1 Introduction
Cardiovascular diseases (CVDs) have been one of the most serious illnesses causing numerous mortalities in recent days worldwide [1]. Multiple reasons are responsible for them, including different geographic locations, modifications in lifestyle choices, shifts in social policies, cultural practices [3] and genetic inheritance. In particular, the latest factor, in which histone modification (HM) could play an essential role via lactylation [4] and phosphorylation [5], has been revealed by recent studies [6].
With mainly chronic and long-term effects, traditional therapies against CVDs, including diuretics, beta-blockers, ACE inhibitors and surgeries, have spent a fortune and put a nonnegligible financial strain on the government [1,7]. In addition, traditional treatments tend to have some side effects; for instance, diuretics may cause hypokalaemia and consequently other disorders and abnormalities [8]. Moreover, these treatments are mainly for post diseases and focus on addressing symptoms instead of eliminating factors contributing to illnesses such as obesity6, which can be attributed to epigenetic traits [9]. Therefore, further targeting treatments with fewer side effects against CVDs are needed to obtain a better therapeutic effect. Hence, we should put our sight into some novel fields in biology and medicine, like epigenetics.
An epigenetic trait is a stably heritable phenotype resulting from changes in a chromosome without alterations in the DNA sequence [10]; for instance, RNA methylation regulates depression-like behaviour in mice [11], and transforming growth factor-β downregulates H4K16 histone acetyltransferase MYST1[12]. HM is one of the fundamental parts of epigenetic inheritance. Histone is the place where genetic material deoxyribonucleic acid (DNA) winds on in a nucleosome, the basic unit for chromosomes or chromatids, through hydrogen bonds or electrostatic forces. There are eight histones in every single nucleosome (see Fig. 1, Fig. 2) [13.] Fig. 2 indicates a crystal structure of a histone octamer with H3 histones colorized in light blue and purple. The DNA surrounds a bundle of histones.
Histone combinations with DNA are normally based on certain amino acids, such as glutamic acids, which are also loci for HM. In comparison with DNA-binding amino acids, HM-targeted amino acids tend to be located at the tails of histones. There are a variety of modifications on histones, such as acetylation, ubiquitination and the addition of other groups to the histone.[13] These variations could make a difference to the transcription of DNAs through changing the global configuration of nucleosomes and hence chromatins. Once the configuration of chromatin has been changed, the affinity of RNA synthetase to DNA may decrease, and RNA synthetase may have difficulty adhering to the DNA molecule and then transcribe [14]. For example, lysine acetylation on histone can neutralise and reduce the residue and therefore weaken the interaction between the DNA molecule and the histone, which will then promote transcription [15].
HM has been put into practice clinically against specific types of tumours (nonmedullary thyroid cancer [16]), with the common means of inhibiting HM enzymes to function. Despite being widely discussed about its potential in praxis, published histone-based drugs against CVD are in the process to be developed. Intriguingly, certain HMs are referred to as profound, for instance, inhibiting methylation via a type of methyltransferase inhibitor, GSK126[17], and inhibiting histone deacetylases (HDACs) through curcumins [7]. Highlighting curcumins, which are a substance abundant in Curcuma sp. Curcumin, a type of spice widely used in Asian regions, has been confirmed to have a high ability to synthesize bioactive materials easily [18].
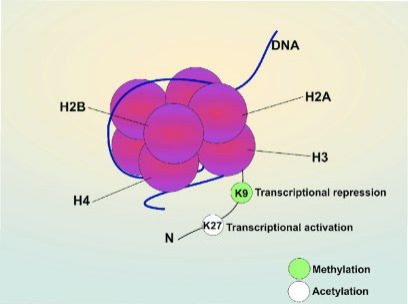
Figure 1. The figure represents a schema for a nucleosome. The DNA molecule winds on the histone octamer, and there are modification sites at the tails of histones. (Fig. 2[19]) H2A, H2B, H3 and H4 are histone monomers and K is lysine.
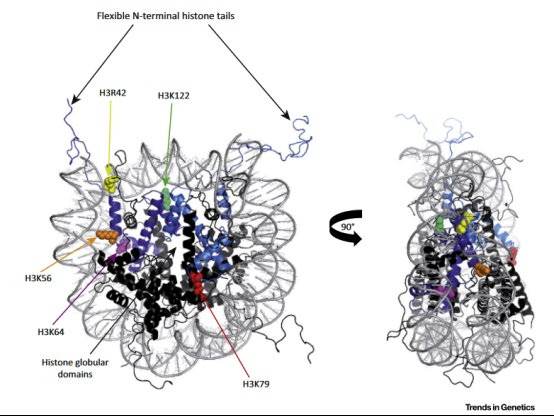
Figure 2. Location of Histone Globular Domain Modifications. Crystal structure of a histone octamer with H3 histones shown in light blue and purple. DNA is depicted in grey. H3K79 is indicated in red and is located on the solvent-exposed faces of the octamer. H3R42 (yellow), H3K56 (orange), H3K64 (pink), and H3K122 (light green) are shown on the lateral surface of the histone. Face-on and side views are shown. Note that H3R42, H3K56, H3K64, and H3K122 are located directly underneath the DNA, (Fig. 2 [13])
Remarkably, a vast number of studies have also suggested the significant role that HM plays in various CVD outbreaks (see Fig. 3) [6]. With the beginning of 2005 when Zhu et al. discovered the role DNA methylation inactivation of MCT3 played in atherosclerosis, a commonly occurring CVD in the older generation, there were all kinds of HM-related signaling pathways reported in the following years, which acclaimed the high significance recent researchers have placed on the relationship between HM and CVDs.
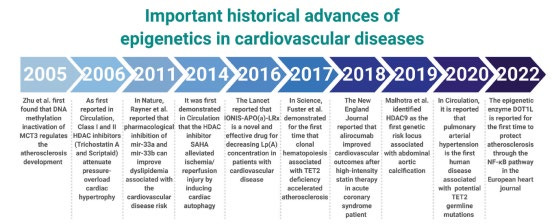
Figure 3. Important historical advances in epigenetics in cardiovascular diseases. (Fig. 3[6])
However, there are no human studies providing data for histone-based drugs in CVD therapies, while they are under evaluation both in vivo and in vitro. The reason could be that there are some potential risks, to exemplify, the cardiotoxicity of the HDAC inhibitors [7] as well as elusive mechanisms for this kind of epigenetics for numerous CVDs. Significantly, some potential histone- based drugs against coronary heart diseases, myocardial infarction and hypertension are ready to be taken into clinical trials6. In addition, histone-based treatments have encountered some challenging barriers in multiple aspects regardless of what types of drugs they are such as target selection, chemical synthesis, assay development, in vivo biology and clinical applications [20]. Hence, effective and adequate alternatives must be found.
Therefore, treatments against CVDs based on HM may play a more positive role in the clinic in the future due to its high effectiveness and accessibility to all patients, yet there are currently multiple limitations including lack of human studies and incompletely studied mechanisms. This dissertation will focus on the comparison between traditional treatments and the HM-based therapies, some mechanisms of histone-regulated signalling pathways to reduce the risk of CVDs experimentally, and the recent and profound potential histone-based drugs like HDAC inhibitors against CVDs, as well as discussing whether this novel idea could be put into clinical practice in the future with suggesting some methods to develop these therapies. Meanwhile, combined treatments will also be taken into consideration.
2 Research review
2.1 Current treatments are double-sided
There are two major fundamental clinical methods against CVDs including operation and drug treatment, both of which have distinct effectiveness varying from diseases to diseases and from individuals to individuals. For example, coronary bypass surgery (CBS) has been a standard revascularization treatment for certain CVDs, such as atherosclerosis [21], since the 1960s. According to Head et al., 2017, there have been an enormous number of patients undergoing CBS in recent years worldwide. (See Fig 4.)
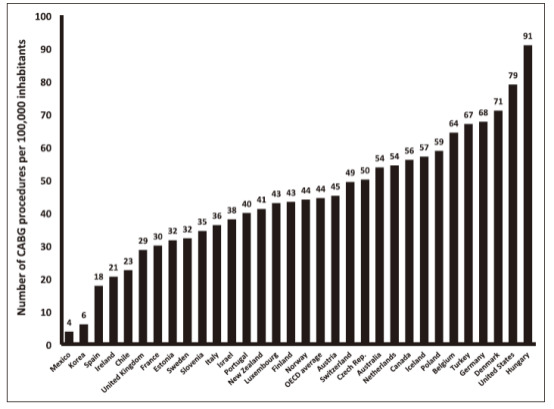
Figure 4. Number of coronary artery bypass graft (CABG) operations per 100 000 inhabitants. All data are from 2013 except for data from Hungary (2012), Belgium (2012), Australia (2012), Canada (2012), Turkey (2012), Chile (2012), the Netherlands (2010), the United States (2010), Iceland (2009), Portugal (2009), and Switzerland (2008). Data are from the Organisation for Economic Cooperation and Development (OECD) (Fig. 1, [22])
However, it was recently confirmed that various surgical procedures, namely, bilateral internal-thoracic-artery grafting and single internal-thoracic artery grafting, will benefit distinct patients; that is, the latter will significantly decrease the mortality and the risk for stroke at 10 years only if the patient will live longer than 7 years after the surgery, which is much more technically challenging to the surgeons [23]. Nevertheless, it has been considered to have side effects on neurons since the emergence of the procedure [24]. With regard to the development of the procedure and increasingly more experienced surgeons for this, it seems that there are still certain concerns about the brain, specifically, cognitive abnormalities due to microembolism, after surgery 2. An association between the suppressed immune response of monocytes and dendritic cells and CBS was also found [25].
