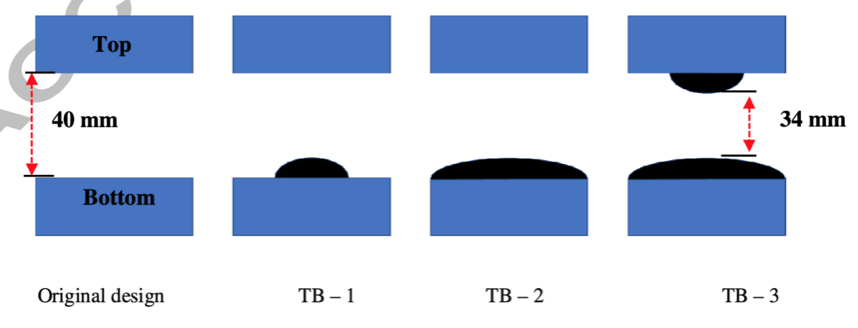
Figure 7. adapted from Mingzhe et al, different shape design of feeder.
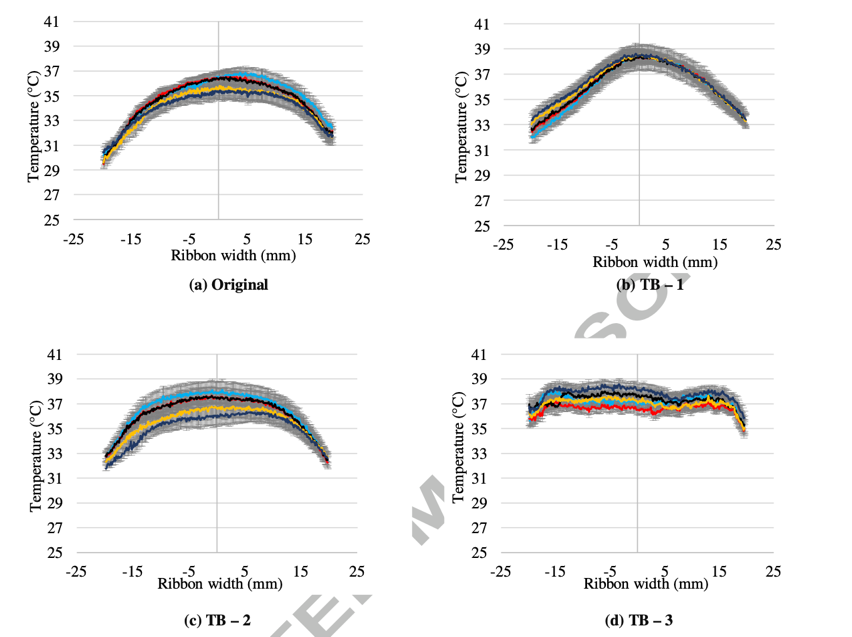
Figure 8. adapted from Mingzhe et al., pressure distribution presented by temperature of MCC PH 101 material ribbons under different feeder shape. TB-3 is the one finally adapted [12].
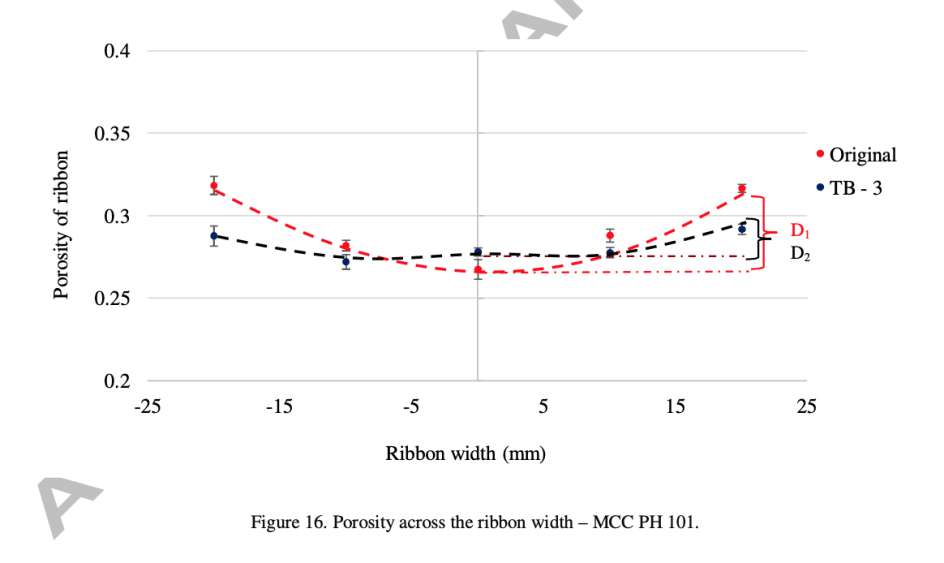
Figure 9. Adapted from Mingzhe et al., porosity distribution of MCC PH 101 ribbon under original feeder and TB-3 feeder [12].
