1. Introduction
Energy and environmental issues are becoming increasingly severe. Humanity is paying more attention to environmental pollution and global warming, with a widespread realization that energy shortages and environmental pollution are critical constraints on sustainable social development and improvements in living standards. The consumption of traditional fossil fuels, such as petroleum, coal, and methane, continues to rise. However, the combustion of these fuels not only releases harmful gases containing elements like C, N, and S into the air but also contributes to global warming. Therefore, to prevent further deterioration of energy shortages and environmental problems, the global consensus is to replace traditional fossil fuels with efficient, green, and renewable energy sources.
New energy sources include solar, hydro, nuclear, wind, hydrogen, geothermal, biomass, and tidal energy. Among these, hydrogen energy is widely regarded as the best solution to address global environmental pollution, energy shortages, and climate change due to its high combustion heat value (1 kg of hydrogen produces 62.8 kJ of energy), high energy density, ease of storage, and clean combustion product (H₂O) [1]. Currently, the main hydrogen production methods worldwide include hydrogen production from fossil fuels, water electrolysis, biological hydrogen production, and solar hydrogen production. Solar hydrogen production, in particular, has garnered attention for its clean, environmentally friendly, and energy-saving advantages. Since solar energy is the most abundant and renewable resource on Earth, it allows for continuous photocatalytic hydrogen production. Photocatalytic water splitting for hydrogen production has received widespread global attention. By combining solar energy with photocatalysts, hydrogen can be efficiently produced in a simple operation. In 1972, Japanese researchers Fujishima and Honda published an article in Nature, demonstrating for the first time that hydrogen could be successfully produced by photocatalytic water splitting using a titanium dioxide single crystal as a photoelectrode under ultraviolet light. This discovery marked the beginning of the era of semiconductor photocatalytic hydrogen production.
The majority of the energy in sunlight is distributed in the visible light spectrum. However, most semiconductors exhibit photocatalytic activity only under ultraviolet light due to their band structure, resulting in low energy utilization efficiency. To enhance the efficiency of solar energy utilization, it is necessary to expand the light absorption range of semiconductors, improve photocatalytic activity, and fully harness the energy in the visible spectrum. Modifications are required to improve the visible light response of semiconductors, including methods such as metal/non-metal deposition, solid-solution treatment, dye sensitization, and the development of novel single-phase visible-light-responsive semiconductor materials. Among these methods, dye sensitization stands out due to its simplicity, low cost, abundant raw materials, and strong ability to harness sunlight. Additionally, dyes possess advantages such as high stability, low price, and environmental friendliness, making them widely applicable in various photocatalytic reaction systems [2].
2. Overview of hydrogen technology
Hydrogen, as a novel energy source, boasts significant advantages such as a high combustion heat value (62.8 kJ per kilogram of hydrogen), high energy density, easy storage, and the production of a single, non-polluting combustion byproduct, H₂O. These features make hydrogen highly promising for global development and application, earning the favor of governments worldwide. Various policies have been introduced to continuously improve hydrogen energy infrastructure and promote its development.
2.1. Hydrogen production technologies
2.1.1. Hydrogen production from fossil fuels
Currently, the majority of global hydrogen production relies on fossil fuels. Although this method is relatively mature, its limitations, including finite resources and environmental pollution, restrict further development. The primary fossil fuel-based hydrogen production technologies include coal gasification and natural gas reforming.
Coal Gasification: This process involves burning coal at high temperatures to produce CO and H₂. While the traditional coal gasification process has advantages such as low production costs and technological maturity, it suffers from drawbacks like operational complexity and low hydrogen production efficiency. To address these issues, innovative concepts and technologies are being developed. For instance, Japan has introduced the Hypr-RING method, which uses material cycles of H₂O-H₂-H₂O and CaO-CaCO₃-CaO to achieve direct coal-to-hydrogen power generation and CO₂ emission control. Similarly, Xi’an Jiaotong University in China has utilized the unique physicochemical properties of water in a supercritical state to convert elements such as C and H in coal into H₂ and CO₂. This approach not only efficiently converts the chemical energy in coal into hydrogen energy but also significantly reduces emissions of oxides, sulfides, and particulate matter.
Natural Gas Reforming: Due to its high C-to-H ratio, natural gas is a superior material for hydrogen production. The process primarily involves two steps. First, steam reacts with natural gas at high temperatures to produce CO and H₂. Second, a shift reactor converts CO into CO₂ and additional H₂.