In comparison with surgery therapy, which is a post disease treatment to a large extent, drugs against CVDs could be a much more fundamental and basic approach in clinical praxis, especially some essential nutrients, or in other words, micronutrients [26]. Highlighting antioxidants such as vitamin C, they have a significant effect on reducing the sensitivity of low-density lipoproteins to be oxidized in patients with coronary artery diseases [27]. Hypo vitamin C was meanwhile confirmed to lead to an increased risk of CVDs, while the dose of vitamin C over RDA still has an elusive effect on the risk of developing CVDs [28,29]. Apart from vitamin C, copper ions were also reported to contribute to CVDs, e.g., atherosclerosis, through copper homeostasis and copper-induced cell death.[30]
Aside from nutrient treatments, diuretics, ACE inhibitors, antiplatelets and beta-blockers are generally applied drugs against hypertension, one of the most serious CVDs.[31] Representative are beta-blockers, which have been considered the first-line antihypertensive medications and are highly recommended for therapies in specific conditions viz. patients with angina pectoris and a history of MI [32]. However, beta blockers cannot be applied to patients with moderate to severe asthma due to the requirement of adrenergic bronchodilatation to intact beta-2 receptors [33]. For diuretics, which are normally classified into loop diuretics and thiazide diuretics, both types of diuretics lower the risk of hypertension by inhibiting the reabsorption of ions and consequently reducing the volume of extracellular fluid [33,34]. In contrast, diuretics, as a type of kidney-influencing medicament, have coutilization influences on other systems in the human body, especially the urinary system. An increased incidence of urinary disorders such as hypokalaemia [34], hypovolaemia, urinary outflow tract obstruction and sepsis after application of diuretics such as furosemide [35]. Comparingly, antiplatelets can also play a positive role in reducing the recurrence risk for stroke, and this effect can be promoted by combined therapy with patent foramen ovale [36]. This popular treatment is massively preferred in prescription for primary CVD prevention in females but secondary CVD prevention in male patients in China [37]. Worldwide, the application of aspirin, a commonly used antiplatelet clinically, is extraordinarily significant in the white population, over 66% of whom have taken a dosage over 150 mg [38], as shown in Fig 5. However, major ischemic events occurred at a rate of 5.75% and major haemorrhage at a rate of 0.68% in a 90-day-long randomized trial, in which 4881 patients with minor ischemic stroke within 12 hours of time last known free of new ischemic symptoms were randomized 1:1 to either clopidogrel or matching placebo that was indistinguishable by appearance and taste, revealing that antiplatelets may lead to miss of the physiological ability to coagulation [39], see Table 1.
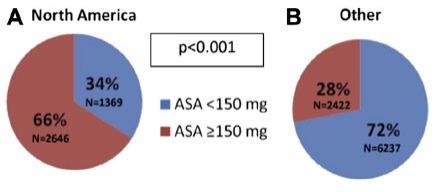
Figure 5. Discharge ASA (aspirin) dose according to Geographical Region (Fig 1[38]). Remarkably, more North American patients tend to receive high dosage of ASA than other regions.
Table 1. Percentages of coagulating disorder occurrence on a weekly basis (Fig 1[39])
Number of Events by week (% of effective sample size) |
||||||||
Total Events |
1st week |
2nd week |
3rd week |
4th week |
5th week |
6th week - Day 90 |
||
Major Ischemic Events |
Aspirin (n=2449) |
160 |
111(4.60%) |
14(0.63%) |
12(0.55%) |
4(0.19%) |
1(0.05%) |
18(1.02%) |
Clopidogrel-aspirin (n=2432) |
121 |
70(2.93%) |
16(0.71%) |
2(0.09%) |
5(0.23%) |
5(0.23%) |
23(1.26%) |
|
Difference |
39 |
41(1.67%) |
-2(-0.08%) |
10(0.46%) |
-1(-0.04%) |
-4(-0.18%) |
-5(-0.25%) |
|
Effective Sample Size |
4805 |
4475 |
4366 |
4345 |
4332 |
3587 |
||
Major Hemorrhage |
Aspirin |
10 |
4(0.17%) |
0(0.00%) |
1(0.04%) |
1(0.04%) |
1(0.04%) |
3(0.16%) |
Clopidogrel-aspirin |
23 |
7(0.29%) |
2(0.09%) |
1(0.04%) |
1(0.04%) |
3(0.13%) |
9(0.48%) |
|
Difference |
-13 |
-3(-0.13%) |
-2(-0.09%) |
0(0%) |
0(0%) |
-2(-0.1%) |
-6(-0.32%) |
|
Effective Sample Szie |
4800 |
4632 |
4547 |
4534 |
4525 |
3742 |
||
2.2 Multiple signalling pathways through HM can affect CVD outbreaks
Recent studies have confirmed numerous HM-based regulation principles of CVD risks, e.g., lactylation [4], phosphorylation [5], deacetylation [7], methylation and demethylation [14,40,41].
Given evidence that histone lysine lactylation with the involvement of glycolysis and lactate can trigger the expression of M2-like genes in M1 macrophages [42], the anti-inflammatory response and proangiogenic activities caused by histone lactylation can contribute to the recovery of immune homeostasis and cardiac repair after myocardial infarction4. M1 macrophages are responsible for proinflammatory response, whilst M2 macrophages are capable of anti-inflammatory responses and can repair damaged tissues [43]. The modification occurs on H3K18la instead of H3K18ac in M1 macrophages, proven by a heatmap (see Fig. 6), and is involved in immune, endocrine, and circulatory systems; cardiovascular diseases; and lipid and carbohydrate metabolism, known by Kyoto Encyclopedia of Gene and Genome analysis. Coherent with the function suggested by the analysis, therapeutic effects after such CVD could be realized through both immune-reparative activities and improvement in cardiac function.
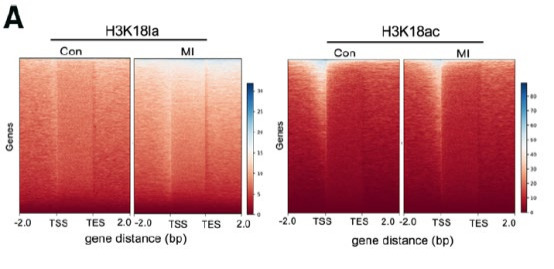
Figure 6. Heatmaps for H3K18la and H3K18ac binding peaks in circulating monocytes from sham-operated (Con) and myocardial infarction mice. Colour depth indicates the relative number of reads, and genes with similar distribution patterns are clustered together through a clustering algorithm to show the binding trends of lactylation modifications on all genes. (Fig. 3A [4])
In contrast, the senescence of vascular endothelial cells (ECs) induced by histone H3T11 phosphorylation seems to have a predominant impact on the formation of CVDs [5]. It was found that serine biosynthesis mediated by phosphoglycerate dehydrogenase (PHGDH) can suppress the premature senescence ofECs, and this process is regulated by the PHGDH-PKM2-H3pT11 axis, which is responsible for the transcription of SIRT1 and senescence-associated genes. PKM2, viz. Pyruvate kinase M2 can phosphorylate H3T11 under stimulation by PHGDH. The SIRT1 gene plays an important role in maintaining EC functions through multiple mechanisms, such as regulating eNOS (endothelial nitric oxide synthase) [44], LKB1 (liver kinase B1) and forkhead box O1 (FOXO1) [45]. These various substrates can exert vasoprotective effects and hence lower CVD risks. Representative is eNOS, which can mediate the synthesis of NO, a chemical signal in vessels, and consequently decrease CVD risk [46].
In addition to H3pT11, deacetylation can also impose a regulating effect on eNOS, albeit in non-ECs47. It was showed that the eNOS mRNA level experienced a significant surge after the application of the HDAC inhibitor TSA, which can promote the acetylation of H3 at the eNOS 5’- flanking region and result in the downregulation of DNA methylation-mediated repression of the eNOS promoter. In fact, the effects of histone deacetylation on the formation of CVDs are complex yet self-contradictory. On the one hand, inhibiting HDACs may change the expression of cell cycle genes and diminish the proliferation of smooth muscle cells, which could be atheroprotective [14,40]. Furthermore, HDACs may also lead to abnormal amounts of ion channels, such as Ca2+ channels, Na+ channels and K+ channels, which is attributed to arrhythmia [48]. On the other hand, HDACs can have anti-CVD potential, as mice with either knocking down Hdac3 or exterior injection of the HDAC inhibitor trichostatin A will consequently suffer from greater CVD hazard [40]. Nevertheless, the prevailing view of the academic community tends to accept that the disadvantages of HDACs significantly outweigh their positive effects, referring to their enhancement in tumorigenesis [48,49], yet it is not biased. There are a vast number of studies indicating the promotive role that HDACs play in CVD development, including cardiac hypertrophy [50–55], hypertension [55,56], arrhythmia [48,55], atrial fibrillation [55,57–59] and atherosclerosis [60]. The debate on HDACs is mainly concentrated on atherosclerosis, a type of CVD that can be affected variously according to different classes of HDACs60. Based on sequence similarity, HDACs have been classified into four classes, where class III HDACs are NAD+ dependent, whereas the remaining classes are zinc dependent. Different classes of HDACs can cause different proatherogenic oscillatory shear stress and atheroprotective pulsatile effects, two shear forces responsible for atherosclerosis outbreaks (see Fig. 7) [60]. Class I increases the oxidation and proliferation of endothelial cells, while class II upregulates inflammation under proatherogenic oscillatory shear. In the environment with another type of shear force, both class I and class II have a reversed function compared to the effects in oscillatory shear, albeit without antioxidation effects in class I. Moreover, class III can contemporarily enhance NO synthesis, which can protect the failing hearts [14]. Altogether, HDACs or histone deacetylation can increase CVD risk, considering that more suppressive effects of HDACs on the physiological functions of cardiovascular cells were found than supportive effects.
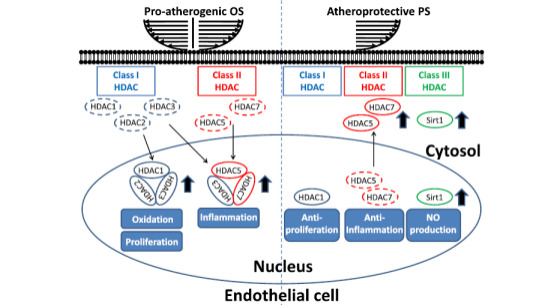
Figure 7. Roles of HDACs in modulating hemodynamics-regulated EC dysfunctions, including proliferation, inflammation, and oxidation. Proatherogenic OS induces the expression and nuclear accumulation of both class I (HDAC-1, -2, and -3), labelled in blue, and class II HDACs (HDAC-5 and -7), labelled in red. Moreover, OS further enhances the formation of HDAC-1/HDAC-2/HDAC-3 and HDAC-3/HDAC-5/HDAC-7 heterocomplexes to promote proliferation, inflammation, and oxidation. In contrast, atheroprotective PS induces phosphorylation-dependent nuclear export of class II HDACs to decrease HDAC levels in the nucleus to inhibit their effects on proliferation, inflammation, and oxidation. On the other hand, PS induces the expression of Class III (Sirt1), labelled in green, to enhance NO production [60].
