1. Introduction
Around half of the world’s population is dependent on synthetic fertilizers for food. Inorganic fertilizers are composed of synthetic chemicals that supply nutrients to crops [1]. These synthetic chemicals contain macronutrients such as nitrogen, phosphorous, and potassium, which improve plant growth [2]. However, these synthetic chemicals degrade soil health over time, emit greenhouse gases, and lowers water quality [3,4].
The combined application of herbicides and inorganic fertilizers is a popular method today [5,6]. However, herbicide overuse accelerates nutrient leaching, making it hazardous for humans and animals to consume plants grown on the plot [7-9]. Akinnawo’s study conducted in the United Kingdom found that 70% of eutrophication came from agricultural run-off and sewage effluent, both of which contained phosphorus [10]. Eutrophication is where a body of water with overabundant nutrients (usually from agricultural runoff) causes excess growth of aquatic plants and algae [11]. In a 2005 to 2012 study conducted in Europe, only an estimate of 40 to 60% of nitrate and phosphate were used for their intended purpose of growing plants. The remaining 40 to 60% had leached into surrounding water bodies with a 0.26-0.30 TgP/yr phosphate discharge and was released into the sea. Moreover, studies have found that farmers are more likely to overuse herbicides due to convenience, cost and the immediate effectiveness [12].
In addition, heavy rainfall increases nutrient runoff, which may exacerbate the eutrophication process [13]. In Shenzhen Bay, China, researchers found that 60 to 80% of total pollution loads were caused by rainfall-runoff pollution. Their results also showed that urban rainfall-runoff pollution has an effect on the concentration of chlorophyll-A in Shenzhen Bay [14]. Inorganic fertilizers additionally release harmful greenhouse gases such as nitrogen oxide and carbon from the soil into the air [15].
An alternative to inorganic fertilizers that avoids releasing greenhouse gases is sustainable green manure. This agricultural practice involves planting a fast-growing, nutrient-rich crop on nutrient-poor land. Once the crop reaches maturity, it is plowed into the soil, where it decomposes and enriches the soil with organic matter. In this way, the nutrients are naturally recycled and the soil is replenished. As experts noted, soil fertility underpins crop yield. However, intense and improper treatment of the cultivated land increases soil degradation [16]. Repeated abuse of the land without replenishing the soil lead to poor crop yield and quality [17]. For example, monocropping depletes soil nutrients and fosters toxic buildup [16] .
In a 2010 study, organic and inorganic fertilizers exhibited the same effectiveness in promoting plant growth and nutritional quality. Both outperformed compost and non-fertilized control plants. When comparing the plant shoot yield, the three commercial organic fertilizers performed the best followed by NPK inorganic fertilizer [18]. NPK is an acronym for nitrogen, phosphorus, and potassium [19]. In addition, the organic compost was able to increase soil fertility by increasing the soil organic matter by 23.3% in the second year and 0.6% in the third, improving crop yield. However, the NPK inorganic fertilizer did not increase soil fertility [20]. Therefore, organic compost was the best option overall.
Plants typically used for green manure such as legumes take up land and resoures that could have otherwise been used to grow the desired crops. As the world’s population steadily increases, the food industry has to increase production to accommodate. However, it remains a huge challenge to produce more on limited land and is bound to become a greater problem in the future [16] .
Duckweed might be the key to solving the problem [21]. Lemna minor, also known as duckweed, is a small aquatic plant that does not compete with food corps and has strong absorption capacity [22]. The aquatic plant responds extremely well to increases in nutrients, often triggering its own eutrophication with rapid growth in abundance [23-25]. Other features of the plant include that it adapts well to a range of climate conditions, has the capacity to absorb a lot of nutrients, and can easily be collected from small bodies of water [26]. Researchers suggested using Lemna minor in places that have an overabundance of nutrients and later turning it into agricultural manure [27]. The captured nutrients such as nitrogen and phosphorus can be reintroduced into the soil as an organic fertilizer [24,25].
Often in waterways and in bodies of water, pollutants and pharmaceuticals can be found [28]. As the world’s third most consumable drug, ibuprofen was found to be at levels ranging from 1417, 15 to 414, and 5.0 to 280 μg/L in China, Korea, and Taiwan, respectively in surface waters [29,30]. In addition, roundup, which contains glyphosate, has increased in popularity as one of the most widespread herbicides in the world. When tested in short-term exposure and at the lowest content found in water, glyphosate was found to affect duckweed [31]. As the food production industry expands, the usage of agricultural related chemicals increases, which emphasizes the need for designing technology to remove these chemicals, including from the waterways [28]. Several researchers have tested and discovered that duckweed could be used as a treatment to pick up anthropogenic pollutants and convert it into plant biomass before the chemicals enter other waterways [32]. However, because duckweed is a good absorber, there is a possibility that it has the ability to take in toxic chemicals. If the duckweed absorbs these pollutants, the duckweed-based manure could negatively affect crops.
Yet, such property of duckweed may still be leveraged to purify polluted water. In developing nations, water and sanitation services are severely inadequate especially compared to countries such as the United States. This also increases the risk of hygiene-related diseases and as a result, millions die from otherwise preventable illnesses [33]. The lack of proper water treatment facilities in developing countries such as Pakistan remains a major cause of mortality with 0.2 to 0.25 million children dying every year due to diseases developed from drinking contaminated water [34]. The practice of using duckweed as both a phytoremediator and green manure can be used in developing nations and places without functional water treatment facilities. Lemnaceae, the subfamily Lemna minor belongs to, can be found almost anywhere on Earth, except in deserts [35]. Duckweed is therefore cost effective and easily accessible [26]. Therefore, Lemna minor could act as both a water remediator and agricultural manure which is a sustainable alternative to inorganic fertilizers.
Despite recent growing knowledge of duckweed as a green manure and as a treatment, this study examines whether duckweed can absorb pollutants and pharmaceuticals and their impact on growing crops. The central hypothesis is that if the contaminated Lemna minor green manure amendment has a wet plant biomass greater than or equal to the inorganic fertilizer, then the green manure could be coupled with the duckweed, which can also function as a water phytoremediator. The experiment answers these two main questions: (1a) Which treatment(s) the duckweed was grown with affected the kale biomass? (1b) What treatment had the greatest positive influence in promoting agricultural crop growth? (2) What is the difference between growing an agricultural crop with inorganic or organic fertilizer? The control duckweed treatment is spectulated to have the greatest increase in duckweed growth and that organic fertilizer would outperform inorganic fertilizer.
