1. Introduction
Emulsion explosives, widely used in industrial applications, are known for their water resistance, high safety, and environmental friendliness. However, due to the presence of approximately 10% water in their composition, the proportion of combustible components is reduced, limiting the energy output of these explosives [1]. Therefore, improving the blasting performance of emulsion explosives and developing high-brisance formulations has been a key focus of research worldwide.
In the process of researching high-brisance emulsion explosives, hydrogen storage materials have been found to serve as both sensitizers and energetic additives [2-3]. Cheng et al. demonstrated that MgH2-based hydrogen storage emulsion explosives significantly improve brisance and work capacity [4]. In addition to hydrogen storage materials, high-energy metal powders can also effectively enhance the performance of emulsion explosives. Mishra et al. noted that emulsion explosives maintain stable detonation performance at an aluminum content of 5% [5]. Cheng et al. found that with a boron powder content of 16%, the detonation performance was optimal. And with the boron powder content from 0% -20% gradually increased, the average explosion temperature, explosion pressure, positive impulse and heat of explosion containing boron emulsion explosives first increased and then decreased [6].
Although the aforementioned materials can effectively enhance the detonation performance of emulsion explosives, they also face issues such as high costs, reduced stability of emulsion explosives, and complex processes. This study, through theoretical analysis and preliminary experimental research, has found that NaBH4 has a hydrogen content of 10.6%. It generates a significant amount of heat and gaseous products during the explosion reaction, which can improve the detonation performance of emulsion explosives. Moreover, its cost is only 10% of that of metal powders. Therefore, this paper proposes to select NaBH4 as a high-energy additive to develop a new type of high-brisance emulsion explosive. The study will conduct brisance experiments to test the detonation performance of the samples and explore the relationship between NaBH4 content and the detonation performance of emulsion explosives. Additionally, it will investigate the impact of NaBH4 on the thermal stability of emulsion explosives through thermal decomposition experiments. The ultimate goal is to develop a new type of high-brisance emulsion explosive that is cost-effective, powerful, and safe. This work will provide a basis for further theoretical and experimental research as well as for large-scale production.