Able to work as the upstream reaction of HDAC in the signaling pathway, histone methylation can also contribute to CVD risks [14]. Accordingly, histone methylation can increase HDAC activity in caveolin-knockout mice, resulting in decreased ventricular function and increased apoptosis of cardiomyocyte cells in the setting of ischemia and reperfusion.
2.3 Current HM-based treatments against CVDs are limited
Principles for HM affecting CVDs provide necessities for the development of HM-based epigenetic treatments against CVDs. Compared with HM-based medicaments applied in cancer, where HDACs show mostly disease-causing effects attributed to the induction of cell proliferation and repression of apoptosis and hence HDAC inhibitors have already made some success [49,61,62], HM- based drugs against CVDs seem to be more slow-paced with respect to more complicated mechanisms, as mentioned above, and potential cardiotoxicity [7]. It is more despairing that a worse CVD situation has been observed during the application of the HDAC inhibitor vorinostat, which causes frequent and serious thrombi as a side effect [49].
In contrast, other indirect influences on HM may also be therapeutic against CVDs. Phytochemicals, bioactive compounds synthesized by plants [63], have already been confirmed to have anti-CVD properties due to their ability to activate histone modification signaling pathways [64,65] and to interact with the gut microbiome [65]. Simultaneous therapeutic effects caused by histone modification interactions and further production of phytochemical-derived short-chain fatty acid metabolites (SCFAs), bioactive substances that can be produced by gut microorganisms, can be observed. Despite the absence of related drugs, substances such as curcumin and resveratrol exist in daily diets, in some types of spices and peanuts, grapes, red wine and some berries, respectively [65]. With developed extraction or synthesis methods, clinical trials can be conducted to check their efficiency in treating CVDs [66,67].
Conclusively, there are currently HM-based drugs against cancers instead of CVDs, and we still have multiple barriers standing in the way to the application of HM-based CVD drugs, including the safety and the effectiveness. Hence, we should carefully evaluate their prospect.
3 Discussion/ Development
Overall, HM-based drugs against CVDs still have a long journey to clinical application despite their numerous positive attributes.
With respect to potential concerns and side effects of traditional therapies against CVDs, targeting treatments, a representative example of which can be HM-based drugs, are in an urgent requirement of CVD patients for both their preventing and therapeutic effects. However, what we can never contemporarily ignore is the current significance of these traditional therapies. They have been confirmed about their validity and ability to reduce CVD risks and hazards with a long clinical application history and experience. Comparingly, the actual role that HM-based drugs will play in modern medicine still underlies their mysterious and complicated mechanism and effects on cardiovascular cells and the circulatory system. According to the recent clinical application of FDA-certified HDAC inhibitors, which are used in cancer therapies rather than CVD therapies, both cardiovascular and other side effects can be observed for some HDAC inhibitors [68], whilst some other kinds of HDAC inhibitors may potentially do not. This limits the development of these drugs for CVD patients. Meanwhile, clinical certification, application in other conditions and theoretically confirmed mechanisms cannot fully confirm the clinical effectiveness, which must be assessed by careful clinical trials.
In contrast, our current understanding of HM mechanisms that can make a difference to CVDs still indicates the potential application of such drugs to a large extent, some types of which have even shown a good capacity to be applied to patients combined with traditional therapies; for instance, histone lactylation activators, which may boost long-term postmyocardial infarction curation, can be combined with traditional treatments, which could have short-term effects [69]. Meanwhile, a combined therapy in this may also mean that the safer lower dosage of traditional drugs, especially beta-blockers, on dosage of which there is a debate [70], can be applied without concerns about the therapeutic effects, as these drugs can fulfil the missed effects through another mechanism. In addition, a change in dietary style may also be a daily HM-based ‘treatment’ that can be suggested to patients simultaneously with traditional CVD drugs, and consequently, multiple aspects of CVD relief can be achieved.
Nevertheless, their side effects should also be taken into consideration. HDAC inhibitors, which have already been confirmed to lead to further CVDs, may even harden the situation, including thrombosis [49], cardiac toxicity [7], and myelosuppression [71]. On the one hand, it can be the difference of different types of HDAC inhibitors. On the other hand, the place where these drugs play a role may be the key, whether at pro-atherogenic OS or atheroprotective PS. Therefore, they may still have a long journey before combined application before their targeting has been improved. However, this is not a concern unique to HDAC inhibitors but other HM-based drugs. It is true that further improvement in the usage of chemicals as HDAC inhibitors or other HM influencers can relieve the dilemma on the one hand, but it seems to be extremely challenging to fully develop a novel bioactive chemical with certain properties [72,73]. An alternative could be developing drug delivery. Both phytochemicals, which are abundant in daily nutrition, and some HDAC inhibitors (panobinostat, vorinostat and chidamide. They are currently used for cancer therapies) are treated orally, which will experience a long transportation to approach the target with a likelihood of affecting irrelevant cells and systems. Therefore, given the difficulties in directly targeting at the focus of infection, some mediators, such as antibody-drug conjugates [74,75], nanovesicles [76,77] and nanoparticles [78–80]. More impressively, these methods carrying HDAC inhibitors have been confirmed to have an enhanced effect with respect to both targeting and effectiveness accordingly, and some of their functionally homologous treatments are already allowed by the FDA in clinical practice [76–80]. However, there have been no relevant investigations on them as CVD drugs confirming their effects. Even if they do show elevated therapeutic effects, CVD-targeting carriers, which can be absolutely different from cancer-drug carriers, should also be developed. Therefore, with respect to this aspect, effective and specific HM-based CVD drugs may still stand far away from our life.
These innovative deliveries may also have some difficulties before their clinical application, in other words, are they capable of novel drug delivery in CVD treatments and have the same level of effects like the HM-based cancer drugs. Exemplifying HDAC inhibitors, restricted published agents can be tightly carried by liposomes or immunoliposomes due to their unique structure [81]. One of them, LAQ824 or Dacinostat, obtains successfully increased therapeutic properties possibly due to their coherent half-life time with the maximum tumour accumulation, according to the investigation of Drummond, D. C. et al. Regarding CVDs, there are no published studies confirming their effects, while similar mechanisms have already been shown to be CVD-protective [82]. However, interestingly, this type of inhibitor may have an indirect effect on CVD risks, viz. anti-obesity properties. However, the capability of nanovesicles and such applications may still be elusive, so further evaluations and research are needed to conclude properly and completely, despite recent success and good prospects. Another concentration can be researching whether other types of histone modifiers, including histone lactylation activators and histone phosphorylation inhibitors, are capable of being carried on delivery molecules or whether only specific types are isolated examples with such properties.
Under simultaneous consideration of the combination with other symptoms and diseases, especially diabetes, obesity and other metabolism or immune disorders, which have already been confirmed to have a tight and strong linkage to CVD risks [83–86]. As written above, it was demonstrated that histone lactylation can enhance reparative gene activation and that this modification is greatly relevant to glycolysis and metabolism [4]. Therefore, patients with diabetes, obesity and other metabolic disorders may differ from those without such conditions in terms of treatment and dosage. According to a randomized controlled trial, there is a strong correlation between an increased risk of Type 2 Diabetes and 1-y changes in lactate [87], which is directly used as the resource for histone lactylation88. Therefore, there is evidence to suggest a potential link between metabolic dysregulation and CVD risks, and hence, it enables indirect HM-based drug development ideas, viz. therapeutic effects via histone modification on cell respiration- or glycolysis-related genes, such as targeting the ENO1 pathway, which has already been proven to have a regulatory role in glycolysis [89]. To some extent, the investigation of Wang N et al. also revealed the connection between the immune system, digestive system and circulatory system, which also makes personalized treatment for a patient with multiple and complex disorders capable and optional. According to Wang N et al., the lactate level can affect the phenotype of macrophages by changing the acidity; hence, varied content in histone lactylation-based treatments can be developed, equipped with sodium lactate elevation contents for patients without immune disorders and with lactate promotion contents for patients with such additional symptoms or serious circulatory system tumours, which require M1 macrophages, the type of cell that can be converted into M2 macrophages due to histone lactylation, polarized to eliminate them[90]. However, the optimal dosage for the two diseases may also vary; hence, we should evaluate the risks and benefits for different dosages carefully, which can also be a point for further investigation.
Therefore, there will be another important issue underneath this future therapy, the relation between cancers and CVDs, or in other words, if we should prioritize the treatment against tumours or CVDs. As both CVDs1 and cancers[91] are jeopardizing increasingly more populations, and these illnesses tend to have collaborative relationships with each other92, the therapy with antagonistic effects on them, HM-based treatments, still has to be more evaluated and discussed, regardless of the HDAC inhibitors, which are already used to treat cancer patients but may aggravate the symptoms of CVDs, once an unexpected class of HDAC is inhibited, or the histone lactylation activator, which can convert M1 macrophages to M2 macrophages, resulting in a lower resistance against cancer. With respect to HDAC inhibitors, Yoon S. et al. reported generally positive results, albeit with concerns about aggravated vascular calcifications and thrombosis49. It was suggested that specific HDAC inhibitors should be developed to minimize the side effects. In contrast, the effects of HDACs in tumours appear to be much more significant in terms of dietary fibres and the microbiome, where the bacteria digest short-chain fatty acids into butyrate and downregulate HDACs [93,94]. Under that consideration, the answer to this question may still be worth discussing. For histone lactylation, this half decade, when this field has been a hot research topic with all 124 relevant articles (69 of 116 essays with their influence factor (IF) have Ifs over 6) posted within the period (see Fig. 8,), has witnessed a vast number of articles reporting the intensive linkage between such type of epigenetic shift and increased tumorigenesis [95], including ocular melanoma [96], lung cancer [97] and cell renal cell carcinoma [98]. However, there have been no scientists discussing this contradictory situation, one of the possible reasons for which could be the novelty of the field. As potential suggestions, specific delivery could be developed, as mentioned above. Antagonists can also be recommended, referring to relative disease mechanisms. For example, certain dosages of agents able to activate the 4 identified anti-H3K18la-ChIP target genes or demethylzeylasteral can be combined with histone lactylation activators when treating patients with ocular melanoma and postmyocardial infarction [4,96,99]. The result for this combination is that a regional depressed conversion of M1 macrophages near the melanoma with simultaneous increased M2 macrophages in the circulatory system contributes to healing after myocardial infarction.