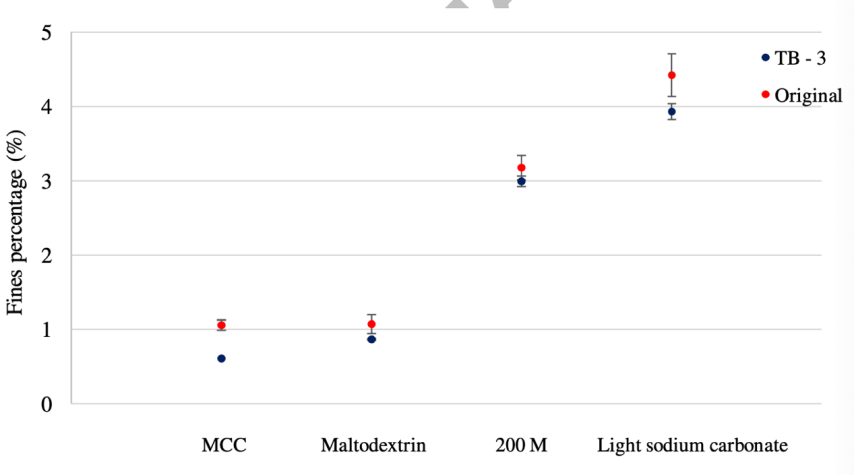
Figure 10. Adapted from Mingzhe et al., fragment percentage for re-designed feeder and original feeder [12].
These special feeders help to fill the gap between the feeders more evenly with raw material, so that the ribbon is also evenly compacted. For most materials in the research, both the horizontal temperature distribution, which represents the pressure distribution (figure 8), and the porosity of the ribbon become more evenly distributed (figure 9). As a result, the physical property of the ribbon is more stable and tensile strength of the ribbon increases significantly, and less fragment is produced, according to figure 10. This progression brings three positive results: the ribbon is less likely to split from weak locations, less fine fragments formed in breaking process, which also reduces the raw material wasting, and tablet more stable and less likely to break. This modification seems reduced most of the limitations of RC greatly.
4. Discussion
According to the research done in the past two decades, much less improvements modified DG than other processing methods such as wet granulation probably because DG is less complex in machine structure and simpler in tablet formation steps. Some scholars think that the lack of the amount of modification hindered the development of DG and leave the limitations unsolved. The main limitation of DG are the ribbon segregation, fragment formation, and fragile tablet. Although some modifications were pointed out and somewhat solved the fragment issue, the main causing factor of all three limitations above was not eliminated until recent time: A newly designed feeder make the physical structure of ribbon more stable and solved all these limitations greatly.
5. Conclusion
Compared to other manufacturing processes, DG and RC have their benefits including high production flow, low material waste, environmentally friendly, etc. These benefits make DG a unique and indispensable processing method and modifications need to be come up with to reduce its limitations, such as ribbon splitting and segregation, fragmentation, and fragile tablets. Although the underlying reason was discovered, the most successful solving method was not pointed out until recent years. Some modifications such as adding fine particle sorting system made limited improvements, and they also brought other issues such as uneven physical property of ribbons when recycling the fragments and make tablets easier to break. One of the most significant improvements in recent years is that by modifying the feeder structure, the raw material is more evenly distributed between the rollers and make the ribbon more stable and even in physical properties. By increasing tensile strength, this modification greatly increases ribbon durability, reduces the likelihood of ribbon segregation, and reduces the amount of fragment. This improvement is regarded as a novel design that solved a long-term-existed issue. However, the low durability to high moisture is still a major issue for DG, because uneven material distribution may still result in uneven pressure distribution and ruins the structure of the ribbon in traditional rollers. Further research and modification are yet to be conducted with modified DG and high-moisture material, and such research is necessary to make DG and RC a wider-applicable and effective method of manufacturing.