2. Methods
2.1. Duckweed propagation set-up
One species of duckweed, Lemna minor, was used for this experiment. The duckweed was provided by the Kohler Environmental Center (KEC) in Wallingford, CT. The optimal range of temperature to keep duckweed is between 63.5 to 86 Fahrenheit [36]. The duckweed was separated into 8 tanks to propagate with temperatures around 66.5 Fahrenheit. The temperature was taken by a digital thermometer with an external sensor and was purchased from Amazon. The tank was made out of glass and was 20 x 10.5 x 12.5 inches. Each tank was filled with water until the 3 inch mark. The water was put through a reverse osmosis system to remove unwanted particles and to add nutrients. Four tanks shared one grow light set at 12 hours purchased from Amazon, which is a total of 2 lights. An additional 2 tanks were placed in the greenhouse at a temperature of 74.3 Fahrenheit and tested to see if it could propagate as well as a tank under grow lights.
2.2. Duckweed container treatments
After 5 weeks, the duckweed was separated into 16 Sterilite 6 Qt. Storage Boxes (34.6 cm x 21 cm x 12.4 cm). There were four replicates per treatment. The 6-quart container could hold a total of 1 gallon of water. The water went through the same reverse osmosis system. An average of 59.90g of water were added to each container, not including any treatments nor duckweed. Additionally, 8mL per 6-quart container of Thrive’s All in One Fertilizer was given to all 16 containers as duckweed requires nutrients to grow [36]. The aquatic fertilizer, purchased from Amazon, contained nitrogen, phosphate, potash, magnesium, sulfur, boron, copper, iron, manganese, molybdenum, and zinc. The application rate was determined based on the nitrogen concentration listed on the bottle, in order to simulate nitrogen levels typically found in untreated sewage entering a municipal wastewater facility. A range from 20-85mg/L of nitrogen were found so 50mg/L of nitrogen were allotted per container [37].
Four of the containers held 1 tablet of ibuprofen. The ibuprofen was purchased from Amazon containing 200 tablets with 200mg per tablet. 1 tablet was put into a beaker with 1L of water and mixed using a magnetic stirrer. After 5 minutes, 12mL of the mixture was allocated to each container. The amount of ibuprofen was based on the concentration of ibuprofen found in aquatic ecosystems in several countries. Another four contained 6 fluid ounces of glyphosate. The glyphosate was purchased from Home Depot as a 35.2oz Concentrate Weed and Grass Killer Roundup Concentrate Plus. 990mL of water and 10mL of roundup were mixed using a magnetic stirrer for 5 minutes. 13.0µL were allocated for each container. Four different containers contained a mixture of both 6 ounces of glyphosate and a 200mg tablet of ibuprofen. A different set of four contained both glyphosate and ibuprofen containing the same amounts of treatments. The last four only had Thrive fertilizer and did not have any other additives. This was the control of the experiment. An average of 59.71 grams of duckweed were placed into each container.
2.3. Kale pots
The green manure used in the final treatment pots for kale consisted of 40 g of treated duckweed and 133.57 g of soil amendment. The soil amendment was prepared as a mixture of peat moss, vermiculite, and perlite in a 2:1:1 ratio. Black Gold Natural & Organic Canadian Sphagnum Peat Moss, Sta-Green Organic Vermiculite, and Viagro Horticulture Perlite were purchased and provided by the KEC.
After the soil amendment was prepared, blue curled scotch kale seeds were planted in each of the starter pots, with 3–4 seeds sown at a depth of ¼ inch. A total of 40 containers were filled with the soil mixture, and 3–4 kale seeds were distributed per pot. The Vates Blue Scotch Curled non-GMO heirloom seeds were obtained from Gardeners Basics.
There were four replicates for each treatment: inorganic fertilizer, glyphosate green manure, ibuprofen green manure, combined glyphosate and ibuprofen green manure, untreated green manure, and soil only. In total, 24 plastic pots (6 × 4 inches) were filled to the 3.5-inch mark with the soil amendment for the final kale treatments. After three weeks of duckweed treatment, 133.6 g of wet duckweed biomass was measured and applied to the top of each kale pot. The contents were mixed with a shovel to incorporate the duckweed into the soil. Over the course of three days, the decomposing duckweed was turned to accelerate the green manure process. Pot placement was randomized using an Excel sheet randomizer. The inorganic fertilizer was composed of the soil mixture and 5mL of Miracle-Gro’s All Purpose Plant Food mixed with a gallon of water, which was sprayed on the pot.
2.4. Biomass measurement
After four weeks of treatment, the plants were harvested and their wet biomass was measured. The total wet biomass per pot was weighed in grams, after which the roots were severed to obtain the aboveground biomass. The belowground wet biomass was then calculated by subtracting the aboveground biomass from the total wet biomass.
The plants were placed into paper bags labeled with the respective treatment names and subsequently dried in an oven to determine the dry kale biomass. After 24 hours of drying, the bags were removed, and the previously separated roots and leaves were measured to obtain the total dry biomass, dry aboveground biomass, and dry belowground biomass.
2.5. Statistical methods
R was used to analyze the data and produce visualizations. Differences between the initial and final duckweed wet biomass were analyzed using two-way analysis of variance (ANOVA) followed by boxplots and an interaction plot. The significance level was considered at p < 0.05. The p-value was significant, so the null hypothesis was rejected. Differences between the kale growth after treatment application were analyzed using two-way ANOVA followed by boxplots and an interaction plot. The significance level was considered at p > 0.05. The p-value was not significant, so the null hypothesis could not be rejected. Differences between inorganic fertilizer and green manure were analyzed using one-way ANOVA. The significance level was considered at p > 0.05. The p-value was not significant, so the null hypothesis was kept.
3. Results
3.1. The effect of treatments on duckweed growth
The duckweed in each container was weighed (in grams) at the time of distribution and again before preparing the green manure. One can see slight differences in mean in all cases except for duckweed grown with ibuprofen (Figure 1A and Figure 1B). Duckweed grown with glyphosate showed a significant difference (p < 0.05), however duckweed grown with ibuprofen did not show a significant difference (p > 0.05). The combination of glyphosate and ibuprofen induced statistically significant growth of duckweed compared to controls. There is a strong correlation showing that duckweed growth increases depending on the presence of glyphosate (Figure 1C).
