1. Introduction
With the widespread adoption of new energy vehicles, the use of batteries in the automotive industry has rapidly developed. In recent years, sodium-ion batteries have gradually entered the public’s view due to their abundant resource reserves, higher safety, and lower cost. In addition, the development of sodium-ion battery technology plays a critical role in achieving energy conservation, emission reduction, and economic development. China’s “14th Five-Year Plan” for new energy storage explicitly points out the development of sodium-ion battery technology. Significant progress has been made in sodium-ion battery research in China. Leading Chinese battery company CATL (Contemporary Amperex Technology Co., Limited) has announced that the second generation of sodium-ion batteries has been successfully developed. Against this backdrop, this paper summarizes recent research on sodium-ion batteries.
2. Advantages of sodium-ion batteries
The abundance of lithium in the Earth’s crust is 0.0065%, whereas sodium’s abundance is 2.6%, which is much higher than that of lithium. Sodium also has advantages such as widespread distribution, low extraction cost, and inexpensive raw materials. This inherent advantage means that the production cost of sodium-ion batteries will be lower than that of lithium-ion batteries. This is why, despite the simultaneous development of sodium and lithium batteries, sodium batteries have gained more attention in recent years.
Sodium batteries are lower in manufacturing cost, have higher safety performance, and are more suited for large-scale applications, making them the optimal choice for large-scale energy storage systems. Furthermore, sodium batteries do not contain precious metals, and their electrode materials can be replaced with the more affordable material, aluminum. The suitability of sodium batteries under low-temperature conditions is another advantage of their development. Even in extremely cold regions, the endurance of sodium-ion batteries is seldom affected.
In 2010, groundbreaking progress was made in the research of two-level materials for sodium-ion batteries. Furthermore, the implementation of a series of supportive policies in recent years has accelerated the industrialization process of sodium-ion batteries.
3. Carbon-based sodium-ion battery electrode materials
Sodium and lithium belong to the same group in the periodic table and share similar chemical properties, making it feasible for sodium-ion batteries to replace lithium-ion batteries. Biomass and its derivatives possess environmental friendliness and sustainability, giving them significant potential as green energy storage materials. This makes research into biomass-based carbon sodium-ion battery electrode materials of unprecedented importance.
3.1. Biomass-based carbon sodium-ion battery electrode materials
Agricultural and forestry biomass resources are rich in cellulose, hemicellulose, and lignin. These substances can form biomass carbon-based materials at high temperatures. After a series of processing treatments, they exhibit excellent sodium-ion storage properties, making them suitable as electrode materials for sodium-ion batteries. In addition, certain animal biomass and their shells can also undergo a series of transformations to become negative electrode materials for sodium-ion batteries, showing excellent rate performance and good cycling stability. These findings demonstrate that biomass carbon-based materials possess good specific capacitance and excellent cycling stability, holding great prospects and potential for the development of sodium-ion batteries. Furthermore, due to the renewability of biomass carbon-based materials, they help reduce the production cost of sodium-ion batteries.
3.2. MOFs and their derivatives as sodium-ion battery electrode materials
Metal-organic frameworks (MOFs) and their derivatives possess a porous structure, a large specific surface area, and strong electrical properties. These characteristics make them suitable for creating sodium-ion battery materials with high efficiency, low cost, and excellent cycling performance, making them a research hotspot in recent years. The core-shell structures formed by MOFs and their derivatives provide a larger contact area for the charge and discharge activities of the sodium battery. This shortens the diffusion path of sodium ions and enhances the conductivity of the battery (see Table 1). Integrating biomass with MOF precursors will further optimize the performance of the battery, making this approach highly significant for the exploration of sodium-ion batteries.