In addition, the cost of HM-based CVD drugs can also be a problem standing in front oftheir future ubiquitous clinical application. Albeit without developed drugs, other HM-based drugs may be indicative. Vorinostat is sold at a price of $131.79 per unit, whereas the inexpensive diuretic Lasix has a low price of at least $5. If only considering medicament expenditure, HM-based drugs may be disadvantaged, excluding the dosage difference due to symptoms. However, indirect HM influencers phytochemicals can be more accessible in price. For example, curcumin as a supplement has a price of only $0.25 per unit. However, supplements can never replace prescriptive medicaments, so the actual financial advantage of the disadvantage of these drugs is still elusive and may be altered once better extraction or synthesis methods are developed. Meanwhile, another important evaluation data point, the average cost for curing, is currently not available due to the absence of clinical application, also resulting in their mysterious status.
Generally, HM-based drugs may still be effective despite current difficulties. First, they are more specific and targeting than traditional therapies, which mainly rely on general human regulation and homeostasis. In contrast, certain types of HM-based treatments focus more on tackling the source of diseases directly, which is likely to minimize damage to irrelevant cells and tissues and has high effectiveness. A potential example could be diastolic dysfunction. This condition currently has limited therapies, including diuretics and beta-blockers [100]. However, animal trials in feline models have already achieved success, although there may be some limitations, like the difference between human and feline [101]. In this example, the HM-based drug treatment SAHA seems to be much more effective without any reported adverse events. Therefore, with simultaneous additional mechanistic evidence [102], these novel treatments could be beneficial to some extent by ignoring species differences, which may lead to different relevant modification positions and differentially affected proteins downstream, but this still has to be confirmed by future research in human beings after safety confirmation.
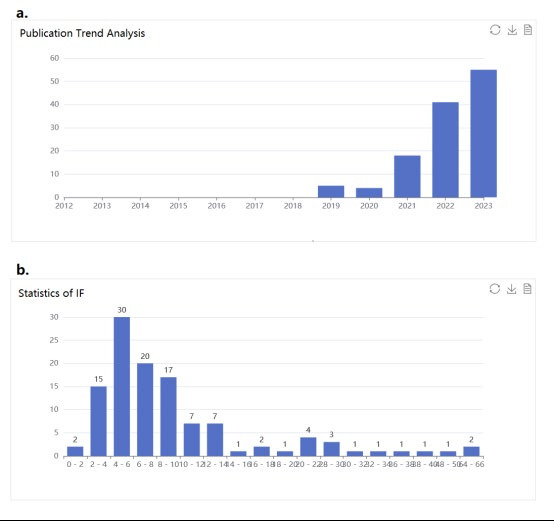
Figure 8. a. The annual number of published papers on histone lactylation. It emerged in 2019 and surged since 2020. b. The graph demonstrates the distribution of IF of essays in this field. A large number of essays are in zone 1 of SCI.
In another aspect, phytochemicals may be low-risk and beneficial substitutes that act as HM-based CVD drugs. Compared with more direct HM-based treatments, some of these chemicals, viz. Curcumin, EGCG and resveratrol are in clinical trials against CVDs. In fact, these substances have numerous advantages in comparison with the remaining potential HM-based treatments. First, they are basically natural molecules that are abundant in our daily nutrients, which means both that all patients have easy access to them and that they are safe to a large extent. The latter attribute for curcumin and resveratrol has also been confirmed by randomized trials, without any serious adverse event occurrence [103,104]. What also benefits from them is that adherence to pill taking may increase, especially for the senior generation, who is likely to be reluctant to take medicaments due to bias. Once these elderly patients are informed that these chemicals are plant-derived, they may take such medicaments or have such a diet more regularly, resulting in a potentially better therapeutic effect. Furthermore, phytochemicals can prevent more outbreaks than other therapies against CVDs. In other words, we have a type of more reliable preventive agent against factors other than vitamin C, whose effects have not yet been proven. One of the results of this may be a relieved financial stress on the government and individuals, who are paying a fortune to postoutbreak therapies, viz. The traditional therapies against CVDs. In addition, these chemicals show other therapeutic effects on other diseases, including chronic kidney diseases [105], tumours [106] and osteoarthritis [107], leading to a great capacity as treatments for patients with multiple conditions other than CVDs, while unexpected adverse events may have an increased probability due to a vast number of targeting mechanisms. Meanwhile, what is also behind these advantages is the reduced immediacy compared to those artificially synthesized chemicals due to the regulation principle. Phytochemicals are more likely to influence CVD risk through gut microorganisms instead of direct effects [65,108]. Hence, there could be multiple factors disrupting the regulation pathway for CVD risks, which may consequently harm the effectiveness of these drugs. Under such consideration, phytochemicals should be evaluated more when they are regarded as CVD drugs.
In addition, these drugs can provide a new idea for CVD therapies through cell-level regulation, which is much more micro- and accurate than systematic therapies, a significant attribute of traditional therapies. With further steps, they may be more therapeutic when combined with traditional treatments. In other words, we may be able to treat CVD patients with different drugs responsible for various mechanisms.
However, all these potential benefits have not been clinically proven, which also points out the direction to which specialized researchers could focus. First, more animal trials in different species, including rats and other human-like species, should be conducted to check the safety of these drugs, especially histone lactylation activators, which are thoroughly newly discovered. Contemporarily, differences between these species and human beings ought to be highly considered, as histone modification is an inheritance substance-related process, which is dependent on histones expressed in chromosomes to a large extent. Given that different species may have various histone attributes, we should carefully identify those histone modifications that have a higher likelihood of occurrence in human bodies and consequently prioritize investigations on them. With the completion of these tasks, they can then be brought into clinical trials. In addition to safety and future clinical trials, experimental progress should also be done in some currently unsolved problems and elusive mechanisms. For instance, SIRT genes, the target of class III HDAC inhibitors and histone phosphorylation, are not only expressed in vascular endothelial cells, where the role of histone modification has been confirmed but also in other types of cardiovascular cells. A question could be whether histone modification can make a difference. Another question could be the actual relation between homoeostasis and the development of these drugs. Both histone lactylation and microorganism-mediated histone deacetylation involve homoeostasis. If direct intervention on these modifications is not realistic, can we modify histones relating to homoeostasis that acts as the upstream signal for the modifications mentioned above? In addition, inspired by the discovery of histone lactylation, are we able to uncover other novel modifications that regulate CVD outbreaks? All these questions may be a direction of future research on this topic. Aside from this, we should also evaluate the significance and importance of the development of these drugs, or is it worth paying for them. Ifworthy, can they be a sufficiently good alternative for traditional therapies? If not, what can we learn from them and apply in the development of other drugs? All these questions are ready to be answered by future studies.
4 Conclusion
As a novel field in medicine development, HM-based drugs themselves are restricted clinically to a large extent because of their complicated mechanisms, accurate functioning levels and diversity. A vast number of amino acids on different histones at various positions can be modified by a diversity of reactions, such as phosphorylation, lactylation and deacetylation, each of which has a unique influence on the expression of some genes and consequently on protein synthesis and cell phenotypes. This situation is even more true for drugs against CVDs, which include various symptoms and involve the entire body, including the immune system, circulatory system and even digestive system. This attribute of such conditions can be a reason why traditional CVD drugs appear to be not successful enough – a disbalance between different systems and their functions. Comparingly, HM-influencing molecules may be able to be treated targetingly and specifically by both unique signaling pathways and developed drug delivery methods, such as nanoparticles and nanovesicles, as well as utilizing the indirect impacts on other systems to function.
Despite advantages in mechanisms, barriers including lack of clinical trials, antagonistic or coordinated effects with other conditions and elusive cost are still obstructing their journey to clinical application. Aside from the novelty-caused shortage of clinical trials and human studies, their side effects on other conditions can be the major concern. This problem and risk may also lead to a reluctance for researchers to conduct human studies for them, resulting in other difficulties for current accurate comments on these drugs.
References
[1]. Timmis, A., et al. (2020). European Society of Cardiology: Cardiovascular disease statistics 2019. European Heart Journal, 41(12), 12–85.
[2]. Spn, M., Newman, S., Newman, S. P., & Harrison, M. J. (2002). Coronary-artery bypass surgery and the brain: Persisting concerns. The Lancet Neurology, 1. http://neurology.thelancet.com
[3]. Bhatnagar, A. (2017). Environmental determinants of cardiovascular disease. Circulation Research, 121, 162–180. https://doi.org/10.1161/CIRCRESAHA.117.306458
[4]. Wang, N., et al. (2022). Histone lactylation boosts reparative gene activation post-myocardial infarction. Circulation Research, 131, 893–908.
[5]. Wu, Y., et al. (2023). Phosphoglycerate dehydrogenase activates PKM2 to phosphorylate histone H3T11 and attenuate cellular senescence. Nature Communications, 14.
[6]. Shi, Y., et al. (2022). Epigenetic regulation in cardiovascular disease: Mechanisms and advances in clinical trials. Signal Transduction and Targeted Therapy, 7. https://doi.org/10.1038/s41392-022-01055-2
[7]. Chistiakov, D. A., Orekhov, A. N., & Bobryshev, Y. V. (2017). Treatment of cardiovascular pathology with epigenetically active agents: Focus on natural and synthetic inhibitors of DNA methylation and histone deacetylation. International Journal of Cardiology, 227, 66–82. https://doi.org/10.1016/j.ijcard.2016.11.204
[8]. Zillich, A. J., Garg, J., Basu, S., Bakris, G. L., & Carter, B. L. (2006). Thiazide diuretics, potassium, and the development of diabetes: A quantitative review. Hypertension, 48(2), 219–224.
[9]. Gutiérrez-Repiso, C., et al. (2021). Epigenetic biomarkers of transition from metabolically healthy obesity to metabolically unhealthy obesity phenotype: A prospective study. International Journal of Molecular Sciences, 22.
[10]. Berger, S. L., Kouzarides, T., Shiekhattar, R., & Shilatifard, A. (2009). An operational definition of epigenetics. Genes & Development, 23(7), 781–783.