References
[1]. Shanmugam, BioImpacts, 5(1), 55-63.(2015) doi: 10.15171/bi.2015.04
[2]. Clarke, J., Gamble, J.F., Jones, J.W. et al. Determining the Impact of RC Processing Conditions on Granule and API Properties. AAPS PharmSciTech 21, 218 (2020). https://doi.org/10.1208/s12249-020-01773-2
[3]. Krista Taipale-Kovalainen, Jarkko Ketolainen, Ossi Korhonen, lation process; a demonstration with ketoprofen tablets, European Journal of Pharmaceutical Sciences (2020), doi: https://doi.org/10.1016/j.ejps.2020.105381
[4]. O. Mahmah, M.J. Adams, C. S.Omar, B. Gururajan, A.D. Salman, RC: Ribbon splitting and sticking, International Journal of Pharmaceutics (2019), doi: https://doi.org/10.1016/j.ijpharm. 2019.01.031
[5]. Wu, C.Y., Hung, W.L., Miguélez-Morán, A.M., Gururajan, B., Seville, J.P.K., 2010. RC of moist pharmaceutical powders. Int. J. Pharm. 391, 90–97. https://doi.org/10.1016/j.ijpharm.2010.02.022
[6]. Niklas Sandler & Robert Frank Lammens. Pneumatic DG: potential to improve RC technology in drug manufacture, Expert Opinion on Drug Delivery, 8:2, 225-236. (2011) DOI: 10.1517/17425247.2011.548382
[7]. Miller R, Sheskey PJ. RC technology for the pharmaceutical industry. In: Parikh DM, editor, Handbook of pharmaceutical granulation technology. Marcel Dekker, New York; 2006. p. 3159-76
[8]. Sun, Wei-Jhe, Sun, Changquan Calvin, Ribbon thickness influences fine generation during dry granulation.International Journal of Pharmaceutics http://dx.doi.org/10.1016/j.ijpharm.2017.06.038
[9]. Saarinen, T., Antikainen, O. & Yliruusi, J. Simultaneous Comparison of Two RC Techniques and Two Particle Size Analysis Methods. AAPS PharmSciTech 18, 3198–3207 (2017). https://doi.org/10.1208/s12249-017-0778-1
[10]. Politi G, Heilakka E. Method and apparatus for DG. Google Patents; 2009.
[11]. Bacher, C., Olsen, P.M., Bertelsen, P., Kristensen, J., Sonnergaard, J.M., 2007. Improving the compaction properties of roller compacted calcium carbonate. International Journal of Pharmaceutics 342, 115- 123.
[12]. M. Yu, C. Omar, A. Schmidt, J.D. Litster, A.D. Salman, Improving feeding powder distribution to the compaction zone in the roller compaction, European Journal of Pharmaceutics and Biopharmaceutics (2018), doi: https://doi.org/10.1016/j.ejpb.2018.04.015
Cite this article
Shen,S. (2023). Research on the challenges and improvements of the roller compaction process for dry granulation. Applied and Computational Engineering,7,677-684.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Materials Chemistry and Environmental Engineering (CONF-MCEE 2023), Part II
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Shanmugam, BioImpacts, 5(1), 55-63.(2015) doi: 10.15171/bi.2015.04
[2]. Clarke, J., Gamble, J.F., Jones, J.W. et al. Determining the Impact of RC Processing Conditions on Granule and API Properties. AAPS PharmSciTech 21, 218 (2020). https://doi.org/10.1208/s12249-020-01773-2
[3]. Krista Taipale-Kovalainen, Jarkko Ketolainen, Ossi Korhonen, lation process; a demonstration with ketoprofen tablets, European Journal of Pharmaceutical Sciences (2020), doi: https://doi.org/10.1016/j.ejps.2020.105381
[4]. O. Mahmah, M.J. Adams, C. S.Omar, B. Gururajan, A.D. Salman, RC: Ribbon splitting and sticking, International Journal of Pharmaceutics (2019), doi: https://doi.org/10.1016/j.ijpharm. 2019.01.031
[5]. Wu, C.Y., Hung, W.L., Miguélez-Morán, A.M., Gururajan, B., Seville, J.P.K., 2010. RC of moist pharmaceutical powders. Int. J. Pharm. 391, 90–97. https://doi.org/10.1016/j.ijpharm.2010.02.022
[6]. Niklas Sandler & Robert Frank Lammens. Pneumatic DG: potential to improve RC technology in drug manufacture, Expert Opinion on Drug Delivery, 8:2, 225-236. (2011) DOI: 10.1517/17425247.2011.548382
[7]. Miller R, Sheskey PJ. RC technology for the pharmaceutical industry. In: Parikh DM, editor, Handbook of pharmaceutical granulation technology. Marcel Dekker, New York; 2006. p. 3159-76
[8]. Sun, Wei-Jhe, Sun, Changquan Calvin, Ribbon thickness influences fine generation during dry granulation.International Journal of Pharmaceutics http://dx.doi.org/10.1016/j.ijpharm.2017.06.038
[9]. Saarinen, T., Antikainen, O. & Yliruusi, J. Simultaneous Comparison of Two RC Techniques and Two Particle Size Analysis Methods. AAPS PharmSciTech 18, 3198–3207 (2017). https://doi.org/10.1208/s12249-017-0778-1
[10]. Politi G, Heilakka E. Method and apparatus for DG. Google Patents; 2009.
[11]. Bacher, C., Olsen, P.M., Bertelsen, P., Kristensen, J., Sonnergaard, J.M., 2007. Improving the compaction properties of roller compacted calcium carbonate. International Journal of Pharmaceutics 342, 115- 123.
[12]. M. Yu, C. Omar, A. Schmidt, J.D. Litster, A.D. Salman, Improving feeding powder distribution to the compaction zone in the roller compaction, European Journal of Pharmaceutics and Biopharmaceutics (2018), doi: https://doi.org/10.1016/j.ejpb.2018.04.015