Table 1. MOF-Derived Carbon Materials for Sodium-Ion Batteries [1]
MOFs | Sample | Current density/ (mA∙g-1) | Number of iterations | Specific capacity/ (mAh∙g-1) |
ZIF-67 | P@N-MPC | 150 | 100 | 600 |
ZIF-67 | CoC | 100 | 100 | 473 |
MOF | CoS | 600 | 2250 | 358 |
MIL-125(Ti) | Porous TiO2 | 100 | 3000 | 196 |
MIL-88(V) | V2O3 | 50 | 1000 | 417 |
MOF-199 | Porous CuxS | 100 | 110 | 372 |
ZIF-8 | ZnS-Sb2S3@C | 100 | 120 | 630 |
MOF-5 | CPC | 100 | 100 | 233 |
MIL-101 | Fe2O3@C | 500 | 200 | 662 |
Ni-MOF | NiO/Ni | 200 | 200 | 290 |
4. Solid-state electrolyte sodium-ion batteries
4.1. \( {Na_{5}}{YSi_{4}}{O_{12}} \) sodium-ion batteries
Compared to traditional liquid-state batteries that use liquid electrolytes, solid-state batteries utilize safer solid electrolytes. This better addresses issues such as electrolyte leakage, flammability, and explosion risks. However, while solving the problems associated with liquid electrolytes, solid-state batteries face a significant drawback in the form of a substantial reduction in ion conductivity. Therefore, to enable the widespread application of solid electrolytes, it is crucial to find ways to improve the ion conductivity at room temperature.
Solid electrolytes \( {Na_{5}}{YSi_{4}}{O_{12}} \) belong to the hexagonal crystal system \( {Na_{5}}{MSi_{4}}{O_{12}} \) (M=Y,In,FeLu \( ~ \) Sm), and under certain doping conditions, they exhibit high room temperature conductivity, making them suitable for sodium-ion transport. As a result, solid electrolytes are considered to have great potential, and a large number of related studies are currently underway. They have become a popular topic in inorganic solid electrolytes. A novel solution-assisted solid-state reaction (SASSR) method is typically used for preparation (see Figure 1).
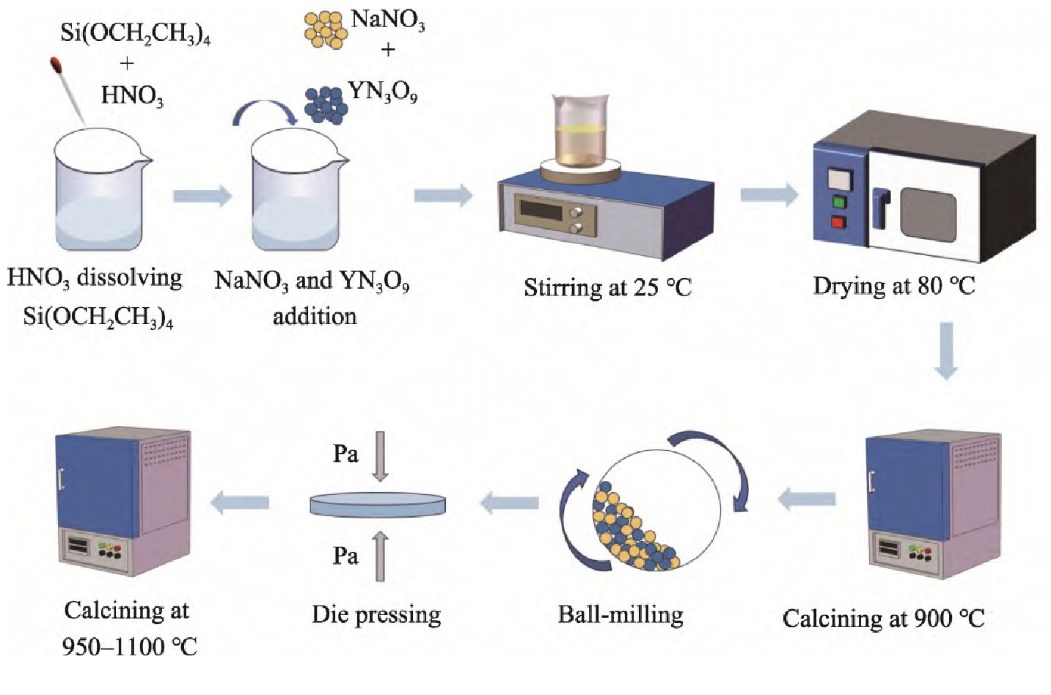
Figure 1. Experimental Flowchart for the preparation of \( {Na_{5}}{YSi_{4}}{O_{12}} \) by SASSR Method [2]
Studies about \( {Na_{5}}{YSi_{4}}{O_{12}} \) have shown that when the sintering temperature is 1050°C with a 6-hour hold time, \( {Na_{5}}{YSi_{4}}{O_{12}} \) achieves the highest shrinkage rate and density, which results in the highest ionic conductivity, thus ensuring an increase in sodium ion transport efficiency. Electrochemical impedance spectroscopy studies of \( {Na_{5}}{YSi_{4}}{O_{12}} \) have revealed that the activation energy is the lowest at the same sintering temperature of 1050°C for 6 hours, and lower activation energy promotes the transfer of sodium ions within the lattice. Due to these characteristics, \( {Na_{5}}{YSi_{4}}{O_{12}} \) are considered to be promising sodium-ion conductors, and research in this area is of significant importance to the development of sodium-ion batteries in China.