2.1.2. Hydrogen production via water electrolysis
The majority of hydrogen atoms globally exist in water. In 1800, Nicholson and Carlisle first confirmed that water electrolysis produces O₂ and H₂. The principle of water electrolysis for hydrogen production involves decomposing water into O₂ and H₂ using voltage across electrodes, with the assistance of catalysts [3]. Based on the type of electrolyzer, this technology can be classified into alkaline water electrolysis, proton exchange membrane (PEM) water electrolysis, and solid oxide water electrolysis.
2.1.3. Biological hydrogen production
Biological hydrogen production involves treating biomass through various pretreatment methods and then producing hydrogen via gasification or microbial catalytic deoxygenation [4]. The two main approaches to biological hydrogen production are biological methods and thermochemical methods. Compared to thermochemical methods, biological methods operate under milder conditions, which results in lower hydrogen production efficiency. Both approaches produce methane and CO, which can then undergo steam reforming to generate additional hydrogen.
2.1.4. Solar hydrogen production
Solar hydrogen production can be divided into thermochemical water splitting, photocatalytic water splitting, and photolysis. Among these, photolysis is the most cost-effective and efficient solar hydrogen production technology. The process involves semiconductor materials absorbing solar energy in an electrolytic environment to produce hydrogen and oxygen. Although this technology is still in its early stages, its excellent economic and production efficiency has attracted worldwide attention and research. It is expected to become a major hydrogen production technology in the future.
Table 1. Characteristics of Hydrogen Production Technology
Hydrogen production technology |
Advantage |
Disadvantage |
Hydrogen production technology from fossil fuels |
Low production cost and mature technology |
Pollution of the environment and limited energy |
Hydrogen production technology through electrolysis of water |
Clean and environmentally friendly |
High production costs and poor stability |
Biohydrogen production technology |
Renewable and low production cost |
Low energy conversion efficiency |
Hydrogen production from solar energy |
Clean and environmentally friendly, with abundant resources |
Immature technology and poor stability |
2.2. Applications of hydrogen
Hydrogen, as a clean and renewable energy source, serves as an essential raw material in industrial production. At present, it has been widely applied worldwide. Hydrogen finds extensive applications in fields such as low-carbon metallurgy, new energy vehicles, biomedicine, and the preparation of high-purity materials.
Table 2. Application Fields of Hydrogen
Application areas of hydrogen gas |
Specific applications |
Low carbon metallurgy field |
Hydrogen direct reduction ironmaking, hydrogen melting reduction ironmaking, etc |
In the field of new energy vehicles |
Hydrogen fuel cell vehicles, etc |
Biomedical field |
Improve safety and stability, treat diseases, etc |
High purity material preparation field |
Preparation of high-purity and small-sized nanoscale tungsten powder, etc |
3. Overview of Dye-Sensitized technology
3.1. Principle of Dye sensitization
The photocatalytic water splitting process via dye sensitization can be divided into four steps: First, after absorbing energy from solar radiation, the valence electrons in the HOMO orbital of the dye molecules transfer to the LUMO orbital, forming excited-state electrons (dye*). Second, a portion of the excited-state electrons (dye*) transfers from the LUMO orbital to the conduction band (CB) of the semiconductor, while another portion transfers to the surface of the dye molecules and recombines with the holes in the HOMO orbital. Third, some of the excited-state electrons (dye*) in the conduction band (CB) of the semiconductor transfer to the surface of the dye molecules and recombine with the holes in the HOMO orbital, while another portion transfers to the cocatalyst. Fourth, the excited-state electrons (dye*) on the cocatalyst transfer to its surface, reducing hydrogen atoms to generate hydrogen gas. Simultaneously, the excited-state electrons (dye*) return to the ground state under the action of a sacrificial agent.