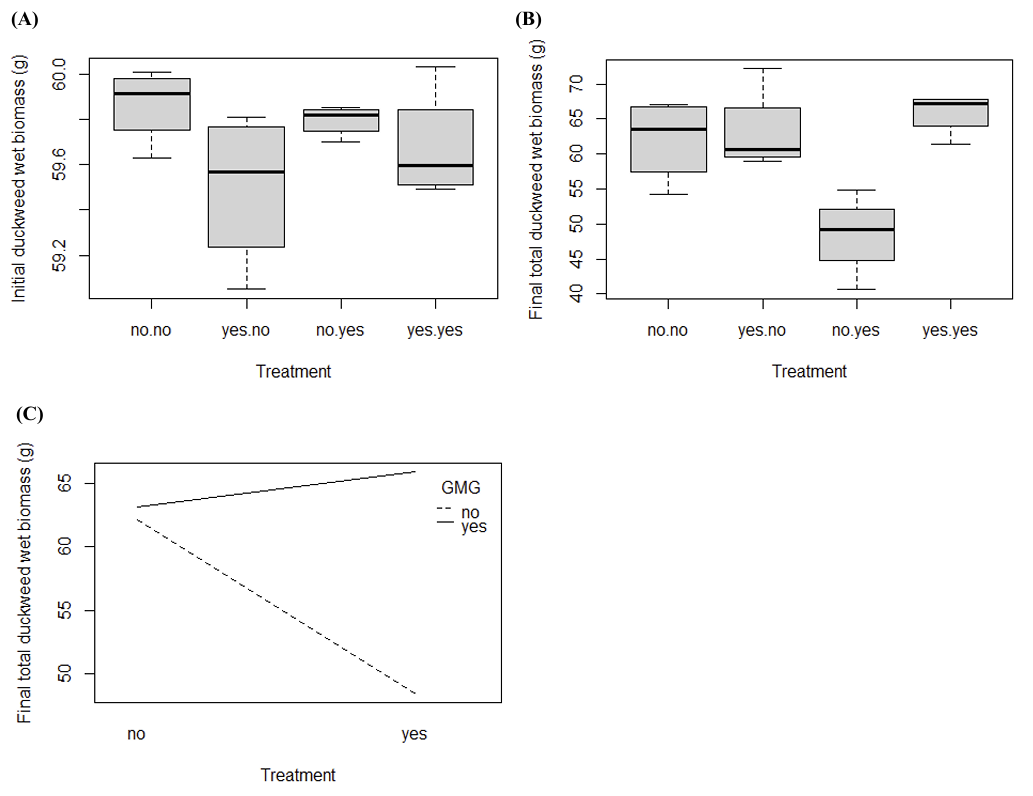
3.2. The effect of treatments on kale using wet biomass
After a few weeks of watering with or without treatment, the total, aboveground, and belowground wet kale biomass per pot was weighed in grams. There was no statistically significant between treatment conditions (p > 0.05, Figure 2A, Figure 2B). In contrast to the duckweed (Figure 1C), application of green manure treated with ibuprofen alone appeared to increase kale growth, however when paired with glyphosate, there was a slight decrease in biomass. The application of glyphosate alone had the highest total biomass, which is unexpected since glyphosate is typically used as a herbicide.
Kale was severed at the root–stem junction, and the leaves were collected for measurement of above-ground wet biomass. Glyphosate had a significant effect on the above ground kale growth (p < 0.05, Figure 2C). Duckweed treated with combined application of glyphosate and ibuprofen induced kale growth (p < 0.05). However, ibuprofen alone did not induce significant increase in the kale biomass (p > 0.05). (There needs to be statistical bars/astericks on the graphs). The below kale wet biomass were not significantly different across conditions (p > 0.05, Figure 2D).
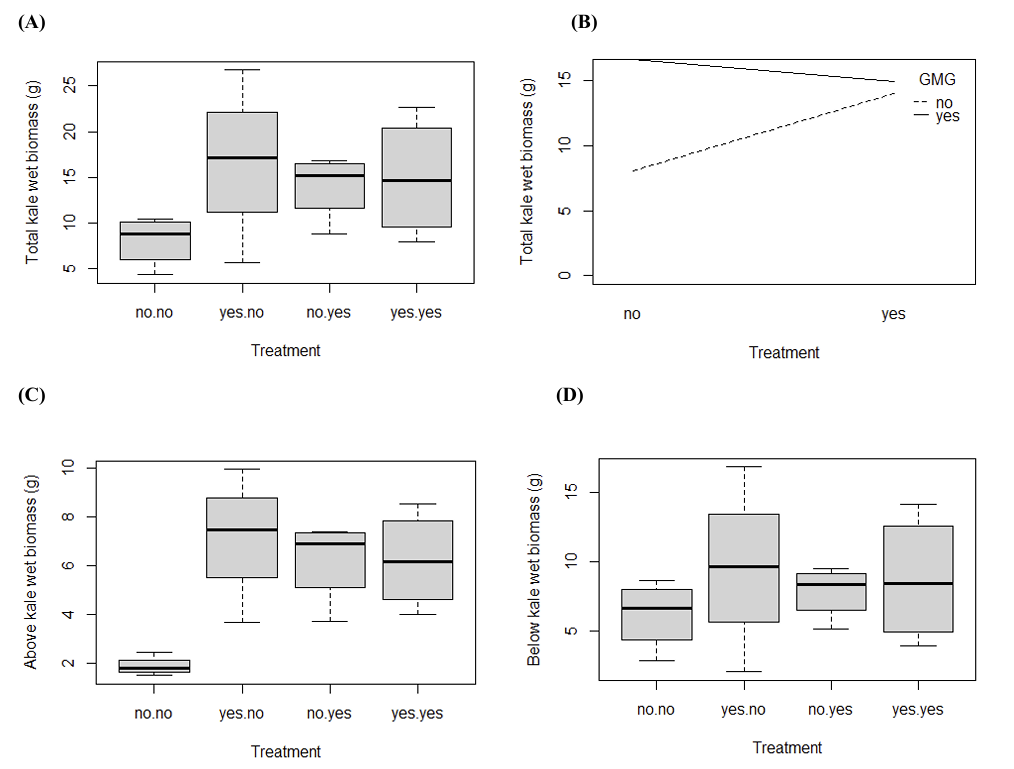
3.3. The effect of treatments on kale using dry biomass
After noting the wet kale biomass, the plants were dried in an oven to determine the dry biomass in grams. The total, aboveground, and belowground dry kale biomass per pot was then weighed. Similar to figures 2A, 2B, and 2D, nothing was statistically significant in figures 3A and 3B (p > 0.05). Figure 3B showed a trend similar to figure 2B, albeit at a smaller scale due to water loss.
The stems and leaves were separated from the roots to measure the aboveground biomass. In Figure 3C, no statistically significant differences were observed (p > 0.05). The belowground biomass was calculated by subtracting the aboveground kale biomass from the total kale biomass. However, no statistically significant differences were detected in Figure 3D (p > 0.05), indicating that the treatments did not have a measurable effect on belowground biomass under the conditions of this experiment.