4.2. Oxide solid electrolytes
Sodium-ion batteries based on oxide solid electrolytes (see Figure 2) currently exhibit the high power density and long lifespan required for stationary energy storage, making them valuable for future applications.
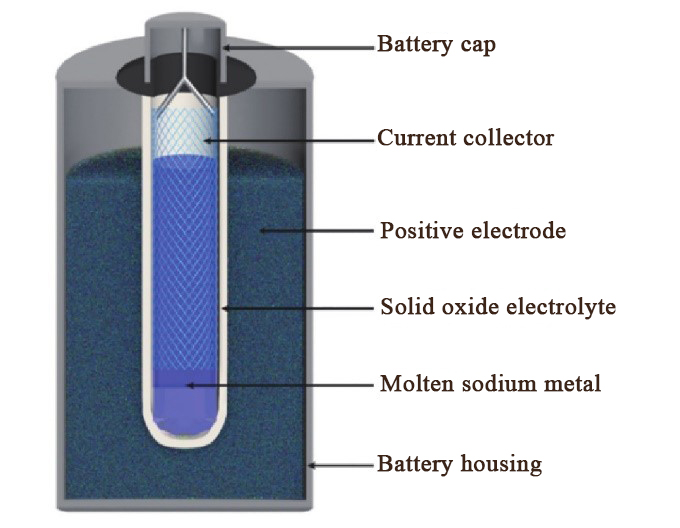
Figure 2. Sodium-Ion Battery Based on Oxide Solid-State Electrolyte for Energy Storage [3]
Sodium ions can be quickly transported within ceramic-based aluminum oxide. The aluminum oxide-based ceramic electrolyte can be divided into two types depending on the Na and Al element ratio: \( β-{Al_{2}}{O_{3}} and {β^{ \prime \prime }}-{Al_{2}}{O_{3}}, {β^{ \prime \prime }}-{Al_{2}}{O_{3}} \) 's unique structure (see Figure 3) allows it to have high ionic conductivity and thermal stability at operating temperatures above 300°C. This characteristic makes it a primary material for high-temperature sodium-sulfur battery solid electrolytes.
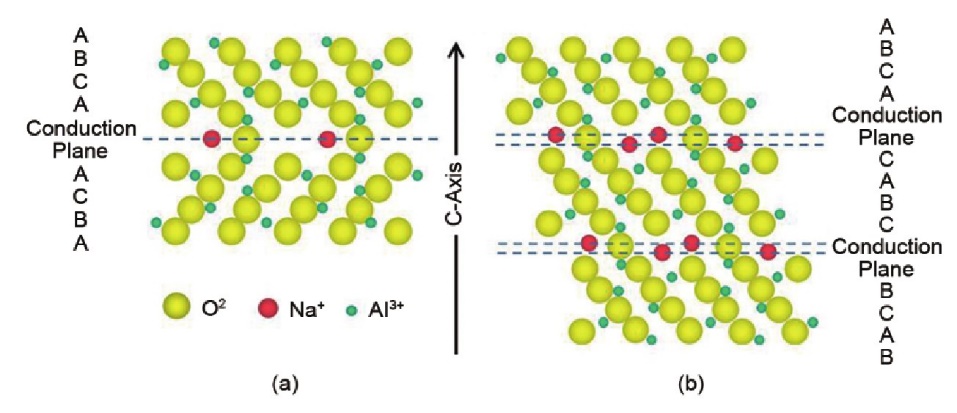
Figure 3. \( β/{β^{ \prime \prime }}-{Al_{2}}{O_{3}} \) Crystal Structure [3]
In 2004, Oshima et al. [4] successfully applied \( {β^{ \prime \prime }}-{Al_{2}}{O_{3}} \) to high-temperature sodium-sulfur batteries. However, its thermodynamic stability is relatively poor, and a small portion of low-conductivity material \( β-{Al_{2}}{O_{3}} \) may be produced during the synthesis process. Therefore, improving the content of \( {β^{ \prime \prime }}-{Al_{2}}{O_{3}} \) in the solid electrolyte is crucial. The primary method for enhancing this content is by adding sintering aids or performing doping modifications during synthesis. For example, Chen et al. [5] enhanced the material by doping with MgO,Yi et al. [6] added \( {TiO_{2}} and {ZrO_{2}} \) during sintering, and Lee et al. [7] improved the ratio by doping with appropriate amounts of Fe and Ti.