2. Experimental part
2.1. Preparation of emulsion explosives
In order to clarify the material properties of industrial grade sodium borohydride and pure sodium borohydride and their applicability, this study was tested using XRD ray diffraction experiments and the results are shown in Figure 1.
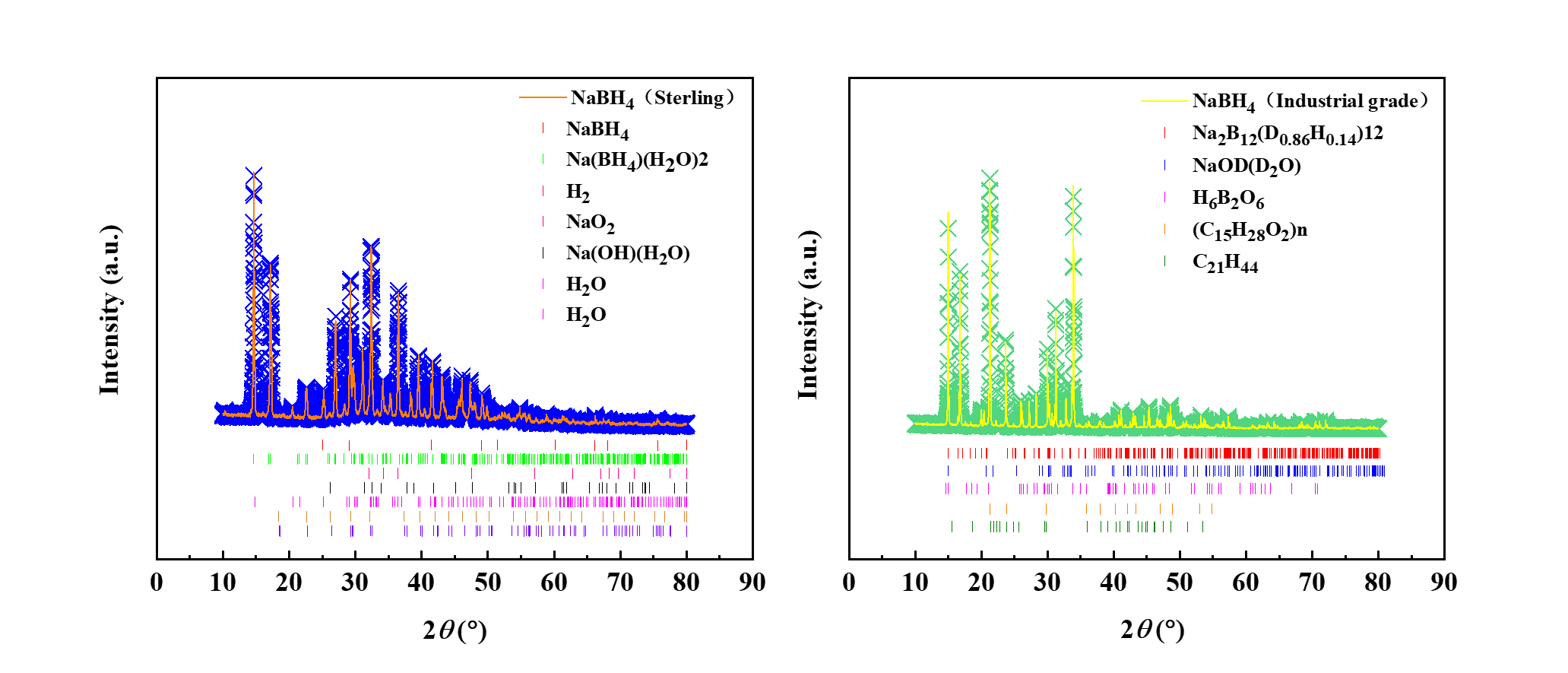
Figure 1. XRD curves of industrial-grade sodium borohydride and pure sodium borohydride
As shown in Figure 1, both materials primarily consist of sodium borohydride, sodium borohydride hydrate, and sodium hydroxide hydrate. Pure sodium borohydride is highly reactive at room temperature and readily reacts with moisture in the air, leading to premature reactions during sample preparation. In contrast, industrial-grade sodium borohydride is coated with carbon-hydrogen organic compounds, which enhance its chemical stability and reduce its cost. Therefore, industrial-grade sodium borohydride is more suitable as an energetic additive.
The composition and mass percentage of the emulsion matrix are as follows: Ammonium Nitrate (AN): Sodium Nitrate (SN): composite oil phase: urea: water = 73:10.7:5.3:2:9. Different mass fractions of NaBH4 were then added to the emulsion matrix to prepare NaBH4-containing emulsion explosives.
The formulations and oxygen balance (OB) of the samples are shown in Table 1.
Table 1. Sample formulations and oxygen balance
Samples | 1# | 2# | 3# | 4# | 5# | 6# |
NaBH4 Content/ % | 0% | 1% | 3% | 5% | 7% | 9% |
OB/ (g∙g-1) | 0.0157 | -0.0014 | -0.0361 | -0.0697 | -0.1039 | -0.1381 |
2.2. Brisance experiment
The brisance was tested using the lead column compression method as specified in GB12440-90. A 50 g explosive column was prepared in a paper tube, fixed on a lead column with a spacer, and detonated in an explosion container. Experiments were conducted at temperatures of 20 ℃, 25 ℃, and 30 ℃ to compare the changes in brisance under different environmental conditions. The diagram of the brisance experiment is shown in Figure 2.