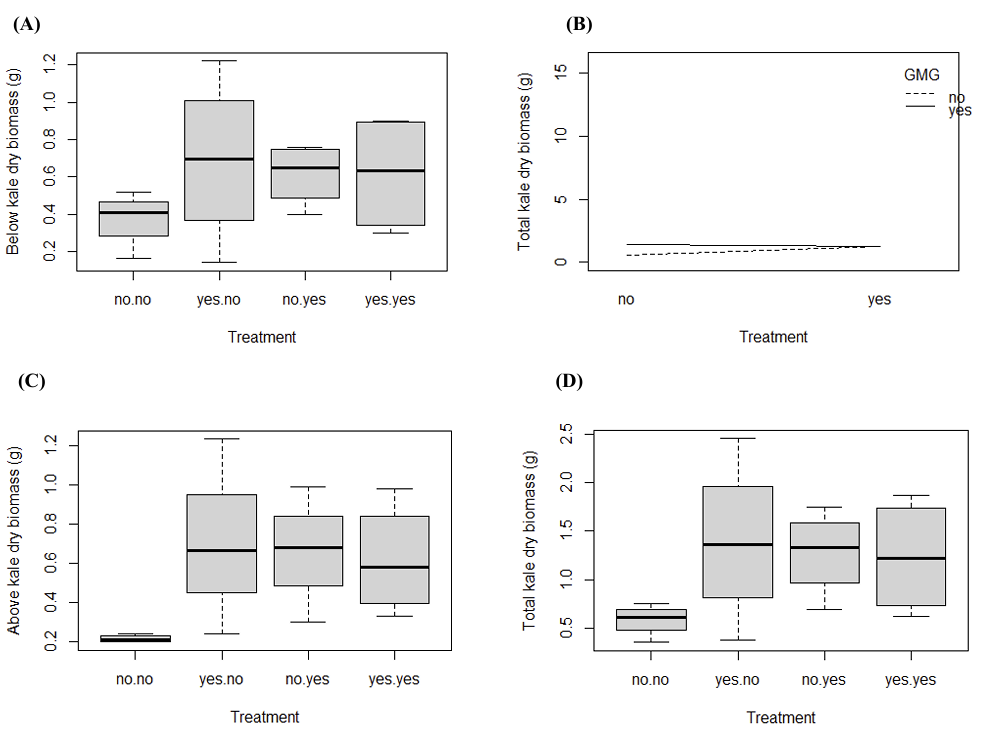
3.4. The effect of fertilizer on kale using wet biomass
Inorganic fertilizer was prepared using Miracle-Gro, while organic fertilizer consisted of untreated green manure. At the end of the kale growth period, the wet biomass per pot was collected and weighed in grams. As shown in Figures 4A, 4B, and 4C, no statistically significant differences were observed between the inorganic and organic fertilizer treatments (p > 0.05). This indicates that, under the conditions of this study, organic green manure performed comparably to the commercial inorganic fertilizer in supporting kale growth. The lack of significant differences may also suggest that the experimental scale and duration were insufficient to capture subtle treatment effects, highlighting the need for larger sample sizes, longer growth periods, and more detailed nutrient analyses in future studies.
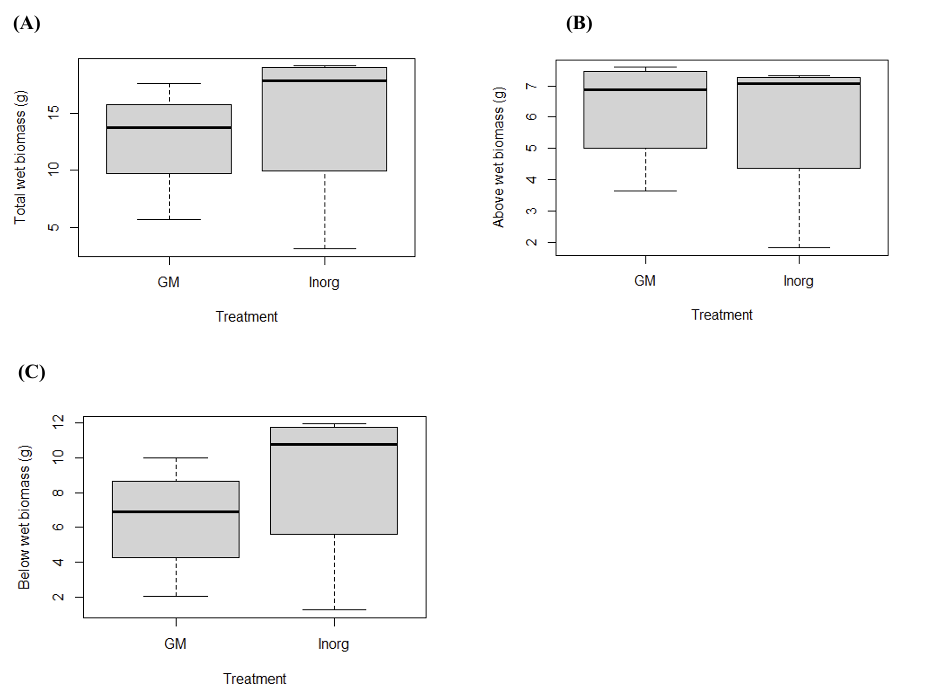
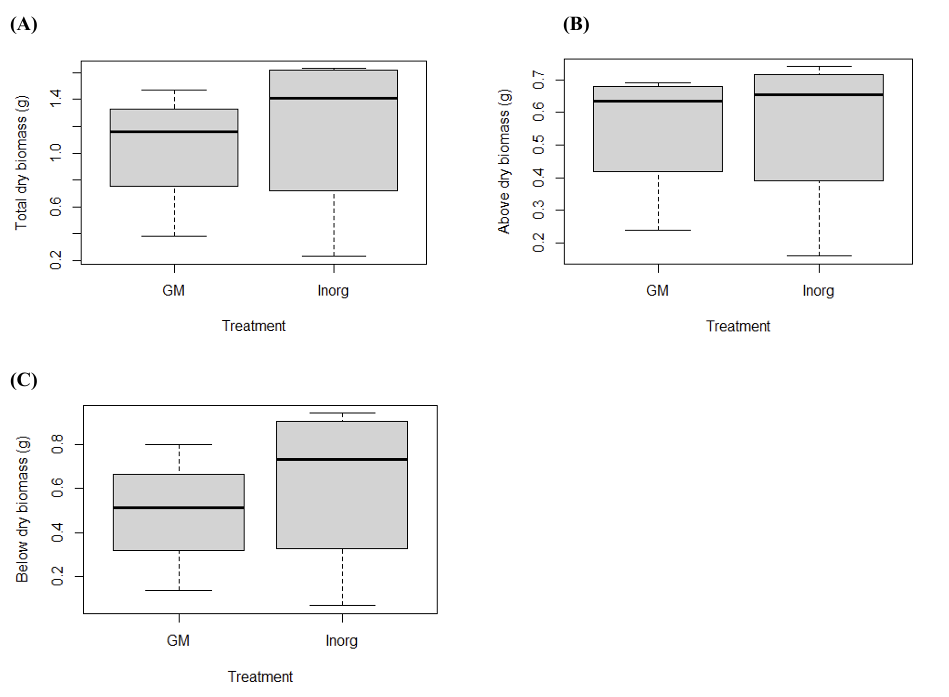
3.5. The effect of fertilizer on kale using dry biomass
After the wet biomass was recorded, kale plants were dried and their dry biomass was determined in grams per pot. Similar to the wet biomass results, no statistically significant differences were detected among treatments for total, aboveground, or belowground dry biomass (Figures 5A–C; p > 0.05). These results suggest that, within the timeframe and scale of this study, organic green manure and inorganic fertilizer provided comparable effects on kale growth. The absence of clear differences may reflect the short duration of the experiment, the relatively small sample size, or external factors such as pest pressure. While the data indicate that both fertilizer types can sustain crop growth in the short term, further investigation using longer experimental periods and more detailed nutrient analyses will be needed to determine whether differences emerge under extended cultivation.