[11]. Liu, S., et al. (2021). Fat mass and obesity-associated protein regulates RNA methylation associated with depression-like behavior in mice. Nature Communications, 12.
[12]. Zehender, A., et al. (2021). TGFβ promotes fibrosis by MYST1-dependent epigenetic regulation of autophagy. Nature Communications, 12.
[13]. Lawrence, M., Daujat, S., & Schneider, R. (2016). Lateral thinking: How histone modifications regulate gene expression. Trends in Genetics.
[14]. Kim, G. H., Ryan, J. J., & Archer, S. L. (2013). The role of redox signaling in epigenetics and cardiovascular disease. Antioxidants & Redox Signaling, 18(14), 1920–1936.
[15]. Koprinarova, M., Schnekenburger, M., & Diederich, M. (2016). Role of histone acetylation in cell cycle regulation. Current Topics in Medicinal Chemistry, 16(7), 732–744.
[16]. Celano, M., et al. (2018). Targeting post-translational histone modifications for the treatment of non-medullary thyroid cancer. Molecular and Cellular Endocrinology, 469, 38–47. https://doi.org/10.1016/j.mce.2017.05.036
[17]. Yang, Y., Luan, Y., Yuan, R. X., & Luan, Y. (2021). Histone methylation-related therapeutic challenge in cardiovascular diseases. Frontiers in Cardiovascular Medicine, 8. https://doi.org/10.3389/fcvm.2021.710053
[18]. Barros, C. H. N., et al. (2021). Synthesis and self-assembly of curcumin-modified amphiphilic polymeric micelles with antibacterial activity. Journal of Nanobiotechnology, 19.
[19]. Mäntylä, E. (2018). Import and impact: Characterization of parvovirus-nucleus interactions.
[20]. Audia, J. E., & Campbell, R. M. (2016). Histone modifications and cancer. Cold Spring Harbor Perspectives in Biology, 8.
[21]. Tompkins, B. A., & Salerno, T. A. (2021). Commentary: What does the future hold for coronary bypass surgery? Journal of Thoracic and Cardiovascular Surgery, 162, 1120–1121. https://doi.org/10.1016/j.jtcvs.2020.01.029
[22]. Head, S. J., Milojevic, M., Taggart, D. P., & Puskas, J. D. (2017). Current practice of state-of-the-art surgical coronary revascularization. Circulation, 136(12), 1331–1345.
[23]. Head, S. J., & Kappetein, A. P. (2019). Coronary bypass surgery — An ART for dedicated surgeons. New England Journal of Medicine, 380, 489–491.
[24]. Brierley, J. B. (1963). Neuropathological findings in patients dying after open-heart surgery. Thorax, 18.
[25]. Perros, A. J., et al. (2020). Coronary artery bypass grafting is associated with immunoparalysis of monocytes and dendritic cells. Journal of Cellular and Molecular Medicine, 24(9), 4791–4803.
[26]. An, P., et al. (2022). Micronutrient supplementation to reduce cardiovascular risk. Journal of the American College of Cardiology, 80(23), 2269–2285.
[27]. Mosca, L., et al. (1997). Antioxidant nutrient supplementation reduces the susceptibility of low-density lipoprotein to oxidation in patients with coronary artery disease.
[28]. Lykkesfeldt, J., Michels, A. J., & Frei, B. (2014). Vitamin C. Advances in Nutrition, 5(1), 16–18.
[29]. Moser, M. A., & Chun, O. K. (2016). Vitamin C and heart health: A review based on findings from epidemiologic studies. International Journal of Molecular Sciences, 17(8). https://doi.org/10.3390/ijms17081328
[30]. Chen, X., et al. (2023). Copper homeostasis and copper-induced cell death in the pathogenesis of cardiovascular disease and therapeutic strategies. Cell Death & Disease, 14. https://doi.org/10.1038/s41419-023-05639-w
[31]. Oparil, S., et al. (2018). Hypertension. Nature Reviews Disease Primers, 4. https://doi.org/10.1038/nrdp.2018.14
[32]. Fihn, S. D., et al. (2012). 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease. Journal of the American College of Cardiology, 60.
[33]. Laurent, S. (2017). Antihypertensive drugs. Pharmacological Research, 124, 116–125. https://doi.org/10.1016/j.phrs.2017.07.026
[34]. Weinberger, M. H. (n.d.). Diuretics and their side effects dilemma in the treatment of hypertension. Hypertension. http://hyper.ahajournals.org/
[35]. Ho, K. M., & Power, B. M. (2010). Benefits and risks of furosemide in acute kidney injury. Anaesthesia, 65(3), 283–293. https://doi.org/10.1111/j.1365-2044.2009.06228.x
[36]. Mas, J.-L., et al. (2017). Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. New England Journal of Medicine, 377(11), 1011–1021.
[37]. Xia, S., et al. (2020). Sex differences in primary and secondary prevention of cardiovascular disease in China. Circulation, 141(7), 530–539.
[38]. Kohli, P., et al. (2014). Discharge aspirin dose and clinical outcomes in patients with acute coronary syndromes treated with prasugrel versus clopidogrel: An analysis from the TRITON-TIMI 38 study (trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-thrombolysis in myocardial infarction 38). Journal of the American College of Cardiology, 63(3), 225–232.
[39]. Johnston, S. C., et al. (2019). Time course for benefit and risk of clopidogrel and aspirin after acute transient ischemic attack and minor ischemic stroke. Circulation, 140(8), 658–664. https://doi.org/10.1161/CIRCULATIONAHA.119.040713
[40]. Khyzha, N., Alizada, A., Wilson, M. D., & Fish, J. E. (2017). Epigenetics of atherosclerosis: Emerging mechanisms and methods. Trends in Molecular Medicine, 23(4), 332–347. https://doi.org/10.1016/j.molmed.2017.02.008
[41]. Xu, S., Pelisek, J., & Jin, Z. G. (2018). Atherosclerosis is an epigenetic disease. Trends in Endocrinology & Metabolism, 29(11), 739–742. https://doi.org/10.1016/j.tem.2018.09.003
[42]. Zhang, D., et al. (2019). Metabolic regulation of gene expression by histone lactylation. Nature, 574(7779), 575–580. https://doi.org/10.1038/s41586-019-1678-1
[43]. Yunna, C., Mengru, H., Lei, W., & Weidong, C. (2020). Macrophage M1/M2 polarization. European Journal of Pharmacology, 877. https://doi.org/10.1016/j.ejphar.2020.173090
[44]. Chen, M. S., Lee, R. T., & Garbern, J. C. (2022). Senescence mechanisms and targets in the heart. Cardiovascular Research, 118(5), 1173–1187. https://doi.org/10.1093/cvr/cvab288
[45]. Man, A. W. C., Li, H., & Xia, N. (2019). The role of Sirtuin1 in regulating endothelial function, arterial remodeling and vascular aging. Frontiers in Physiology, 10. https://doi.org/10.3389/fphys.2019.00918
[46]. Sessa, W. C. (2004). eNOS at a glance. Journal of Cell Science, 117(12), 2427–2429. https://doi.org/10.1242/jcs.01245
[47]. Gan, Y., et al. (2005). Role of histone deacetylation in cell-specific expression of endothelial nitric-oxide synthase. Journal of Biological Chemistry, 280(17), 16467–16475. https://doi.org/10.1074/jbc.M414065200
[48]. Hesham, H. M., Lasheen, D. S., & Abouzid, K. A. M. (2018). Chimeric HDAC inhibitors: Comprehensive review on the HDAC-based strategies developed to combat cancer. Medicinal Research Reviews, 38(6), 2058–2109. https://doi.org/10.1002/med.21488
[49]. Yoon, S., & Eom, G. H. (2016). HDAC and HDAC inhibitor: From cancer to cardiovascular diseases. Chonnam Medical Journal, 52(1), 1–11. https://doi.org/10.4068/cmj.2016.52.1.1
[50]. Ooi, J. Y. Y., et al. (2015). HDAC inhibition attenuates cardiac hypertrophy by acetylation and deacetylation of target genes. Epigenetics, 10(5), 418–430. https://doi.org/10.1080/15592294.2015.1039227
[51]. Han, Y., Nie, J., Wang, D. W., & Ni, L. (2022). Mechanism of histone deacetylases in cardiac hypertrophy and its therapeutic inhibitors. Frontiers in Cardiovascular Medicine, 9. https://doi.org/10.3389/fcvm.2022.887258
[52]. Liu, C. F., & Tang, W. H. W. (2019). Epigenetics in cardiac hypertrophy and heart failure. JACC: Basic to Translational Science, 4(8), 976–993. https://doi.org/10.1016/j.jacbts.2019.09.005
[53]. Kurdi, M., & Booz, G. W. (2011). Three 4-letter words of hypertension-related cardiac hypertrophy: TRPC, mTOR, and HDAC. Journal of Molecular and Cellular Cardiology, 50(6), 964–971. https://doi.org/10.1016/j.yjmcc.2010.11.016
[54]. Zhao, T., et al. (2021). Selective HDAC8 inhibition attenuates isoproterenol-induced cardiac hypertrophy and fibrosis via p38 MAPK pathway. Frontiers in Pharmacology, 12. https://doi.org/10.3389/fphar.2021.673859
[55]. Li, P., Ge, J., & Li, H. (2020). Lysine acetyltransferases and lysine deacetylases as targets for cardiovascular disease. Nature Reviews Cardiology, 17(2), 96–115. https://doi.org/10.1038/s41569-019-0256-5
[56]. Chen, C.-N., et al. (2023). Restoration of Foxp3+ regulatory T cells by HDAC-dependent epigenetic modulation plays a pivotal role in resolving pulmonary arterial hypertension pathology. American Journal of Respiratory and Critical Care Medicine, 208. https://doi.org/10.1164/rccm.202305-0843OC
[57]. Molife, R., et al. (2007). HDAC inhibitors and cardiac safety. Clinical Cancer Research, 13(4), 1068. https://doi.org/10.1158/1078-0432.CCR-06-2283
[58]. Lkhagva, B., et al. (2016). Targeting histone deacetylases: A novel therapeutic strategy for atrial fibrillation. European Journal of Pharmacology, 781, 250–257. https://doi.org/10.1016/j.ejphar.2016.04.027
[59]. Scholz, B., et al. (2019). HDAC (histone deacetylase) inhibitor valproic acid attenuates atrial remodeling and delays the onset of atrial fibrillation in mice. Circulation: Arrhythmia and Electrophysiology, 12. https://doi.org/10.1161/CIRCEP.118.007071