5. Other sodium-ion batteries
5.1. Sodium-sulfur batteries
Sodium-sulfur batteries (see Figure 4) were invented in 1967 and use molten liquid sodium and elemental sulfur as the positive and negative electrode materials. These batteries require relatively high operating temperatures, which places significant demands on the performance of materials and the battery structure. Sodium-sulfur batteries also feature higher energy storage per unit volume, making them widely used in load balancing and emergency power supplies.
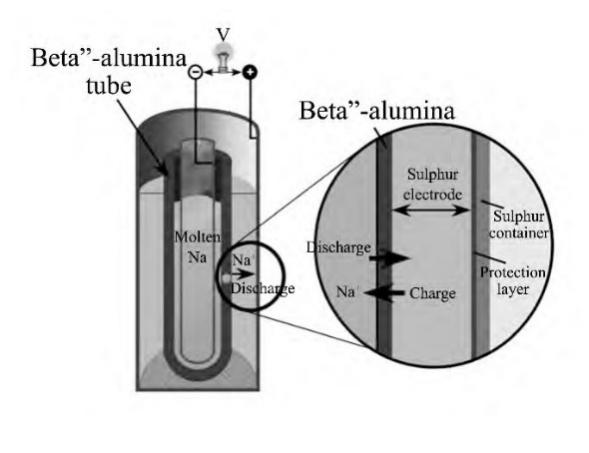
Figure 4. Sodium-Sulfur Battery Principle Diagram [8]
Under high-temperature operating conditions, if the ceramic oxide electrolyte breaks, sodium and sulfur can come into direct contact, leading to a short circuit. This results in a rapid temperature rise, potentially causing thermal runaway. This safety issue has limited the development of sodium-sulfur batteries. Therefore, research on low-temperature sodium-sulfur batteries focuses on resolving safety concerns, which could expand the application market for sodium-sulfur batteries.
5.2. ZEBRA batteries (sodium-metal chloride batteries)
ZEBRA batteries, also known as sodium-salt batteries (see Figure 5), have a similar structure to sodium-sulfur batteries. They use liquid sodium for the negative electrode and less corrosive materials for the positive electrode, improving the safety of the battery. However, they still operate under high-temperature conditions. Nevertheless, ZEBRA batteries are relatively safe, with no significant safety risks even if the battery is damaged. Additionally, they operate at a higher voltage, which makes it possible to address technical challenges (such as developing better sodium-ion solid-state electrolytes) and potentially achieve applications in the automotive field.
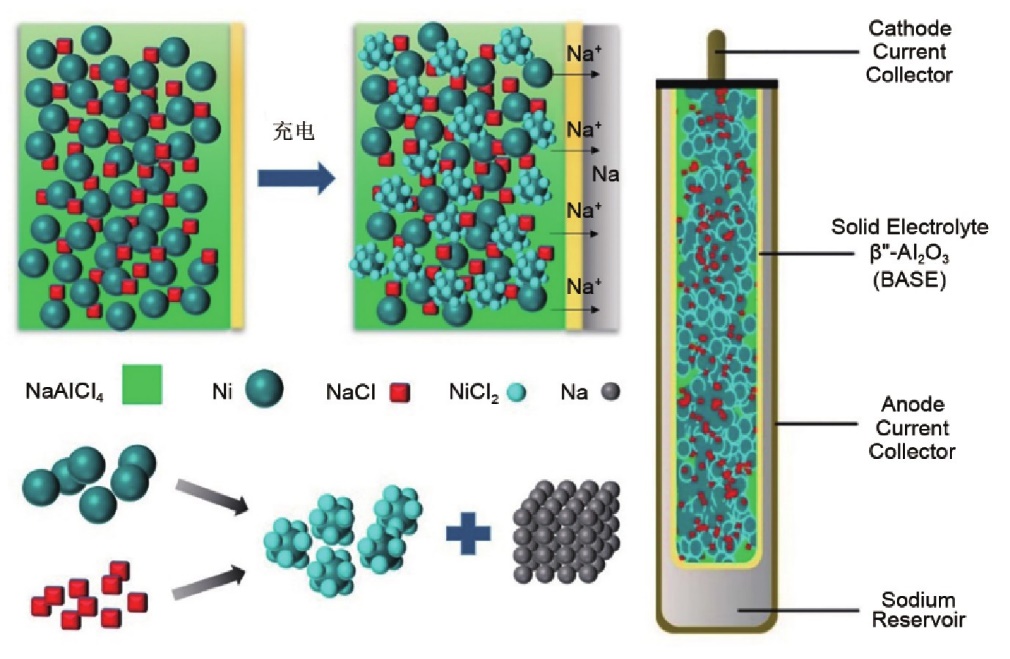
Figure 5. ZEBRA Battery Reaction Mechanism [3]
5.3. Sodium-air batteries