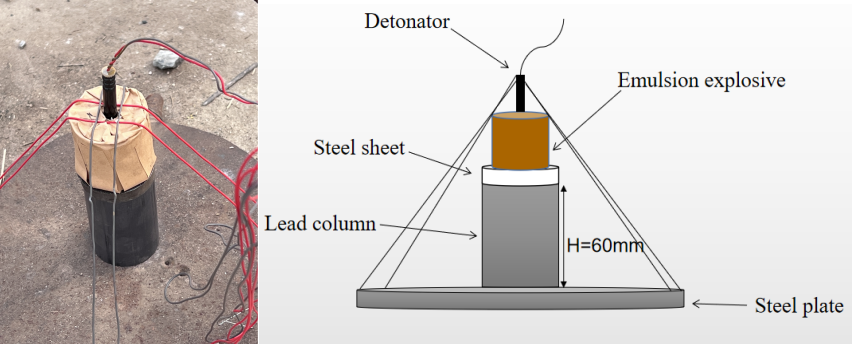
Figure 2. Schematic diagram of the brisance experiment
2.3. Thermal decomposition characteristics experiment
The thermal decomposition experiments were conducted using a TGA2 thermogravimetric analyzer manufactured by Mettler Toledo, Switzerland. Alumina crucibles were used during the experiments, with nitrogen (N2) as the experimental atmosphere at a flow rate of 50 mL/min. Approximately 2.5 mg of each sample was placed into the alumina crucibles. Heating rates of 10 °C/min and 15 °C/min were applied, with the temperature range set between 30 °C and 400 °C. The mass changes of the samples before and after the experiments were recorded and compared to analyze the thermal decomposition characteristics.
3. Results and discussion
3.1. Effect of NaBH4 content on the brisance of emulsion explosives
To further analyze the mechanism of the detonation performance of NaBH4 emulsion explosives, this paper also calculates the theoretical heat of explosion of the emulsion explosive samples using MATLAB software, based on the detonation parameters of the Becker-Williams Method and the calculation method of Hess's Law. Theoretical calculation of heat of explosion and the actual heat of explosion comparison figure is shown in Figure 3 below.
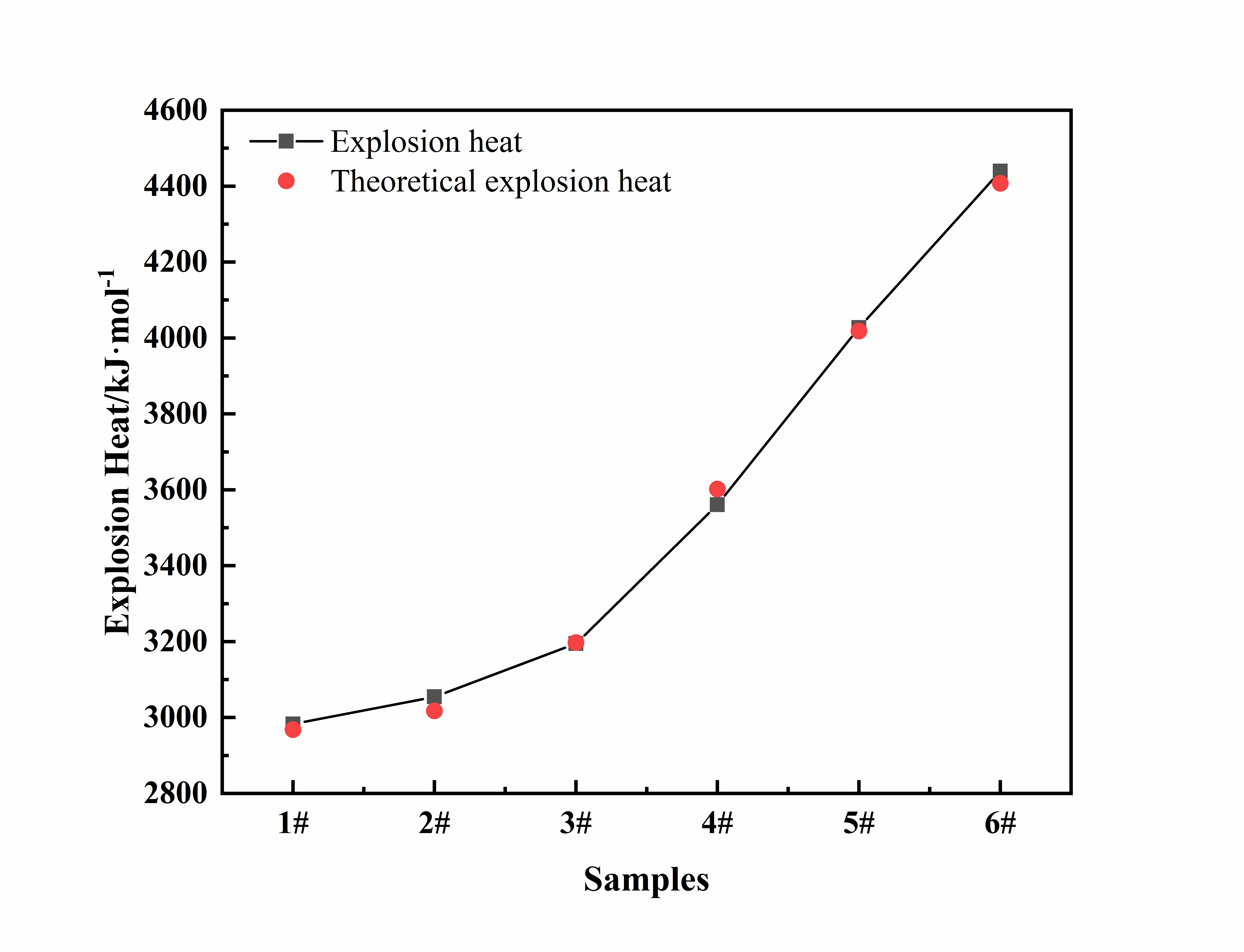
Figure 3. Comparison of actual heat of explosion and theoretically calculated heat of explosion
As can be seen from the comparison figure, the explosion heat value calculated according to the detonation equation is basically consistent with the actual explosion heat of the emulsion explosives samples, indicating that the actual detonation performance of NaBH4-containing emulsion explosives is basically consistent with the predictive theoretical model. The results of the brisance experiment and the calculated heat of explosion for emulsion explosive samples with varying NaBH4 contents are presented in Table 2.
Table 2. Detonation parameters of emulsion explosive samples
Samples | 1# | 2# | 3# | 4# | 5# | 6# |
Density/g∙cm-3 | 1.32 | 1.36 | 1.41 | 1.59 | 1.48 | 1.35 |
Heat/kJ·mol-1 | 2982.69 | 3054.83 | 3194.92 | 3560.75 | 4027.46 | 4439.57 |
aBrisance/mm | 14.6 | 18.0 | 20.0 | 21.8 | 18.9 | 17.3 |
bBrisance/mm | 14.9 | 18.6 | 20.3 | 22.4 | 19.8 | 17.2 |