[60]. Lee, D. Y., & Chiu, J. J. (2019). Atherosclerosis and flow: Roles of epigenetic modulation in vascular endothelium. Journal of Biomedical Science, 26. https://doi.org/10.1186/s12929-019-0522-x
[61]. Ho, T. C. S., Chan, A. H. Y., & Ganesan, A. (2020). Thirty years of HDAC inhibitors: 2020 insight and hindsight. Journal of Medicinal Chemistry, 63(21), 12460–12484. https://doi.org/10.1021/acs.jmedchem.0c00487
[62]. Dawson, M. A., & Kouzarides, T. (2012). Cancer epigenetics: From mechanism to therapy. Cell, 150(1), 12–27. https://doi.org/10.1016/j.cell.2012.06.013
[63]. Kumar, A., et al. (2023). Major phytochemicals: Recent advances in health benefits and extraction method. Molecules, 28. https://doi.org/10.3390/molecules28010324
[64]. Petrovski, G., Gurusamy, N., & Das, D. K. (2011). Resveratrol in cardiovascular health and disease. Annals of the New York Academy of Sciences, 1215, 22–33. https://doi.org/10.1111/j.1749-6632.2010.05850.x
[65]. Evans, L. W., Athukorala, M., Martinez-Guryn, K., & Ferguson, B. S. (2020). The role of histone acetylation and the microbiome in phytochemical efficacy for cardiovascular diseases. International Journal of Molecular Sciences, 21(23), 1–18. https://doi.org/10.3390/ijms21239249
[66]. Indira Priyadarsini, K. (2014). The chemistry of curcumin: From extraction to therapeutic agent. Molecules, 19. https://doi.org/10.3390/molecules190912300
[67]. Tian, B., & Liu, J. (2020). Resveratrol: A review of plant sources, synthesis, stability, modification and food application. Journal of the Science of Food and Agriculture, 100(4), 1392–1404. https://doi.org/10.1002/jsfa.10152
[68]. Kumar Singh, A., Bishayee, A., & Pandey, A. K. (2018). Targeting histone deacetylases with natural and synthetic agents: An emerging anticancer strategy. Nutrients, 10(6), 731. https://doi.org/10.3390/nu10060731
[69]. Lu, L., Liu, M., Sun, R. R., Zheng, Y., & Zhang, P. (2015). Myocardial infarction: Symptoms and treatments. Cell Biochemistry and Biophysics, 72(3), 865–867. https://doi.org/10.1007/s12013-015-0533-4
[70]. Taqueti, V. R., & O’Gara, P. T. (2015). Beta-blocker therapy after myocardial infarction: More questions than answers. Journal of the American College of Cardiology, 66(13), 1442–1444. https://doi.org/10.1016/j.jacc.2015.08.002
[71]. Arslan, F. B., Ozturk, K., & Calis, S. (2021). Antibody-mediated drug delivery. International Journal of Pharmaceutics, 596.
[72]. Chang, S. F., et al. (2022). Blood reflux-induced epigenetic factors HDACs and DNMTs are associated with the development of human chronic venous disease. International Journal of Molecular Sciences, 23.
[73]. David Strain, W., & Paldánius, P. M. (2018). Diabetes, cardiovascular disease and the microcirculation. Cardiovascular Diabetology, 17(57).
[74]. Ding, Y., et al. (2022). Application of lipid nanovesicle drug delivery system in cancer immunotherapy. Journal of Nanobiotechnology, 20, 214.
[75]. Drummond, D. C., et al. (2005). Enhanced pharmacodynamic and antitumor properties of a histone deacetylase inhibitor encapsulated in liposomes or ErbB2-targeted immunoliposomes. Clinical Cancer Research, 11, 3392–3401.
[76]. Dyck, G. J. B., Raj, P., Zieroth, S., Dyck, J. R. B., & Ezekowitz, J. A. (2019). The effects of resveratrol in patients with cardiovascular disease and heart failure: A narrative review. International Journal of Molecular Sciences, 20.
[77]. Eaton, D. M., et al. (2022). HDAC inhibition regulates cardiac function by increasing myofilament calcium sensitivity and decreasing diastolic tension. Pharmaceutics, 14.
[78]. Elagizi, A., Kachur, S., Carbone, S., Lavie, C. J., & Blair, S. N. (2020). A review of obesity, physical activity, and cardiovascular disease. Current Obesity Reports, 9, 571–581.
[79]. Evans, L. W., Athukorala, M., Martinez-Guryn, K., & Ferguson, B. S. (2020). The role of histone acetylation and the microbiome in phytochemical efficacy for cardiovascular diseases. International Journal of Molecular Sciences, 21.
[80]. Fu, Z., Li, S., Han, S., Shi, C., & Zhang, Y. (2022). Antibody drug conjugate: The ‘biological missile’ for targeted cancer therapy. Signal Transduction and Targeted Therapy, 7.
[81]. Guasch-Ferré, M., et al. (2020). Glycolysis/gluconeogenesis- and tricarboxylic acid cycle-related metabolites, Mediterranean diet, and type 2 diabetes. American Journal of Clinical Nutrition, 111, 835–844.
[82]. Gunassekaran, G. R., Poongkavithai Vadevoo, S. M., Baek, M. C., & Lee, B. (2021). M1 macrophage exosomes engineered to foster M1 polarization and target the IL-4 receptor inhibit tumor growth by reprogramming tumor-associated macrophages into M1-like macrophages. Biomaterials, 278.
[83]. Hong, J., et al. (2021). F. nucleatum targets lncRNA ENO1-IT1 to promote glycolysis and oncogenesis in colorectal cancer. Gut, 70, 2123–2137.
[84]. Jiang, J., et al. (2021). Lactate modulates cellular metabolism through histone lactylation-mediated gene expression in non-small cell lung cancer. Frontiers in Oncology, 11.
[85]. Li, H., et al. (2021). Artificial exosomes mediated spatiotemporal-resolved and targeted delivery of epigenetic inhibitors. Journal of Nanobiotechnology, 19, 364.
[86]. Liberti, M. V., & Locasale, J. W. (2020). Histone lactylation: A new role for glucose metabolism. Trends in Biochemical Sciences, 45, 179–182.
[87]. Lindemann, H., et al. (2020). Polysaccharide nanoparticles bearing HDAC inhibitor as nontoxic nanocarrier for drug delivery. Macromolecular Bioscience, 20.
[88]. Lindemann, H., et al. (2023). HDACi delivery systems based on cellulose valproate nanoparticles. Methods in Molecular Biology, 2589, 195–205.
[89]. Lovelock, S. L., et al. (2022). The road to fully programmable protein catalysis. Nature, 606, 49–58.
[90]. Maniu, C. V., & Redfield, M. M. (2001). Diastolic dysfunction: Insights into pathophysiology and pharmacotherapy. Expert Opinion on Pharmacotherapy, 2, 997–1008.
[91]. Melissaropoulos, K., et al. (2020). Primary Sjögren’s syndrome and cardiovascular disease. Current Vascular Pharmacology, 18, 447–454.
[92]. Pan, L., et al. (2022). Demethylzeylasteral targets lactate by inhibiting histone lactylation to suppress the tumorigenicity of liver cancer stem cells. Pharmacological Research, 181.
[93]. Pivari, F., et al. (2022). Curcumin supplementation (Meriva®) modulates inflammation, lipid peroxidation and gut microbiota composition in chronic kidney disease. Nutrients, 14.
[94]. Qin, S., et al. (2017). Efficacy and safety of turmeric and curcumin in lowering blood lipid levels in patients with cardiovascular risk factors: A meta-analysis of randomized controlled trials. Nutrition Journal, 16.
[95]. Rashid, M., Brim, H., & Ashktorab, H. (2022). Saffron, its active components, and their association with DNA and histone modification: A narrative review of current knowledge. Nutrients. https://doi.org/10.3390/nu14163317
[96]. Shah, R. R. (2019). Safety and tolerability of histone deacetylase (HDAC) inhibitors in oncology. Drug Safety, 42, 235–245.
[97]. Silveira Rossi, J. L., et al. (2022). Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes/Metabolism Research and Reviews, 38.
[98]. Sturgeon, K. M., et al. (2019). A population-based study of cardiovascular disease mortality risk in US cancer patients. European Heart Journal, 40, 3889–3897.
[99]. Tong, X., et al. (2021). Generative models for de novo drug design. Journal of Medicinal Chemistry, 64, 14011–14027.
[100]. Torre, L. A., Siegel, R. L., Ward, E. M., & Jemal, A. (2016). Global cancer incidence and mortality rates and trends: An update. Cancer Epidemiology, Biomarkers & Prevention, 25, 16–27.
[101]. Waddell, I. S., & Orfila, C. (2023). Dietary fiber in the prevention of obesity and obesity-related chronic diseases: From epidemiological evidence to potential molecular mechanisms. Critical Reviews in Food Science and Nutrition, 63.
[102]. Wang, J., et al. (2022). Enterobacterial LPS-inducible LINC00152 is regulated by histone lactylation and promotes cancer cells invasion and migration. Frontiers in Cellular and Infection Microbiology, 12.
[103]. Yang, J., et al. (2022). A positive feedback loop between inactive VHL-triggered histone lactylation and PDGFRβ signaling drives clear cell renal cell carcinoma progression. International Journal of Biological Sciences, 18, 3470–3483.
[104]. Yoon, S., et al. (2021). Circulation, 143, 1912–1925.
[105]. Yu, J., et al. (2021). Histone lactylation drives oncogenesis by facilitating m6A reader protein YTHDF2 expression in ocular melanoma. Genome Biology, 22.
Cite this article
Li,J. (2024). What is the impact of histone modification-based treatment in cardiovascular diseases in modern medicine?. Journal of Food Science, Nutrition and Health,2,40-53.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Journal:Journal of Food Science, Nutrition and Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Timmis, A., et al. (2020). European Society of Cardiology: Cardiovascular disease statistics 2019. European Heart Journal, 41(12), 12–85.