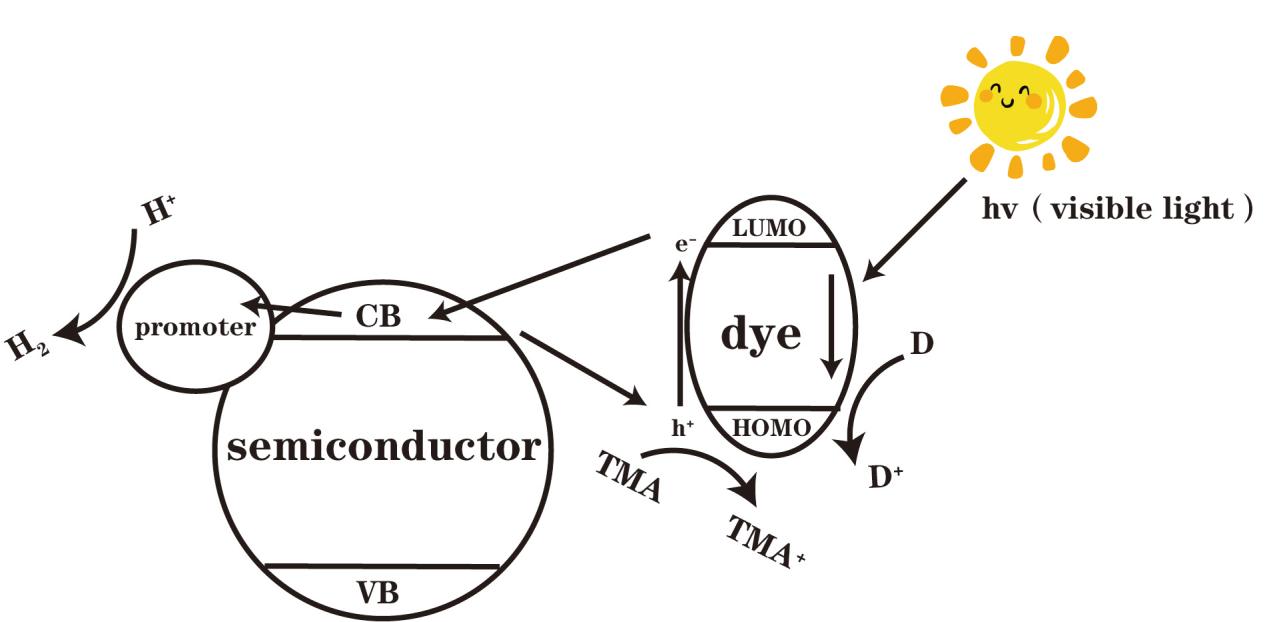
Figure 1. Principle of Dye-Sensitized Photocatalytic Hydrogen Production
3.2. Dye-Sensitized photocatalytic systems
The main components of a dye-sensitized photocatalytic system include semiconductor photocatalysts, dye sensitizers, and sacrificial agents. To enhance photocatalytic performance, cocatalysts are sometimes added. The effects of these four main components and some external factors on photocatalytic efficiency are analyzed below.
3.2.1. Semiconductor photocatalysts
Semiconductor photocatalysts can be classified into the following categories: single semiconductors (e.g., titanium dioxide, zinc oxide, tin oxide, tungsten oxide, cadmium sulfide), composite semiconductors (e.g., TiO₂/WO₃, TiO₂/SnO₂, TiO₂/CdS), metal-doped semiconductors (e.g., Ag, Au, Pt), and semiconductors doped with transition metals and their oxides (e.g., oxynitrides, bismuth oxides, indium sulfides). Semiconductor photocatalysts serve as substrate materials in dye-sensitized photocatalytic systems. Currently, titanium dioxide (TiO₂) is the most extensively studied semiconductor in the world. However, in recent years, carbon nanomaterials have gradually become a new research focus. Carbon nanomaterials, such as graphene and carbon nanotubes, have been increasingly applied to various dye-sensitized photocatalytic systems due to their excellent electrical conductivity and large specific surface area.
3.2.2. Dye sensitizers
Dye sensitizers are generally divided into two categories based on their structures: organic dyes and inorganic dyes. Inorganic dyes mainly include ruthenium- and osmium-based porphyrin derivatives, metalloporphyrins, phthalocyanine dyes, metal oxides (e.g., ZnO, TiO₂), sulfides (e.g., CdS, ZnS), solid solutions (e.g., ZnxInySz), composite metal oxides (e.g., MgxTi₁-xO₂), and polypyridine ruthenium complexes. Organic dyes primarily include metalloporphyrin compounds [5], metal phthalocyanine compounds, xanthenes (e.g., eosin (EY), fluorescein (FL), rhodamine (RHB)), coumarins, perylene dyes, anthocyanins, chlorophyll, and quinolines. Dye sensitizers act as solar energy receptors in dye-sensitized photocatalytic systems, capturing as much solar energy as possible and efficiently transferring electrons to the conduction band of semiconductors. This makes them the most critical component in dye-sensitized photocatalytic systems.