Sodium-air batteries are still in the early stages of development. Their working principle involves sodium ions and oxygen reacting at the air electrode, producing oxides to drive the battery, as shown in Figure 6. Oxygen plays an essential role in the air battery, so it is crucial to ensure oxygen transport within the battery. For this reason, sodium-air battery positive electrode materials are often chosen to be porous carbon materials or porous metal materials. Sodium-air batteries still suffer from the common issue of low energy density, but the process of sodium oxidation to form oxides is more stable.
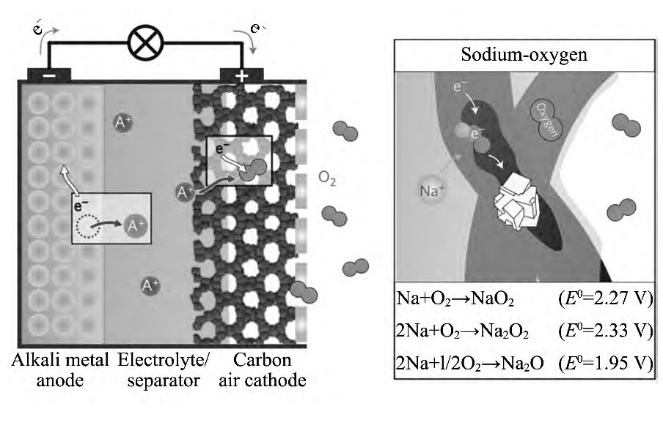
Figure 6. Sodium-Air Battery Principle Diagram [8]
Sun et al. [9] have developed the first sodium-air battery that operates at room temperature. If room-temperature sodium-air batteries can be mass-produced during industrialization, they will play a significant role in the future development of new battery technologies.
5.4. Aqueous sodium-ion batteries
Aqueous electrolyte sodium-ion batteries offer advantages such as higher ion migration rates, lower costs, and greater safety compared to organic electrolyte sodium-ion batteries. The only drawback is that the working voltage window for water-based electrolytes is narrower than that of organic electrolytes. Using aqueous electrolytes is currently the most effective way to reduce the production cost of sodium-ion batteries, which has led to increasing participation from various research teams in aqueous sodium-ion battery studies.
The Wessells team [10-11] proposed using \( KCuFe {(CN)_{6}} and KNiFe{ (CN)_{6}} \) as positive electrode materials for aqueous sodium-ion batteries. While many teams are conducting research on positive electrode materials, relatively fewer studies focus on negative electrode materials.
6. The role of sodium-ion batteries in the development of the new energy vehicle industry
In order to achieve energy conservation and emission reduction goals as well as China’s carbon peak and carbon neutrality targets, new energy vehicles (NEVs) are gradually replacing traditional fuel vehicles, making it an inevitable trend in development. NEVs, with electric vehicles (EVs) as the primary type, are becoming the mainstream in the automotive sector. In this context, rechargeable batteries play a crucial role in the development of NEVs.