[2]. Spn, M., Newman, S., Newman, S. P., & Harrison, M. J. (2002). Coronary-artery bypass surgery and the brain: Persisting concerns. The Lancet Neurology, 1. http://neurology.thelancet.com
[3]. Bhatnagar, A. (2017). Environmental determinants of cardiovascular disease. Circulation Research, 121, 162–180. https://doi.org/10.1161/CIRCRESAHA.117.306458
[4]. Wang, N., et al. (2022). Histone lactylation boosts reparative gene activation post-myocardial infarction. Circulation Research, 131, 893–908.
[5]. Wu, Y., et al. (2023). Phosphoglycerate dehydrogenase activates PKM2 to phosphorylate histone H3T11 and attenuate cellular senescence. Nature Communications, 14.
[6]. Shi, Y., et al. (2022). Epigenetic regulation in cardiovascular disease: Mechanisms and advances in clinical trials. Signal Transduction and Targeted Therapy, 7. https://doi.org/10.1038/s41392-022-01055-2
[7]. Chistiakov, D. A., Orekhov, A. N., & Bobryshev, Y. V. (2017). Treatment of cardiovascular pathology with epigenetically active agents: Focus on natural and synthetic inhibitors of DNA methylation and histone deacetylation. International Journal of Cardiology, 227, 66–82. https://doi.org/10.1016/j.ijcard.2016.11.204
[8]. Zillich, A. J., Garg, J., Basu, S., Bakris, G. L., & Carter, B. L. (2006). Thiazide diuretics, potassium, and the development of diabetes: A quantitative review. Hypertension, 48(2), 219–224.
[9]. Gutiérrez-Repiso, C., et al. (2021). Epigenetic biomarkers of transition from metabolically healthy obesity to metabolically unhealthy obesity phenotype: A prospective study. International Journal of Molecular Sciences, 22.
[10]. Berger, S. L., Kouzarides, T., Shiekhattar, R., & Shilatifard, A. (2009). An operational definition of epigenetics. Genes & Development, 23(7), 781–783.
[11]. Liu, S., et al. (2021). Fat mass and obesity-associated protein regulates RNA methylation associated with depression-like behavior in mice. Nature Communications, 12.
[12]. Zehender, A., et al. (2021). TGFβ promotes fibrosis by MYST1-dependent epigenetic regulation of autophagy. Nature Communications, 12.
[13]. Lawrence, M., Daujat, S., & Schneider, R. (2016). Lateral thinking: How histone modifications regulate gene expression. Trends in Genetics.
[14]. Kim, G. H., Ryan, J. J., & Archer, S. L. (2013). The role of redox signaling in epigenetics and cardiovascular disease. Antioxidants & Redox Signaling, 18(14), 1920–1936.
[15]. Koprinarova, M., Schnekenburger, M., & Diederich, M. (2016). Role of histone acetylation in cell cycle regulation. Current Topics in Medicinal Chemistry, 16(7), 732–744.
[16]. Celano, M., et al. (2018). Targeting post-translational histone modifications for the treatment of non-medullary thyroid cancer. Molecular and Cellular Endocrinology, 469, 38–47. https://doi.org/10.1016/j.mce.2017.05.036
[17]. Yang, Y., Luan, Y., Yuan, R. X., & Luan, Y. (2021). Histone methylation-related therapeutic challenge in cardiovascular diseases. Frontiers in Cardiovascular Medicine, 8. https://doi.org/10.3389/fcvm.2021.710053
[18]. Barros, C. H. N., et al. (2021). Synthesis and self-assembly of curcumin-modified amphiphilic polymeric micelles with antibacterial activity. Journal of Nanobiotechnology, 19.
[19]. Mäntylä, E. (2018). Import and impact: Characterization of parvovirus-nucleus interactions.
[20]. Audia, J. E., & Campbell, R. M. (2016). Histone modifications and cancer. Cold Spring Harbor Perspectives in Biology, 8.
[21]. Tompkins, B. A., & Salerno, T. A. (2021). Commentary: What does the future hold for coronary bypass surgery? Journal of Thoracic and Cardiovascular Surgery, 162, 1120–1121. https://doi.org/10.1016/j.jtcvs.2020.01.029
[22]. Head, S. J., Milojevic, M., Taggart, D. P., & Puskas, J. D. (2017). Current practice of state-of-the-art surgical coronary revascularization. Circulation, 136(12), 1331–1345.
[23]. Head, S. J., & Kappetein, A. P. (2019). Coronary bypass surgery — An ART for dedicated surgeons. New England Journal of Medicine, 380, 489–491.
[24]. Brierley, J. B. (1963). Neuropathological findings in patients dying after open-heart surgery. Thorax, 18.
[25]. Perros, A. J., et al. (2020). Coronary artery bypass grafting is associated with immunoparalysis of monocytes and dendritic cells. Journal of Cellular and Molecular Medicine, 24(9), 4791–4803.
[26]. An, P., et al. (2022). Micronutrient supplementation to reduce cardiovascular risk. Journal of the American College of Cardiology, 80(23), 2269–2285.
[27]. Mosca, L., et al. (1997). Antioxidant nutrient supplementation reduces the susceptibility of low-density lipoprotein to oxidation in patients with coronary artery disease.
[28]. Lykkesfeldt, J., Michels, A. J., & Frei, B. (2014). Vitamin C. Advances in Nutrition, 5(1), 16–18.
[29]. Moser, M. A., & Chun, O. K. (2016). Vitamin C and heart health: A review based on findings from epidemiologic studies. International Journal of Molecular Sciences, 17(8). https://doi.org/10.3390/ijms17081328
[30]. Chen, X., et al. (2023). Copper homeostasis and copper-induced cell death in the pathogenesis of cardiovascular disease and therapeutic strategies. Cell Death & Disease, 14. https://doi.org/10.1038/s41419-023-05639-w
[31]. Oparil, S., et al. (2018). Hypertension. Nature Reviews Disease Primers, 4. https://doi.org/10.1038/nrdp.2018.14
[32]. Fihn, S. D., et al. (2012). 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease. Journal of the American College of Cardiology, 60.
[33]. Laurent, S. (2017). Antihypertensive drugs. Pharmacological Research, 124, 116–125. https://doi.org/10.1016/j.phrs.2017.07.026
[34]. Weinberger, M. H. (n.d.). Diuretics and their side effects dilemma in the treatment of hypertension. Hypertension. http://hyper.ahajournals.org/
[35]. Ho, K. M., & Power, B. M. (2010). Benefits and risks of furosemide in acute kidney injury. Anaesthesia, 65(3), 283–293. https://doi.org/10.1111/j.1365-2044.2009.06228.x
[36]. Mas, J.-L., et al. (2017). Patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. New England Journal of Medicine, 377(11), 1011–1021.
[37]. Xia, S., et al. (2020). Sex differences in primary and secondary prevention of cardiovascular disease in China. Circulation, 141(7), 530–539.
[38]. Kohli, P., et al. (2014). Discharge aspirin dose and clinical outcomes in patients with acute coronary syndromes treated with prasugrel versus clopidogrel: An analysis from the TRITON-TIMI 38 study (trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-thrombolysis in myocardial infarction 38). Journal of the American College of Cardiology, 63(3), 225–232.
[39]. Johnston, S. C., et al. (2019). Time course for benefit and risk of clopidogrel and aspirin after acute transient ischemic attack and minor ischemic stroke. Circulation, 140(8), 658–664. https://doi.org/10.1161/CIRCULATIONAHA.119.040713
[40]. Khyzha, N., Alizada, A., Wilson, M. D., & Fish, J. E. (2017). Epigenetics of atherosclerosis: Emerging mechanisms and methods. Trends in Molecular Medicine, 23(4), 332–347. https://doi.org/10.1016/j.molmed.2017.02.008
[41]. Xu, S., Pelisek, J., & Jin, Z. G. (2018). Atherosclerosis is an epigenetic disease. Trends in Endocrinology & Metabolism, 29(11), 739–742. https://doi.org/10.1016/j.tem.2018.09.003
[42]. Zhang, D., et al. (2019). Metabolic regulation of gene expression by histone lactylation. Nature, 574(7779), 575–580. https://doi.org/10.1038/s41586-019-1678-1
[43]. Yunna, C., Mengru, H., Lei, W., & Weidong, C. (2020). Macrophage M1/M2 polarization. European Journal of Pharmacology, 877. https://doi.org/10.1016/j.ejphar.2020.173090
[44]. Chen, M. S., Lee, R. T., & Garbern, J. C. (2022). Senescence mechanisms and targets in the heart. Cardiovascular Research, 118(5), 1173–1187. https://doi.org/10.1093/cvr/cvab288
[45]. Man, A. W. C., Li, H., & Xia, N. (2019). The role of Sirtuin1 in regulating endothelial function, arterial remodeling and vascular aging. Frontiers in Physiology, 10. https://doi.org/10.3389/fphys.2019.00918
[46]. Sessa, W. C. (2004). eNOS at a glance. Journal of Cell Science, 117(12), 2427–2429. https://doi.org/10.1242/jcs.01245
[47]. Gan, Y., et al. (2005). Role of histone deacetylation in cell-specific expression of endothelial nitric-oxide synthase. Journal of Biological Chemistry, 280(17), 16467–16475. https://doi.org/10.1074/jbc.M414065200
[48]. Hesham, H. M., Lasheen, D. S., & Abouzid, K. A. M. (2018). Chimeric HDAC inhibitors: Comprehensive review on the HDAC-based strategies developed to combat cancer. Medicinal Research Reviews, 38(6), 2058–2109. https://doi.org/10.1002/med.21488
[49]. Yoon, S., & Eom, G. H. (2016). HDAC and HDAC inhibitor: From cancer to cardiovascular diseases. Chonnam Medical Journal, 52(1), 1–11. https://doi.org/10.4068/cmj.2016.52.1.1
[50]. Ooi, J. Y. Y., et al. (2015). HDAC inhibition attenuates cardiac hypertrophy by acetylation and deacetylation of target genes. Epigenetics, 10(5), 418–430. https://doi.org/10.1080/15592294.2015.1039227