cBrisance/mm | 15.8 | 19.1 | 20.8 | 22.7 | 20.6 | 18.4 |
a. The brisance of the emulsion explosive sample at an experimental temperature of 20 ℃. b. The brisance of the emulsion explosive sample at an experimental temperature of 25 ℃. c. The brisance of the emulsion explosive sample at an experimental temperature of 30 ℃.
The experimental results indicate that the brisance of emulsion explosive samples containing NaBH4 is higher than that of ordinary emulsion explosives. As the NaBH4 content increases, the brisance and density of the explosives initially increase and then decrease. Under the three different temperatures, the trend of brisance change of the emulsion explosive samples is consistent. It can be seen that within the temperature range from 20 ℃ to 30 ℃, the brisance performance of the emulsion explosive samples is stable, without significant fluctuations.
The brisance of emulsion explosives containing 1%, 3%, 5%, 7%, and 9% NaBH4 increased by 24.83%, 36.24%, 50.33%, 32.89%, and 15.44%, respectively, compared to Sample #1 (ordinary emulsion explosive) at 25 °C. The brisance reaches its maximum value of 22.4 mm in Sample 4# (with a NaBH4 content of 5%), representing a 50.33% increase compared to Sample 1#. Additionally, the heat of explosion of the emulsion explosive samples increases progressively with the addition of NaBH4.
The analysis reveals that the brisance of emulsion explosives is closely related to their density. When the NaBH4 content ranges from 0% to 5%, the density of the explosive gradually increases. The relatively high hydrogen content in NaBH4 leads to the release of more heat during the detonation reaction, which in turn raises the temperature within the explosion reaction zone. This elevated temperature enhances the work capability of the detonation products on the surrounding medium, thereby gradually increasing the brisance of the explosive. However, when the NaBH4 content exceeds 5%, the density of the explosive begins to decrease. Although the heat of explosion continues to increase, an excessive amount of NaBH4 drives the explosive towards a negative oxygen balance, disrupting the stoichiometric ratio between oxidizers and fuel within the system. This imbalance hinders the complete release of energy from the explosive, resulting in a decline in brisance.