Due to the excellent performance of eosin (EY), it has been widely applied in photocatalytic systems. For instance, Du et al. used eosin (EY) as the dye sensitizer and triethanolamine (TEOA) as the sacrificial agent, achieving an optimal H₂ production rate of 15.76 μmol h⁻¹, which is 90 times higher than that of a pure TiO₂ system.
3.2.3. Sacrificial agents
Sacrificial agents are generally classified into three categories: amines, alcohols, and other electron donors. Sacrificial agents play a role in reducing excited-state electrons (dye*) in dye-sensitized photocatalytic systems, which is critical for sustaining water-splitting reactions. Commonly used sacrificial agents include I⁻, triethanolamine (TEOA), ethylenediaminetetraacetic acid (EDTA), acetonitrile, and methanol (CH₃OH). Fe²⁺ is also often used as an electron donor in dye-sensitized solar cells.
Japanese researcher R. Abe and colleagues prepared hydrogen using TiO₂ as the semiconductor photocatalyst, eosin (EY) as the dye sensitizer, and TEOA as the sacrificial agent. They also found that under the same conditions, methanol and ethanol (CH₃CH₂OH) as sacrificial agents also produced hydrogen. Peng T. et al. from Wuhan University used TiO₂ as the semiconductor photocatalyst and various dye sensitizers, including N719, Ru₂(bpy)₄L₁-PF₆, and Ru(bpy)₂(him)₂-NO₃, with methanol as the sacrificial agent, and observed hydrogen production in all cases. However, Japanese scholar K. Hirano reported that when using methanol as the sacrificial agent and Ru(bpy)₃²⁺ as the dye sensitizer, no hydrogen was produced. This indicates that not all dye sensitizers can facilitate photocatalytic water-splitting for hydrogen production in the same sacrificial agent solution.
Li et al. used TiO₂ as the semiconductor photocatalyst and a porphyrin derivative, tetra-(4-carboxyphenyl) porphyrin (TCPP), as the dye sensitizer. They tested four structurally similar alcohols (methanol, ethanol, n-propanol, and isopropanol) as sacrificial agents under different pH conditions to analyze their photocatalytic efficiency. The results showed that hydrogen production was lower under alkaline conditions when alcohols were used as sacrificial agents. Maximum hydrogen production was observed at pH 7 or pH 5 (for ethanol). This may be attributed to factors such as high dye desorption rates, low dye adsorption, decreased semiconductor conduction band potential, and reduced electron injection efficiency under alkaline conditions, leading to lower hydrogen production. Thus, in dye-sensitized photocatalytic systems using alcohols as sacrificial agents, photocatalytic activity is lower under alkaline conditions.
3.2.4. Cocatalysts
Cocatalysts include transition metals, non-metal oxides, and noble metals (e.g., Pt, Ag). Cocatalysts in dye-sensitized photocatalytic systems facilitate electron separation, thereby enhancing photocatalytic activity. Currently, noble metals such as Pt, Rh, Au, and Ag are widely used as cocatalysts to improve photocatalytic efficiency. However, their low abundance, high cost, and toxicity limit large-scale applications. As a result, abundant, inexpensive, and non-toxic cocatalysts have attracted increasing attention. Transition metal-based cocatalysts, such as phosphides, carbides, sulfides, hydroxides, g-C₃N₄, borides, and selenides, have been extensively studied.
Yang et al. achieved a hydrogen production activity of 144.8 mmol h⁻¹ g⁻¹ using boride NiCoB as the cocatalyst, which was significantly higher than the 36.56 mmol h⁻¹ g⁻¹ achieved with noble metal Pt as the cocatalyst. Therefore, NiCoB demonstrates superior hydrogen production performance compared to noble metals.
Yang also tested the photocatalytic hydrogen production activity using transition metal-coated Fe-B alloys (Fe-B@Ni and Fe-B@Co) as cocatalysts, with trimethylamine as the sacrificial agent and eosin (EY) as the dye sensitizer. The results showed that replacing Fe with transition metals Ni or Co significantly enhanced the photocatalytic activity of the catalyst.