4. Discussion
4.1. Treatment interactions with duckweed and kale
Glyphosate, which is an active ingredient in Concentrate Weed and Grass Killer Roundup Concentrate Plus, was created with the purpose of killing weeds and grass. The herbicide has been found at high concentrations in run-off such as 54 μg × L−1 in Australia and 40.8 μg × L−1 in Canada [31]. In addition, ibuprofen, which is a pain reliever medicine, has been found at average concentrations of 0.98, 1417, and 15 to 414 μg/L for Canada, China, and Korea, respectively in surface waters [30]. Plants such as Lemna minor or duckweed have been looked into as potential wastewater treatment filtration systems [38]. When exposed to glyphosate, there was an increase in growth compared to when exposed to ibuprofen, which decreased the amount of growth. However, when combined, there was an increase in growth higher than when exposed to ibuprofen alone. The interaction was statistically significant.
After application of the treatments on kale, there was statistically significant p-value in the above ground wet biomass. Glyphosate and the combination of glyphosate and ibuprofen had p-values less than 0.05. Glyphosate was shown to increase kale growth; however, ibuprofen was not able to change growth clearly. The interaction between the two meant that ibuprofen can change plant growth, but only when glyphosate is present. Thus, ibuprofen has the ability to modify how glyphosate affects the plant. The effect of ibuprofen on crop growth needs to be further evaluated. Unfortunately, the rest of the graphs were all statistically insignificant with p-values being greater than 0.05.
4.2. Implications
The presence of aphids during the last few weeks of the kale part of the experiment could have hindered the growth of the kale due to selected residing on certain individual plants. One of the inorganic fertilizer plants died and had two plants instead of three which was the standard number per pot. The lack of significance could be because of the small size of the experiment and would need to be done on a larger scale. Larger concentrations of duckweed to create the green manure would most likely change the p-value and produce more accurate results. Furthermore, the wet and dry biomass of individual kale plants were not measured separately; data collection was conducted based on the pot as a whole. Although each average pot mass could be divided into the number of individual plants, it would not be accurate. Analyzing individual kale plants would allow better insight into the effects of the treatments had on duckweed and kale. Contrary to the experiment, glyphosate was not supposed to increase plant growth but rather decrease it [31].
Overall, the data suggests that glyphosate positively influences kale growth. Further research is needed to assess long-term effects and individual interactions between kale and the treatments. A wider range of treatment levels such as glyphosate and ibuprofen will need to be explored as well.
5. Conclusion
This study aimed to analyze the impact glyphosate and ibuprofen had on the growth of Lemna minor, a fast-growing, native aquatic plant. Application to kale in two different types of fertilizer which were inorganic fertilizer or green manure. The findings showed that glyphosate and the mixture of the two popular wastewater pollutants can positively influence kale growth. These results suggest that duckweed may utilize toxic chemicals in polluted water to promote plant growth and may serve as both a phytoremediator and a sustainable green manure. Future experiments should focus on taking a more detailed approach in addressing long-term approaches, larger experimental size, limiting access to predators, and examining individual plants. Glyphosate is meant to decrease and not increase plant growth. In conclusion, research have suggested that Lemna minor is a good candidate for acting as a potential wastewater filter to pick up pollutants. It also has the potential to be applied to other agricultural crops and replace harmful industrial practices.
Acknowledgement
The author would like to thank Lena Nicolai, Joseph Scanio, Dr. William Zhang for their valuable guidance and support throughout this study. Appreciation is also extended to Evelyn Kim who contributed to data collection. The experiments were conducted at the Kohler Environmental Center, and the facilities and resources provided are gratefully acknowledged. All data generated or analyzed during this study are available from the corresponding author upon reasonable request.
References
[1]. Ritchie, H., Roser, M., & Rosado, P. (2022). Fertilizers. Our World in Data. Retrieved November 10, 2024, from https: //ourworldindata.org/fertilizers
[2]. Djibril Moussa, M. I., Alashi, A. M., Sossa-Vihotogbé, C. N., Akponikpè, P. B., Baco, M. N., Djènontin, A. J., Aluko, R. E., & Akissoé, N. H. (2023). Developments in research on the nutritional health-promoting properties of three traditional leafy vegetables commonly consumed in sub-Saharan africa.Journal of Herbal Medicine, 40, 100668. https: //doi.org/10.1016/j.hermed.2023.100668
[3]. Tripathi, S., Srivastava, P., Devi, R. S., & Bhadouria, R. (2020). Influence of synthetic fertilizers and pesticides on soil health and soil microbiology.Agrochemicals Detection, Treatment and Remediation, 25-54. https: //doi.org/10.1016/b978-0-08-103017-2.00002-7
[4]. Lalah, J. O., Omwoma, S., Osano, F. O., Mukunda, E., Shivoga, W. S., Wafubwa, G., Muyekho, D., & Schramm, K.-W. (2018). Assessment of potential risks and effectiveness of agrochemical usage in a catchment.Integrated Analytical Approaches for Pesticide Management, 235-259. https: //doi.org/10.1016/b978-0-12-816155-5.00016-6
[5]. Benbrook, C. M. (2016). Trends in glyphosate herbicide use in the united states and globally.Environmental Sciences Europe, 28(1). https: //doi.org/10.1186/s12302-016-0070-0
[6]. Baweja, P., Kumar, S., & Kumar, G. (2020). Fertilizers and Pesticides: Their Impact on Soil Health and Environment. Soil Health. https: //link.springer.com/chapter/10.1007/978-3-030-44364-1_15
[7]. Formaglio, G., Veldkamp, E., Duan, X., Tjoa, A., & Corre, M. D. (2020). Herbicide weed control increases nutrient leaching compared to mechanical weeding in a large-scale oil palm plantation.Biogeosciences, 17(21), 5243-5262. https: //doi.org/10.5194/bg-17-5243-2020
[8]. Mohd Ghazi, R., Nik Yusoff, N. R., Abdul Halim, N. S., Wahab, I. R. A., Ab Latif, N., Hasmoni, S. H., Ahmad Zaini, M. A., & Zakaria, Z. A. (2023).Health effects of herbicides and its current removal strategies. Bioengineered, 14(1). https: //doi.org/10.1080/21655979.2023.2259526
[9]. Todd, B., & Suter, G., II. (2025, February 7). Herbicides. United States Environmental Protection Agency. Retrieved May 29, 2025, from https: //www.epa.gov/caddis/herbicides#: ~: text=Linking%20Stressors%20to%20Biological%20Responses& text=The%20most%20direct%20effects%20of, competitive%20pressure%20from%20affected%20plants.