3.2. Effect of NaBH4 on the thermal decomposition characteristics of emulsion explosives
Emulsion explosives are hazardous materials with enormous energy stored in a metastable state. When subjected to external stimuli, thermal decomposition reactions occur within the explosive, releasing a large amount of heat in a very short time [7]. If the generated heat accumulates within the explosive and is not released promptly, it may lead to severe consequences such as detonation. While improving the explosive performance of emulsion explosives, changes in their thermal stability must not be overlooked.
To study the effect of NaBH4 on the thermal decomposition characteristics of emulsion explosives, thermal decomposition experiments were conducted on the samples containing different mass fractions of NaBH4. The TG curves of the samples are shown in Figure 4.
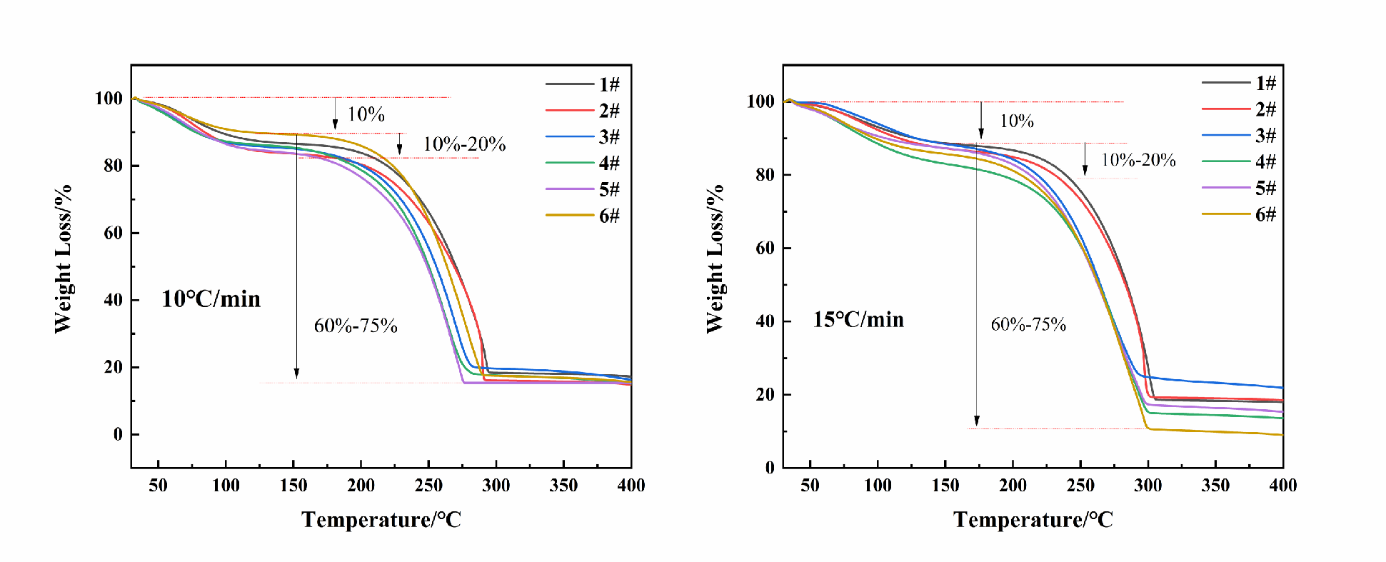
Figure 4. TG curves of emulsion explosives with different NaBH4 contents
From Figure 4, the TG curves of the emulsion explosive samples with varying NaBH4 contents exhibit a consistent trend. Based on theoretical analysis, the thermal decomposition characteristics of the emulsion explosives can be divided into three distinct stages. In the first stage, from 30 °C to 175 °C, the mass loss of the samples is approximately 15%, primarily attributed to the evaporation of water from the emulsion matrix and the decomposition of a small fraction of unstable emulsion components. In the second stage, from 175 °C to 300 °C, the mass loss is between 60% and 75%, mainly resulting from the vigorous decomposition reactions of ammonium nitrate, sodium nitrate, and the composite oil phase within the emulsion explosive system. In the third stage, from 300 °C to 400 °C, the TG curve remains stable with no further mass loss, indicating that the decomposition process has been completed. To further investigate the influence of NaBH4 on the thermal stability of emulsion explosives, the thermal decomposition parameters of the samples were determined under two different temperature conditions based on the TG curves. The data are presented in Table 3.