3.2.5. Other factors
(1) Semiconductor Crystal Structure
When TiO₂ is used as a semiconductor photocatalyst, the influence of its crystal structure on the dye-sensitized photocatalytic system is quite significant. TiO₂ exists in three crystal forms: anatase, brookite, and rutile. When TiO₂ with these three different crystal structures is used as a semiconductor photocatalyst under the same dye-sensitized photocatalytic conditions, the three different crystal structures show varying photocatalytic efficiencies. Current research indicates that only the anatase and rutile forms of TiO₂ exhibit photocatalytic activity, with anatase showing better photocatalytic performance than rutile. This difference may be due to the distinct lattice structures of the two forms: compared to rutile, anatase has more defects in its crystal structure, which results in a greater number of oxygen vacancies, thereby improving the efficiency of electron capture. As a result, anatase TiO₂ exhibits superior photocatalytic activity.
Recent studies have found that when anatase and rutile TiO₂ nanoparticles are mixed in a specific ratio, the photocatalytic activity of the mixture is significantly better than that of a single crystal form. This is likely because the rutile TiO₂ on the surface of anatase TiO₂ can promote the separation of photogenerated charges, thus enhancing photocatalytic activity. Degussa P25 available on the market is an example of a product derived from a mixture of these two crystal forms.
(2) Particle Size
It was previously believed that smaller particle sizes were always better, and some nanoparticle semiconductors have gradually been applied to dye-sensitized photocatalytic systems. Indeed, nanoparticles with smaller sizes have a larger specific surface area, providing more active sites for the adsorption of water molecules. Additionally, the smaller particle diameter shortens the distance for electrons to diffuse to the surface, improving charge separation efficiency, all of which further enhance photocatalytic efficiency. However, subsequent studies have shown that smaller particle sizes are not always better. When the particle size reaches a certain threshold, its ability to capture sunlight decreases significantly [6]. Therefore, semiconductor particle size has an optimal range.
3.3. Dye-Sensitized technology applications
3.3.1. Solar cells
In 1976, the dye-sensitized solar cell (DSSC) was first prepared by Tsubomura and colleagues, achieving a photoelectric conversion efficiency of 1.5%. After several improvements and experiments by scholars both domestically and internationally, in 2022, Gratzel and others successfully fabricated a DSSC with a photoelectric conversion efficiency exceeding 15% by adsorbing two different dyes with distinct absorption spectra onto a TiO₂ film. At the same time, due to issues such as poor stability caused by liquid electrolytes in traditional DSSCs, scholars innovatively developed solid-state dye-sensitized solar cells (ss-DSSCs) and flexible dye-sensitized solar cells.
Currently, the market for solar cells is dominated by silicon-based solar cells. However, these have limitations such as high production costs, low energy conversion efficiency, and environmental concerns, which prevent them from being widely adopted. In contrast, dye-sensitized solar cells (DSSCs) present a promising alternative to silicon-based solar cells. They not only have lower production costs but also can be applied in a variety of settings. DSSCs are currently used in fields like new energy vehicles and smartphones.
3.3.2. Semiconductor modification
The primary energy from sunlight is distributed in the visible light region, but most semiconductors only respond to ultraviolet light, with only a few semiconductors responding to visible light. To enhance photocatalytic efficiency, the dye-sensitized method is used to modify semiconductors, enabling them to respond to visible light.
Dye molecules have strong absorption in the visible light region. By methods such as physical adsorption, these dye molecules can be attached to the semiconductor surface, sensitizing the surface and improving the semiconductor’s utilization of solar energy. This method has been applied in the fields of photocatalytic water splitting for hydrogen production and photocatalytic pollutant degradation, showing significant results.
3.4. Evaluation criteria for photocatalytic activity
Generally, stronger photocatalytic activity and more stable hydrogen production rates indicate a more excellent photocatalytic hydrogen production system, typically measured in μmol/h or μmol/h/g catalyst. However, since there is no universally accepted and official unit for photocatalytic hydrogen production rates, comparing values using units like μmol/h or μmol/h/g catalyst in many studies has limited significance. Therefore, other evaluation indicators are needed, including the apparent quantum yield (AQY), which reflects the system’s light-to-chemical energy conversion efficiency, and the turnover number (TON), which reflects the cyclic utilization capacity of dye molecules.