[10]. Akinnawo, S. O. (2023). Eutrophication: Causes, consequences, physical, chemical and biological techniques for mitigation strategies.Environmental Challenges, 12, 100733. https: //doi.org/10.1016/j.envc.2023.100733
[11]. Khan, M. N., & Mohammad, F. (2013). Eutrophication: Challenges and Solutions. Eutrophication: Causes, Consequences and Control, 2. https: //link.springer.com/chapter/10.1007/978-94-007-7814-6_1
[12]. Moss, S. (2019). Integrated weed management (IWM): Why are farmers reluctant to adopt non‐chemical alternatives to herbicides?Pest Management Science, 75(5), 1205-1211. https: //doi.org/10.1002/ps.5267
[13]. Stricklin, T. (2023, January 16). Effects of Fertilizer Runoff on Drinking Water Quality. SpringWell. Retrieved November 10, 2024, from https: //www.springwellwater.com/effects-of-fertilizer-runoff-on-water/
[14]. Xu, H., Zhang, Y., Zhu, X., & Zheng, M. (2019). Effects of rainfall-runoff pollution on eutrophication in coastal zone: A case study in shenzhen bay, southern china.Hydrology Research, 50(4), 1062-1075. https: //doi.org/10.2166/nh.2019.012
[15]. Worms, P. (n.d.). Why is inorganic fertilizer so bad for the climate? One Earth. Retrieved November 8, 2024, from https: //www.oneearth.org/why-inorganic-fertilizer-is-so-bad-for-the-climate/#: ~: text=The%20waste%20product%20of%20that, %2C%20too%E2%80%94a%20horrible%20outcome.
[16]. Ma, D., Yin, L., Ju, W., Li, X., Liu, X., Deng, X., & Wang, S. (2021). Meta-analysis of green manure effects on soil properties and crop yield in northern china.Field Crops Research, 266, 108146. https: //doi.org/10.1016/j.fcr.2021.108146
[17]. Belete, T., & Yadete, E. (2023). Effect of mono cropping on soil health and fertility management for sustainable agriculture practices: A review.Journal of Plant Sciences. https: //doi.org/10.11648/j.jps.20231106.13
[18]. Akanbi, W. B., Ilupeju, E.A. O., Togun, A. O., O, A. O.A., & Adeyeye, S. A. (2010). Effects of Nigerian commercial organic fertilizers, compost and NPK on growth, shoot yield and nutritional quality of Solanum macrocarpon.The International Journal of Organic Agriculture Research and Development, 1. https: //www.ijoardjournal.org/index.php/ijoardjournal/article/view/26
[19]. Malghani, A. L., Malik, A. U., Sattar, A., Hussain, F., Abbas, G., & Hussain, J. (2010). Response of Growth and Yield of Wheat to NPK Fertilizer.Science International (Lahore).
[20]. Adekayode, F. O., & Ogunkoya, M. O. (2011). Comparative effects of organic compost and NPK fertilizer on soil fertility, yield and quality of amaranth in southwest Nigeria.International Journal of Biological and Chemical Sciences, 5(2). https: //doi.org/10.4314/ijbcs.v5i2.72087
[21]. Ali, Z., Waheed, H., Kazi, A. G., Hayat, A., & Ahmad, M. (2016). Duckweed.Plant Metal Interaction, 411-429. https: //doi.org/10.1016/b978-0-12-803158-2.00016-3
[22]. Sims, A., Gajaraj, S., & Hu, Z. (2013). Nutrient removal and greenhouse gas emissions in duckweed treatment ponds.Water Research, 47(3). https: //doi.org/10.1016/j.watres.2012.12.009
[23]. Retuta, F. (2023, May 31). Duckweed: A Superfood from the Wetlands. Alberta Institute for Wildlife Conservation. Retrieved November 8, 2024, from https: //www.aiwc.ca/blog/duckweed-a-superfood-from-the-wetlands/#: ~: text=Duckweed%20thrives%20in%20environments%20rich, such%20as%20nitrogen%20and%20phosphorus.& text=As%20a%20result%2C%20they%20can, overaccumulation%20of%20nutrients%20in%20waterbodies.
[24]. Gosling, N. (2024, July 15). Using Duckweed as a Green Manure. University of New Hampshire. Retrieved February 26, 2025, from https: //www.unh.edu/unhtoday/2024/07/using-duckweed-green-manure
[25]. What is Green Manure? (2024, August 2). University of New Hampshire. Retrieved November 10, 2024, from https: //colsa.unh.edu/blog/2024/08/what-green-manure
[26]. Yao, Y., Zhang, M., Tian, Y., Zhao, M., Zhang, B., Zhao, M., Zeng, K., & Yin, B. (2017). Duckweed ( spirodela polyrhiza ) as green manure for increasing yield and reducing nitrogen loss in rice production.Field Crops Research, 214, 273-282. https: //doi.org/10.1016/j.fcr.2017.09.021
[27]. Pulido, C. R. F., Caballero, J., Bruns, M. A., & Brennan, R. A. (2021). Recovery of waste nutrients by duckweed for reuse in sustainable agriculture: Second-year results of a field pilot study with sorghum.Ecological Engineering, 168. https: //doi.org/10.1016/j.ecoleng.2021.106273
[28]. Schwarzenbach, R. P., Egli, T., Hofstetter, T. B., von Gunten, U., & Wehrli, B. (2010). Global water pollution and human health.Annual Review of Environment and Resources, 35(1), 109-136. https: //doi.org/10.1146/annurev-environ-100809-125342
[29]. Chopra, S., & Kumar, D. (2020). Ibuprofen as an emerging organic contaminant in environment, distribution and remediation.Heliyon, 6(6), e04087. https: //doi.org/10.1016/j.heliyon.2020.e04087
[30]. Farhadi, N., Tabatabaie, T., Ramavandi, B., & Amiri, F. (2021). Ibuprofen elimination from water and wastewater using sonication/ultraviolet/hydrogen peroxide/zeolite-titanate photocatalyst system.Environmental Research, 198, 111260. https: //doi.org/10.1016/j.envres.2021.111260
[31]. Sikorski, Ł., Baciak, M., Bęś, A., & Adomas, B. (2019). The effects of glyphosate-based herbicide formulations on Lemna minor, a non-target species.Aquatic Toxicology, 209, 70-80. https: //doi.org/10.1016/j.aquatox.2019.01.021
[32]. Zhou, Y., Stepanenko, A., Kishchenko, O., Xu, J., & Borisjuk, N. (2023). Duckweeds for phytoremediation of polluted water.Plants, 12(3), 589. https: //doi.org/10.3390/plants12030589