Table 3. Thermal decomposition parameters of emulsion explosive samples
Heating Rate | Initial Decomposition Temperature /℃ | |||||
1# 2# 3# 4# 5# 6# | ||||||
10 ℃/min 15 ℃/min | 153.16 151.30 | 157.46 153.16 | 161.76 158.90 | 164.43 165.14 | 167.50 169.86 | 173.66 176.53 |
Heating Rate | Maximum decomposition rate temperature /℃ | |||||
1# 2# 3# 4# 5# 6# | ||||||
10 ℃/min 15 ℃/min | 274.96 262.25 | 276.42 286.82 | 276.97 281.62 | 284.17 288.82 | 275.96 286.82 | 262.01 276.97 |
According to Table 3, the initial decomposition temperatures of NaBH4-containing emulsion explosives are all higher than those of ordinary emulsion explosives. Furthermore, at the same heating rate, the initial decomposition temperature increases progressively with the increasing NaBH4 content. Emulsion explosives are water-in-oil multiphase systems where interfacial properties play a critical role. The BH4- ion of NaBH4 is strongly reducing, and the hydrogen atoms in its structure can participate in the chemical reaction, prompting the formation of hydrogen bonds in the system [8]. Hydrogen bonding helps stabilize the interfacial membrane between the water and oil phases. The hydrogen bonds derived from sodium borohydride act in the interfacial region, enhancing the strength and toughness of the interfacial membrane. Under thermal shock, a stable interfacial membrane prevents the separation of the oil and water phases, thereby avoiding the aggregation of active components and uncontrolled reactions caused by phase separation. This provides a safeguard for thermal stability.
As a result, during thermal decomposition experiments, the emulsion explosive system requires higher energy to disrupt the intermolecular forces, leading to an increase in the initial decomposition temperature. In conclusion, NaBH4 enhances the thermal stability of emulsion explosives.
4. Conclusion
• Comparison of the theoretical computational model with the actual results concluded that the predicted detonation model is consistent with the actual. The brisance performance of the emulsion explosives samples is stable in the temperature range of 20-30℃. NaBH4 significantly improves the brisance of emulsion explosives. The brisance of emulsion explosives containing 1%, 3%, 5%, 7%, and 9% NaBH4 increased by 24.83%, 36.24%, 50.33%, 32.89%, and 15.44%, respectively, compared to Sample #1 (ordinary emulsion explosive) at 25 °C. At a NaBH4 content of 5%, the brisance reached a maximum value of 22.4 mm.
• The TG curves of emulsion explosive samples exhibit consistent trends. The initial decomposition temperatures of NaBH4 emulsion explosives are higher than ordinary emulsion explosives. NaBH4 enhances the thermal stability of emulsion explosives to a certain extent.
Acknowledgments
The work is supported by the Foundation of Anhui Engineering Research Center of New Explosive Materials and Blasting Technology of Anhui University of Science and Technology (No. AHBP2023B- 03).
References
[1]. Kramarczyk, B., Suda, K., Kowalik, P., Swiatek, K., Jaszcz, K., & Jarosz, T. (2022). Emulsion explosives: A tutorial review and highlight of recent progress. Materials, 15(14), 49-52.
[2]. Chen, J. P., Ma, H. H., Wang, Y. X., Huang, L. L., & Shen, Z. W. (2022). Effect of hydrogen-storage pressure on the detonation characteristics of emulsion explosives sensitized by glass microballoons. Defence Technology, 18(5), 747–754.