Although solid-state lithium batteries exhibit excellent performance in applications, the limited availability of lithium (Li) on Earth means that solely relying on lithium batteries for the development of NEVs will inevitably encounter bottlenecks in the future. In contrast, sodium, which shares similar chemical properties with lithium, is abundant on Earth. This abundance will significantly reduce the production cost of batteries, thus enhancing the feasibility of implementing energy-saving and emission-reduction strategies. Liquid electrolyte sodium-ion batteries, however, pose potential safety hazards that could lead to serious safety incidents and social economic losses. Replacing liquid electrolytes with solid-state electrolytes is an effective way to ensure sufficient energy density and meet safety requirements. Additionally, solid electrolytes reduce the heat generated by external temperature fluctuations, thus improving energy conversion efficiency. Once all-solid-state sodium-ion batteries are successfully industrialized, global warming and the secondary disasters triggered by it will be effectively mitigated.
On January 5, 2024, the world's first sodium-ion battery-powered vehicle was delivered to users in bulk in Anhui, marking the breakthrough from “0” to “1” in sodium-ion battery-powered new energy vehicles.
7. The development prospects of sodium-ion batteries
2023 marked the beginning of the development of sodium-ion batteries, with many enterprises joining the production and research of sodium batteries, including traditional lithium battery manufacturers. Sodium batteries have already entered various fields. At the recent Third Sodium-Ion Battery Industry Chain and Standards Development Forum, Yu Zhenhua pointed out that sodium batteries are showing strong development trends, with more than 20 sodium battery projects underway. In the future, sodium batteries will see better development in the energy storage sector. Liu Ranran, a senior engineer at the China Electronics Standardization Institute, noted that compared to lithium batteries, sodium batteries are safer, with better aging safety. In addition, sodium batteries also offer advantages in terms of high-rate and low-temperature charge and discharge performance.
Currently, several domestic enterprises have achieved large-scale production of sodium batteries. In the future, sodium batteries will seize a significant market share with their advantages of low cost, high performance, and safety, gradually replacing lithium and lead-acid batteries to become a leading material in the battery industry.
The high cycle efficiency and high conversion efficiency of sodium batteries make the future of the sodium battery industry promising. It is expected that sodium-ion batteries will achieve mass production, with the industry crossing the "GW-level shipment" threshold this year, and effective production capacity will further break through.
Although sodium batteries have lower energy density, which results in less driving range compared to lithium-ion batteries, if this issue can be resolved in the future, sodium batteries may fully replace lithium batteries. Currently, it may be possible to expand the application market for sodium-ion batteries by using an AB battery model, combining sodium-ion and lithium-ion batteries to complement each other’s shortcomings.
At present, the sodium-ion battery industry chain has already taken shape, and relevant investments have been made. The government has also introduced a series of policies to support sodium-ion battery development, with continuous breakthroughs in research and development. In the future, there is potential to improve energy density and resolve the limitations of sodium-ion batteries.
8. Conclusion
The development of sodium-ion batteries will become a major trend in the future of the battery industry. Sodium-ion batteries have inherent advantages, such as low production costs and abundant resources. However, it is undeniable that sodium-ion batteries currently face challenges such as low energy density and high operating temperatures. Once these issues are resolved, sodium-ion batteries will occupy a significant market share in the battery industry and become a leading force in the field. The future of sodium-ion battery research looks promising.
References
[1]. He, Y. Q., Zhang, Y. T., Wang, Y. X., Du, G. B., & Xu, K. M. (2023). Research progress on MOF-derived/biomass carbon-based sodium-ion battery electrode materials. Forest Products Industry, 60(9), 44–50. https://doi.org/10.19531/j.issn1001-5299.202309008
[2]. Xu, Y., Liu, L. M., Zhou, X. L., Guo, W. L., Li, J., & Guo, X. R. (2024). Preparation and conductivity behavior of Na₅YSi₄O₁₂ - based ceramic solid-state sodium battery electrolytes. Journal of Ceramics, 45(3), 483–491. https://doi.org/10.13957/j.cnki.tcxb.2024.03.006
[3]. Wang, J. Z., Han, X. L., Xu, C. F., Zhao, J. W., Tang, Y., & Cui, G. L. (2022). Research progress on energy storage sodium batteries based on oxide solid-state electrolytes. Energy Storage Science and Technology, 11(9), 2834–2846. https://doi.org/10.19799/j.cnki.2095-4239.2022.0424
[4]. Oshima, T., Kajita, M., & Okuno, A. (2005). Development of sodium-sulfur batteries. International Journal of Applied Ceramic Technology, 1(3), 269 - 276.
[5]. Chen, G., Lu, J., & Zhou, X. (2016). Solid-state synthesis of high performance Na-β″-Al₂O₃ solid electrolyte doped with MgO. Ceramics International, 42(14), 16055 - 16062.