[33]. Montgomery, M. A., & Elimelech, M. (2007). Water and Sanitation in Developing Countries: Including Health in the Equation.Environmental Science & Technology. https: //pubs.acs.org/doi/pdf/10.1021/es072435t
[34]. Zahoor, I., & Mushtaq, A. (n.d.). Water Pollution from Agricultural Activities: A Critical Global Review.International Journal of Chemical and Biochemical Sciences. https: //www.iscientific.org/wp-content/uploads/2023/05/19-IJCBS-23-23-24.pdf
[35]. Asolekar, S., Kalbar, P., Chaturvedi, M., & Maillacheruvu, K. (2014). Rejuvenation of rivers and lakes in india: Balancing societal priorities with technological possibilities.Comprehensive Water Quality and Purification, 181-229. https: //doi.org/10.1016/b978-0-12-382182-9.00075-x
[36]. Yahaya, N., Hamdan, N. H., Zabidi, A. R., Mohamad, A. M., Suhaimi, M. L. H., Johari, M. A. A. M., Yahya, H. N., & Yahya, H. (2022). Duckweed as a future food: Evidence from metabolite profile, nutritional and microbial analyses.Future Foods, 5, 100128. https: //doi.org/10.1016/j.fufo.2022.100128
[37]. Wastewater Technology Fact Sheet Trickling Filter Nitrification. (2000, September). United States Environmental Protection Agency. Retrieved May 26, 2025, from https: //www3.epa.gov/npdes/pubs/trickling_filt_nitrification.pdf
[38]. Dalu, J., & Ndamba, J. (2003). Duckweed based wastewater stabilization ponds for wastewater treatment (a low cost technology for small urban areas in zimbabwe).Physics and Chemistry of the Earth, Parts A/B/C, 28(20-27), 1147-1160. https: //doi.org/10.1016/j.pce.2003.08.036
Cite this article
Kim,B. (2025). Usage of lemna minor as a water phytoremediator converted into green manure. Advances in Engineering Innovation,16(11),1-9.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Journal:Advances in Engineering Innovation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Ritchie, H., Roser, M., & Rosado, P. (2022). Fertilizers. Our World in Data. Retrieved November 10, 2024, from https: //ourworldindata.org/fertilizers
[2]. Djibril Moussa, M. I., Alashi, A. M., Sossa-Vihotogbé, C. N., Akponikpè, P. B., Baco, M. N., Djènontin, A. J., Aluko, R. E., & Akissoé, N. H. (2023). Developments in research on the nutritional health-promoting properties of three traditional leafy vegetables commonly consumed in sub-Saharan africa.Journal of Herbal Medicine, 40, 100668. https: //doi.org/10.1016/j.hermed.2023.100668
[3]. Tripathi, S., Srivastava, P., Devi, R. S., & Bhadouria, R. (2020). Influence of synthetic fertilizers and pesticides on soil health and soil microbiology.Agrochemicals Detection, Treatment and Remediation, 25-54. https: //doi.org/10.1016/b978-0-08-103017-2.00002-7
[4]. Lalah, J. O., Omwoma, S., Osano, F. O., Mukunda, E., Shivoga, W. S., Wafubwa, G., Muyekho, D., & Schramm, K.-W. (2018). Assessment of potential risks and effectiveness of agrochemical usage in a catchment.Integrated Analytical Approaches for Pesticide Management, 235-259. https: //doi.org/10.1016/b978-0-12-816155-5.00016-6
[5]. Benbrook, C. M. (2016). Trends in glyphosate herbicide use in the united states and globally.Environmental Sciences Europe, 28(1). https: //doi.org/10.1186/s12302-016-0070-0
[6]. Baweja, P., Kumar, S., & Kumar, G. (2020). Fertilizers and Pesticides: Their Impact on Soil Health and Environment. Soil Health. https: //link.springer.com/chapter/10.1007/978-3-030-44364-1_15
[7]. Formaglio, G., Veldkamp, E., Duan, X., Tjoa, A., & Corre, M. D. (2020). Herbicide weed control increases nutrient leaching compared to mechanical weeding in a large-scale oil palm plantation.Biogeosciences, 17(21), 5243-5262. https: //doi.org/10.5194/bg-17-5243-2020
[8]. Mohd Ghazi, R., Nik Yusoff, N. R., Abdul Halim, N. S., Wahab, I. R. A., Ab Latif, N., Hasmoni, S. H., Ahmad Zaini, M. A., & Zakaria, Z. A. (2023).Health effects of herbicides and its current removal strategies. Bioengineered, 14(1). https: //doi.org/10.1080/21655979.2023.2259526
[9]. Todd, B., & Suter, G., II. (2025, February 7). Herbicides. United States Environmental Protection Agency. Retrieved May 29, 2025, from https: //www.epa.gov/caddis/herbicides#: ~: text=Linking%20Stressors%20to%20Biological%20Responses& text=The%20most%20direct%20effects%20of, competitive%20pressure%20from%20affected%20plants.
[10]. Akinnawo, S. O. (2023). Eutrophication: Causes, consequences, physical, chemical and biological techniques for mitigation strategies.Environmental Challenges, 12, 100733. https: //doi.org/10.1016/j.envc.2023.100733
[11]. Khan, M. N., & Mohammad, F. (2013). Eutrophication: Challenges and Solutions. Eutrophication: Causes, Consequences and Control, 2. https: //link.springer.com/chapter/10.1007/978-94-007-7814-6_1
[12]. Moss, S. (2019). Integrated weed management (IWM): Why are farmers reluctant to adopt non‐chemical alternatives to herbicides?Pest Management Science, 75(5), 1205-1211. https: //doi.org/10.1002/ps.5267
[13]. Stricklin, T. (2023, January 16). Effects of Fertilizer Runoff on Drinking Water Quality. SpringWell. Retrieved November 10, 2024, from https: //www.springwellwater.com/effects-of-fertilizer-runoff-on-water/
[14]. Xu, H., Zhang, Y., Zhu, X., & Zheng, M. (2019). Effects of rainfall-runoff pollution on eutrophication in coastal zone: A case study in shenzhen bay, southern china.Hydrology Research, 50(4), 1062-1075. https: //doi.org/10.2166/nh.2019.012
[15]. Worms, P. (n.d.). Why is inorganic fertilizer so bad for the climate? One Earth. Retrieved November 8, 2024, from https: //www.oneearth.org/why-inorganic-fertilizer-is-so-bad-for-the-climate/#: ~: text=The%20waste%20product%20of%20that, %2C%20too%E2%80%94a%20horrible%20outcome.