[3]. Du, M. R., Huang, Y., Chen, C. Y., Xuan, T. D., Li, W. W., Yuan, L., & Zheng, B. X. (2024). Formulation design and performance study of titanium hydride high-power emulsion explosive based on zero oxygen balance. Journal of Molecular Modeling, 30(12), 414.
[4]. Cheng, Y. F., Wang, Q., Gong, Y., Shen, Z. W., Tang, Y. F., Yuan, H. P., & Qian, H. (2017). Effect of sensitizing methods on the detonation performances of MgH₂-based hydrogen storage emulsion explosives. CIESC Journal, 68, 1734–1739.
[5]. Mishra, A. K., Agrawal, H., & Raut, M. (2019). Effect of aluminum content on detonation velocity and density of emulsion explosives. Journal of Molecular Modeling, 25, 1–5.
[6]. Cheng, Y. F., Yao, Y. L., Li, D. Y., Li, Z. H., & Yao, Y. K. (2023). Effects of boron powders on the detonation performance of emulsion explosives. Propellants, Explosives, Pyrotechnics, 48(3), e202200277.
[7]. Liu, R., Wang, X. G., Wang, H., Wang, Q., & Cheng, Y. F. (2025). Thermal decomposition behaviors and reaction mechanism of emulsion explosive with the addition of TiH₂ powders. Case Studies in Thermal Engineering, 65, 105583.
[8]. Khatoon, U. T., Velidandi, A., & Rao, G. V. S. N. (2023). Sodium borohydride mediated synthesis of nano-sized silver particles: Their characterization, anti-microbial and cytotoxicity studies. Materials Chemistry and Physics, 294, 12699.
Cite this article
Xuan,T.;Du,M.;Dong,Y. (2025). Study on the performance of sodium borohydride-containing emulsion explosives. Advances in Engineering Innovation,16(5),100-105.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Journal:Advances in Engineering Innovation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Kramarczyk, B., Suda, K., Kowalik, P., Swiatek, K., Jaszcz, K., & Jarosz, T. (2022). Emulsion explosives: A tutorial review and highlight of recent progress. Materials, 15(14), 49-52.
[2]. Chen, J. P., Ma, H. H., Wang, Y. X., Huang, L. L., & Shen, Z. W. (2022). Effect of hydrogen-storage pressure on the detonation characteristics of emulsion explosives sensitized by glass microballoons. Defence Technology, 18(5), 747–754.
[3]. Du, M. R., Huang, Y., Chen, C. Y., Xuan, T. D., Li, W. W., Yuan, L., & Zheng, B. X. (2024). Formulation design and performance study of titanium hydride high-power emulsion explosive based on zero oxygen balance. Journal of Molecular Modeling, 30(12), 414.
[4]. Cheng, Y. F., Wang, Q., Gong, Y., Shen, Z. W., Tang, Y. F., Yuan, H. P., & Qian, H. (2017). Effect of sensitizing methods on the detonation performances of MgH₂-based hydrogen storage emulsion explosives. CIESC Journal, 68, 1734–1739.
[5]. Mishra, A. K., Agrawal, H., & Raut, M. (2019). Effect of aluminum content on detonation velocity and density of emulsion explosives. Journal of Molecular Modeling, 25, 1–5.
[6]. Cheng, Y. F., Yao, Y. L., Li, D. Y., Li, Z. H., & Yao, Y. K. (2023). Effects of boron powders on the detonation performance of emulsion explosives. Propellants, Explosives, Pyrotechnics, 48(3), e202200277.
[7]. Liu, R., Wang, X. G., Wang, H., Wang, Q., & Cheng, Y. F. (2025). Thermal decomposition behaviors and reaction mechanism of emulsion explosive with the addition of TiH₂ powders. Case Studies in Thermal Engineering, 65, 105583.
[8]. Khatoon, U. T., Velidandi, A., & Rao, G. V. S. N. (2023). Sodium borohydride mediated synthesis of nano-sized silver particles: Their characterization, anti-microbial and cytotoxicity studies. Materials Chemistry and Physics, 294, 12699.