[6]. Yie, E., Temeche, E., & Laine, R. M. (2018). Superionically conducting β″-Al₂O₃ thin films processed using flame synthesized nanopowders. Journal of Materials Chemistry A, 6(26), 12411 - 12419.
[7]. Lee, S. T., Lee, D. H., & Kim, J. S. (2017). Influence of Fe and Ti addition on properties of Na⁺ - β/β″ - alumina solid electrolytes. Metals and Materials International, 23(2), 246 - 253.
[8]. Chen, Q. F., Fang, C., & Zhang, Y. (2014). Research progress on sodium batteries and their applications in power storage. East China Electric Power, 42(8), 1579–1585.
[9]. Sun, Q., Yang, Y., & Fu, Z. - W. (2012). Electro - chemical properties of room temperature sodium - air batteries with non - aqueous electrolyte. Electrochemistry Communications, 16, 22.
[10]. Wessells, C. D., Huggins, R. A., & Cui, Y. (2011). Copper hexacyanoferrate battery electrodes with long cycle life and high power. Nature Communications, 2, 550.
[11]. Wessells, C. D., Peddada, S. V., & Huggins, R. A. (2011). Nickel hexacyanoferrate nanoparticle electrodes for aqueous sodium and potassium ion batteries. Nano Letters, 11, 5421–5425.
Cite this article
Li,Y. (2025). Review of sodium-ion battery research. Advances in Engineering Innovation,16(3),31-37.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Journal:Advances in Engineering Innovation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. He, Y. Q., Zhang, Y. T., Wang, Y. X., Du, G. B., & Xu, K. M. (2023). Research progress on MOF-derived/biomass carbon-based sodium-ion battery electrode materials. Forest Products Industry, 60(9), 44–50. https://doi.org/10.19531/j.issn1001-5299.202309008
[2]. Xu, Y., Liu, L. M., Zhou, X. L., Guo, W. L., Li, J., & Guo, X. R. (2024). Preparation and conductivity behavior of Na₅YSi₄O₁₂ - based ceramic solid-state sodium battery electrolytes. Journal of Ceramics, 45(3), 483–491. https://doi.org/10.13957/j.cnki.tcxb.2024.03.006
[3]. Wang, J. Z., Han, X. L., Xu, C. F., Zhao, J. W., Tang, Y., & Cui, G. L. (2022). Research progress on energy storage sodium batteries based on oxide solid-state electrolytes. Energy Storage Science and Technology, 11(9), 2834–2846. https://doi.org/10.19799/j.cnki.2095-4239.2022.0424
[4]. Oshima, T., Kajita, M., & Okuno, A. (2005). Development of sodium-sulfur batteries. International Journal of Applied Ceramic Technology, 1(3), 269 - 276.
[5]. Chen, G., Lu, J., & Zhou, X. (2016). Solid-state synthesis of high performance Na-β″-Al₂O₃ solid electrolyte doped with MgO. Ceramics International, 42(14), 16055 - 16062.
[6]. Yie, E., Temeche, E., & Laine, R. M. (2018). Superionically conducting β″-Al₂O₃ thin films processed using flame synthesized nanopowders. Journal of Materials Chemistry A, 6(26), 12411 - 12419.
[7]. Lee, S. T., Lee, D. H., & Kim, J. S. (2017). Influence of Fe and Ti addition on properties of Na⁺ - β/β″ - alumina solid electrolytes. Metals and Materials International, 23(2), 246 - 253.
[8]. Chen, Q. F., Fang, C., & Zhang, Y. (2014). Research progress on sodium batteries and their applications in power storage. East China Electric Power, 42(8), 1579–1585.
[9]. Sun, Q., Yang, Y., & Fu, Z. - W. (2012). Electro - chemical properties of room temperature sodium - air batteries with non - aqueous electrolyte. Electrochemistry Communications, 16, 22.
[10]. Wessells, C. D., Huggins, R. A., & Cui, Y. (2011). Copper hexacyanoferrate battery electrodes with long cycle life and high power. Nature Communications, 2, 550.
[11]. Wessells, C. D., Peddada, S. V., & Huggins, R. A. (2011). Nickel hexacyanoferrate nanoparticle electrodes for aqueous sodium and potassium ion batteries. Nano Letters, 11, 5421–5425.