 \( AQY(\%)=\frac{2×number of evolved H2 molecules}{Number of incident photons}×1 \) 00 \( \% \)
\( AQY(\%)=\frac{2×number of evolved H2 molecules}{Number of incident photons}×1 \) 00 \( \% \)
 \( TON=\frac{2×number of H2 molecules evolved}{Number of dye molecules absorbed} \)
\( TON=\frac{2×number of H2 molecules evolved}{Number of dye molecules absorbed} \)
4. Conclusion
This paper discusses the current research on hydrogen production and dye-sensitized technology by scholars both domestically and internationally. It summarizes the four main hydrogen production technologies currently in use worldwide: fossil fuel hydrogen production, electrolysis of water, biological hydrogen production, and solar hydrogen production. Each of these technologies has its own advantages and disadvantages. Among them, solar hydrogen production, as an emerging technology, is extensively researched and tested due to its renewable, resource-rich, clean, and environmentally friendly characteristics. With ongoing research into hydrogen, its applications are becoming increasingly diverse and are being used across various fields, making it an indispensable part of global energy strategies. During this research, scholars have keenly observed that using dye-sensitized technology to build dye-sensitized photocatalytic systems can significantly increase hydrogen production and has become a leading direction in the world.
Building a dye-sensitized photocatalytic system can enhance photocatalytic hydrogen production efficiency, but this technology is still in the research stage and is not yet fully mature. There are many issues that need to be addressed. It is recommended to focus development in the following areas: Reduce production costs and improve economic viability for large-scale market application. Explore innovative approaches to enhance photocatalytic hydrogen production efficiency.
References
[1]. Xie, J. W. (2008). Application of photocatalytic oxidation technology in pollutant treatment. Tianjin Chemical Industry, (2), 48-51.
[2]. Ni, M., Leung, M. K. H., & Leung, D. Y. C. (2006). Progress in dye-sensitized water splitting hydrogen production technology. Power Supply Technology, (10), 856-859.
[3]. Zhang, C. J., Cai, J., Zhang, Y. K., et al. (2021). Thermodynamic equilibrium-based simulation of high-temperature solid oxide electrolysis water splitting for hydrogen production. Journal of Solar Energy, 42(9), 210-217.
[4]. Luan, T. X., & Zhao, W. W. (2022). Review on the current development and research progress of new energy hydrogen production technologies. Petrochemical Technology, 29(8), 153-154.
[5]. Wang, P., Luo, G. F., Cao, T. T., et al. (2011). Properties, photocatalytic mechanisms, and applications of metal porphyrin compounds. Journal of Three Gorges University (Natural Science Edition), 33(5), 84-92.
[6]. Cui, J. Y., Yao, J., He, Y. J., et al. (2024). Study on heat treatment and cooling rate of FGH96 alloy with different grain sizes. Rare Metal Materials and Engineering, 53(1), 242-249.
Cite this article
Liu,J. (2025). Dye sensitization and hydrogen production. Advances in Engineering Innovation,15,79-84.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Journal:Advances in Engineering Innovation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Xie, J. W. (2008). Application of photocatalytic oxidation technology in pollutant treatment. Tianjin Chemical Industry, (2), 48-51.
[2]. Ni, M., Leung, M. K. H., & Leung, D. Y. C. (2006). Progress in dye-sensitized water splitting hydrogen production technology. Power Supply Technology, (10), 856-859.
[3]. Zhang, C. J., Cai, J., Zhang, Y. K., et al. (2021). Thermodynamic equilibrium-based simulation of high-temperature solid oxide electrolysis water splitting for hydrogen production. Journal of Solar Energy, 42(9), 210-217.
[4]. Luan, T. X., & Zhao, W. W. (2022). Review on the current development and research progress of new energy hydrogen production technologies. Petrochemical Technology, 29(8), 153-154.
[5]. Wang, P., Luo, G. F., Cao, T. T., et al. (2011). Properties, photocatalytic mechanisms, and applications of metal porphyrin compounds. Journal of Three Gorges University (Natural Science Edition), 33(5), 84-92.
[6]. Cui, J. Y., Yao, J., He, Y. J., et al. (2024). Study on heat treatment and cooling rate of FGH96 alloy with different grain sizes. Rare Metal Materials and Engineering, 53(1), 242-249.