[16]. Ma, D., Yin, L., Ju, W., Li, X., Liu, X., Deng, X., & Wang, S. (2021). Meta-analysis of green manure effects on soil properties and crop yield in northern china.Field Crops Research, 266, 108146. https: //doi.org/10.1016/j.fcr.2021.108146
[17]. Belete, T., & Yadete, E. (2023). Effect of mono cropping on soil health and fertility management for sustainable agriculture practices: A review.Journal of Plant Sciences. https: //doi.org/10.11648/j.jps.20231106.13
[18]. Akanbi, W. B., Ilupeju, E.A. O., Togun, A. O., O, A. O.A., & Adeyeye, S. A. (2010). Effects of Nigerian commercial organic fertilizers, compost and NPK on growth, shoot yield and nutritional quality of Solanum macrocarpon.The International Journal of Organic Agriculture Research and Development, 1. https: //www.ijoardjournal.org/index.php/ijoardjournal/article/view/26
[19]. Malghani, A. L., Malik, A. U., Sattar, A., Hussain, F., Abbas, G., & Hussain, J. (2010). Response of Growth and Yield of Wheat to NPK Fertilizer.Science International (Lahore).
[20]. Adekayode, F. O., & Ogunkoya, M. O. (2011). Comparative effects of organic compost and NPK fertilizer on soil fertility, yield and quality of amaranth in southwest Nigeria.International Journal of Biological and Chemical Sciences, 5(2). https: //doi.org/10.4314/ijbcs.v5i2.72087
[21]. Ali, Z., Waheed, H., Kazi, A. G., Hayat, A., & Ahmad, M. (2016). Duckweed.Plant Metal Interaction, 411-429. https: //doi.org/10.1016/b978-0-12-803158-2.00016-3
[22]. Sims, A., Gajaraj, S., & Hu, Z. (2013). Nutrient removal and greenhouse gas emissions in duckweed treatment ponds.Water Research, 47(3). https: //doi.org/10.1016/j.watres.2012.12.009
[23]. Retuta, F. (2023, May 31). Duckweed: A Superfood from the Wetlands. Alberta Institute for Wildlife Conservation. Retrieved November 8, 2024, from https: //www.aiwc.ca/blog/duckweed-a-superfood-from-the-wetlands/#: ~: text=Duckweed%20thrives%20in%20environments%20rich, such%20as%20nitrogen%20and%20phosphorus.& text=As%20a%20result%2C%20they%20can, overaccumulation%20of%20nutrients%20in%20waterbodies.
[24]. Gosling, N. (2024, July 15). Using Duckweed as a Green Manure. University of New Hampshire. Retrieved February 26, 2025, from https: //www.unh.edu/unhtoday/2024/07/using-duckweed-green-manure
[25]. What is Green Manure? (2024, August 2). University of New Hampshire. Retrieved November 10, 2024, from https: //colsa.unh.edu/blog/2024/08/what-green-manure
[26]. Yao, Y., Zhang, M., Tian, Y., Zhao, M., Zhang, B., Zhao, M., Zeng, K., & Yin, B. (2017). Duckweed ( spirodela polyrhiza ) as green manure for increasing yield and reducing nitrogen loss in rice production.Field Crops Research, 214, 273-282. https: //doi.org/10.1016/j.fcr.2017.09.021
[27]. Pulido, C. R. F., Caballero, J., Bruns, M. A., & Brennan, R. A. (2021). Recovery of waste nutrients by duckweed for reuse in sustainable agriculture: Second-year results of a field pilot study with sorghum.Ecological Engineering, 168. https: //doi.org/10.1016/j.ecoleng.2021.106273
[28]. Schwarzenbach, R. P., Egli, T., Hofstetter, T. B., von Gunten, U., & Wehrli, B. (2010). Global water pollution and human health.Annual Review of Environment and Resources, 35(1), 109-136. https: //doi.org/10.1146/annurev-environ-100809-125342
[29]. Chopra, S., & Kumar, D. (2020). Ibuprofen as an emerging organic contaminant in environment, distribution and remediation.Heliyon, 6(6), e04087. https: //doi.org/10.1016/j.heliyon.2020.e04087
[30]. Farhadi, N., Tabatabaie, T., Ramavandi, B., & Amiri, F. (2021). Ibuprofen elimination from water and wastewater using sonication/ultraviolet/hydrogen peroxide/zeolite-titanate photocatalyst system.Environmental Research, 198, 111260. https: //doi.org/10.1016/j.envres.2021.111260
[31]. Sikorski, Ł., Baciak, M., Bęś, A., & Adomas, B. (2019). The effects of glyphosate-based herbicide formulations on Lemna minor, a non-target species.Aquatic Toxicology, 209, 70-80. https: //doi.org/10.1016/j.aquatox.2019.01.021
[32]. Zhou, Y., Stepanenko, A., Kishchenko, O., Xu, J., & Borisjuk, N. (2023). Duckweeds for phytoremediation of polluted water.Plants, 12(3), 589. https: //doi.org/10.3390/plants12030589
[33]. Montgomery, M. A., & Elimelech, M. (2007). Water and Sanitation in Developing Countries: Including Health in the Equation.Environmental Science & Technology. https: //pubs.acs.org/doi/pdf/10.1021/es072435t
[34]. Zahoor, I., & Mushtaq, A. (n.d.). Water Pollution from Agricultural Activities: A Critical Global Review.International Journal of Chemical and Biochemical Sciences. https: //www.iscientific.org/wp-content/uploads/2023/05/19-IJCBS-23-23-24.pdf
[35]. Asolekar, S., Kalbar, P., Chaturvedi, M., & Maillacheruvu, K. (2014). Rejuvenation of rivers and lakes in india: Balancing societal priorities with technological possibilities.Comprehensive Water Quality and Purification, 181-229. https: //doi.org/10.1016/b978-0-12-382182-9.00075-x
[36]. Yahaya, N., Hamdan, N. H., Zabidi, A. R., Mohamad, A. M., Suhaimi, M. L. H., Johari, M. A. A. M., Yahya, H. N., & Yahya, H. (2022). Duckweed as a future food: Evidence from metabolite profile, nutritional and microbial analyses.Future Foods, 5, 100128. https: //doi.org/10.1016/j.fufo.2022.100128
[37]. Wastewater Technology Fact Sheet Trickling Filter Nitrification. (2000, September). United States Environmental Protection Agency. Retrieved May 26, 2025, from https: //www3.epa.gov/npdes/pubs/trickling_filt_nitrification.pdf
[38]. Dalu, J., & Ndamba, J. (2003). Duckweed based wastewater stabilization ponds for wastewater treatment (a low cost technology for small urban areas in zimbabwe).Physics and Chemistry of the Earth, Parts A/B/C, 28(20-27), 1147-1160. https: //doi.org/10.1016/j.pce.2003.08.036