[51]. Han, Y., Nie, J., Wang, D. W., & Ni, L. (2022). Mechanism of histone deacetylases in cardiac hypertrophy and its therapeutic inhibitors. Frontiers in Cardiovascular Medicine, 9. https://doi.org/10.3389/fcvm.2022.887258
[52]. Liu, C. F., & Tang, W. H. W. (2019). Epigenetics in cardiac hypertrophy and heart failure. JACC: Basic to Translational Science, 4(8), 976–993. https://doi.org/10.1016/j.jacbts.2019.09.005
[53]. Kurdi, M., & Booz, G. W. (2011). Three 4-letter words of hypertension-related cardiac hypertrophy: TRPC, mTOR, and HDAC. Journal of Molecular and Cellular Cardiology, 50(6), 964–971. https://doi.org/10.1016/j.yjmcc.2010.11.016
[54]. Zhao, T., et al. (2021). Selective HDAC8 inhibition attenuates isoproterenol-induced cardiac hypertrophy and fibrosis via p38 MAPK pathway. Frontiers in Pharmacology, 12. https://doi.org/10.3389/fphar.2021.673859
[55]. Li, P., Ge, J., & Li, H. (2020). Lysine acetyltransferases and lysine deacetylases as targets for cardiovascular disease. Nature Reviews Cardiology, 17(2), 96–115. https://doi.org/10.1038/s41569-019-0256-5
[56]. Chen, C.-N., et al. (2023). Restoration of Foxp3+ regulatory T cells by HDAC-dependent epigenetic modulation plays a pivotal role in resolving pulmonary arterial hypertension pathology. American Journal of Respiratory and Critical Care Medicine, 208. https://doi.org/10.1164/rccm.202305-0843OC
[57]. Molife, R., et al. (2007). HDAC inhibitors and cardiac safety. Clinical Cancer Research, 13(4), 1068. https://doi.org/10.1158/1078-0432.CCR-06-2283
[58]. Lkhagva, B., et al. (2016). Targeting histone deacetylases: A novel therapeutic strategy for atrial fibrillation. European Journal of Pharmacology, 781, 250–257. https://doi.org/10.1016/j.ejphar.2016.04.027
[59]. Scholz, B., et al. (2019). HDAC (histone deacetylase) inhibitor valproic acid attenuates atrial remodeling and delays the onset of atrial fibrillation in mice. Circulation: Arrhythmia and Electrophysiology, 12. https://doi.org/10.1161/CIRCEP.118.007071
[60]. Lee, D. Y., & Chiu, J. J. (2019). Atherosclerosis and flow: Roles of epigenetic modulation in vascular endothelium. Journal of Biomedical Science, 26. https://doi.org/10.1186/s12929-019-0522-x
[61]. Ho, T. C. S., Chan, A. H. Y., & Ganesan, A. (2020). Thirty years of HDAC inhibitors: 2020 insight and hindsight. Journal of Medicinal Chemistry, 63(21), 12460–12484. https://doi.org/10.1021/acs.jmedchem.0c00487
[62]. Dawson, M. A., & Kouzarides, T. (2012). Cancer epigenetics: From mechanism to therapy. Cell, 150(1), 12–27. https://doi.org/10.1016/j.cell.2012.06.013
[63]. Kumar, A., et al. (2023). Major phytochemicals: Recent advances in health benefits and extraction method. Molecules, 28. https://doi.org/10.3390/molecules28010324
[64]. Petrovski, G., Gurusamy, N., & Das, D. K. (2011). Resveratrol in cardiovascular health and disease. Annals of the New York Academy of Sciences, 1215, 22–33. https://doi.org/10.1111/j.1749-6632.2010.05850.x
[65]. Evans, L. W., Athukorala, M., Martinez-Guryn, K., & Ferguson, B. S. (2020). The role of histone acetylation and the microbiome in phytochemical efficacy for cardiovascular diseases. International Journal of Molecular Sciences, 21(23), 1–18. https://doi.org/10.3390/ijms21239249
[66]. Indira Priyadarsini, K. (2014). The chemistry of curcumin: From extraction to therapeutic agent. Molecules, 19. https://doi.org/10.3390/molecules190912300
[67]. Tian, B., & Liu, J. (2020). Resveratrol: A review of plant sources, synthesis, stability, modification and food application. Journal of the Science of Food and Agriculture, 100(4), 1392–1404. https://doi.org/10.1002/jsfa.10152
[68]. Kumar Singh, A., Bishayee, A., & Pandey, A. K. (2018). Targeting histone deacetylases with natural and synthetic agents: An emerging anticancer strategy. Nutrients, 10(6), 731. https://doi.org/10.3390/nu10060731
[69]. Lu, L., Liu, M., Sun, R. R., Zheng, Y., & Zhang, P. (2015). Myocardial infarction: Symptoms and treatments. Cell Biochemistry and Biophysics, 72(3), 865–867. https://doi.org/10.1007/s12013-015-0533-4
[70]. Taqueti, V. R., & O’Gara, P. T. (2015). Beta-blocker therapy after myocardial infarction: More questions than answers. Journal of the American College of Cardiology, 66(13), 1442–1444. https://doi.org/10.1016/j.jacc.2015.08.002
[71]. Arslan, F. B., Ozturk, K., & Calis, S. (2021). Antibody-mediated drug delivery. International Journal of Pharmaceutics, 596.
[72]. Chang, S. F., et al. (2022). Blood reflux-induced epigenetic factors HDACs and DNMTs are associated with the development of human chronic venous disease. International Journal of Molecular Sciences, 23.
[73]. David Strain, W., & Paldánius, P. M. (2018). Diabetes, cardiovascular disease and the microcirculation. Cardiovascular Diabetology, 17(57).
[74]. Ding, Y., et al. (2022). Application of lipid nanovesicle drug delivery system in cancer immunotherapy. Journal of Nanobiotechnology, 20, 214.
[75]. Drummond, D. C., et al. (2005). Enhanced pharmacodynamic and antitumor properties of a histone deacetylase inhibitor encapsulated in liposomes or ErbB2-targeted immunoliposomes. Clinical Cancer Research, 11, 3392–3401.
[76]. Dyck, G. J. B., Raj, P., Zieroth, S., Dyck, J. R. B., & Ezekowitz, J. A. (2019). The effects of resveratrol in patients with cardiovascular disease and heart failure: A narrative review. International Journal of Molecular Sciences, 20.
[77]. Eaton, D. M., et al. (2022). HDAC inhibition regulates cardiac function by increasing myofilament calcium sensitivity and decreasing diastolic tension. Pharmaceutics, 14.
[78]. Elagizi, A., Kachur, S., Carbone, S., Lavie, C. J., & Blair, S. N. (2020). A review of obesity, physical activity, and cardiovascular disease. Current Obesity Reports, 9, 571–581.
[79]. Evans, L. W., Athukorala, M., Martinez-Guryn, K., & Ferguson, B. S. (2020). The role of histone acetylation and the microbiome in phytochemical efficacy for cardiovascular diseases. International Journal of Molecular Sciences, 21.
[80]. Fu, Z., Li, S., Han, S., Shi, C., & Zhang, Y. (2022). Antibody drug conjugate: The ‘biological missile’ for targeted cancer therapy. Signal Transduction and Targeted Therapy, 7.
[81]. Guasch-Ferré, M., et al. (2020). Glycolysis/gluconeogenesis- and tricarboxylic acid cycle-related metabolites, Mediterranean diet, and type 2 diabetes. American Journal of Clinical Nutrition, 111, 835–844.
[82]. Gunassekaran, G. R., Poongkavithai Vadevoo, S. M., Baek, M. C., & Lee, B. (2021). M1 macrophage exosomes engineered to foster M1 polarization and target the IL-4 receptor inhibit tumor growth by reprogramming tumor-associated macrophages into M1-like macrophages. Biomaterials, 278.
[83]. Hong, J., et al. (2021). F. nucleatum targets lncRNA ENO1-IT1 to promote glycolysis and oncogenesis in colorectal cancer. Gut, 70, 2123–2137.
[84]. Jiang, J., et al. (2021). Lactate modulates cellular metabolism through histone lactylation-mediated gene expression in non-small cell lung cancer. Frontiers in Oncology, 11.
[85]. Li, H., et al. (2021). Artificial exosomes mediated spatiotemporal-resolved and targeted delivery of epigenetic inhibitors. Journal of Nanobiotechnology, 19, 364.
[86]. Liberti, M. V., & Locasale, J. W. (2020). Histone lactylation: A new role for glucose metabolism. Trends in Biochemical Sciences, 45, 179–182.
[87]. Lindemann, H., et al. (2020). Polysaccharide nanoparticles bearing HDAC inhibitor as nontoxic nanocarrier for drug delivery. Macromolecular Bioscience, 20.
[88]. Lindemann, H., et al. (2023). HDACi delivery systems based on cellulose valproate nanoparticles. Methods in Molecular Biology, 2589, 195–205.
[89]. Lovelock, S. L., et al. (2022). The road to fully programmable protein catalysis. Nature, 606, 49–58.
[90]. Maniu, C. V., & Redfield, M. M. (2001). Diastolic dysfunction: Insights into pathophysiology and pharmacotherapy. Expert Opinion on Pharmacotherapy, 2, 997–1008.
[91]. Melissaropoulos, K., et al. (2020). Primary Sjögren’s syndrome and cardiovascular disease. Current Vascular Pharmacology, 18, 447–454.
[92]. Pan, L., et al. (2022). Demethylzeylasteral targets lactate by inhibiting histone lactylation to suppress the tumorigenicity of liver cancer stem cells. Pharmacological Research, 181.
[93]. Pivari, F., et al. (2022). Curcumin supplementation (Meriva®) modulates inflammation, lipid peroxidation and gut microbiota composition in chronic kidney disease. Nutrients, 14.
[94]. Qin, S., et al. (2017). Efficacy and safety of turmeric and curcumin in lowering blood lipid levels in patients with cardiovascular risk factors: A meta-analysis of randomized controlled trials. Nutrition Journal, 16.
[95]. Rashid, M., Brim, H., & Ashktorab, H. (2022). Saffron, its active components, and their association with DNA and histone modification: A narrative review of current knowledge. Nutrients. https://doi.org/10.3390/nu14163317
[96]. Shah, R. R. (2019). Safety and tolerability of histone deacetylase (HDAC) inhibitors in oncology. Drug Safety, 42, 235–245.
[97]. Silveira Rossi, J. L., et al. (2022). Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes/Metabolism Research and Reviews, 38.
[98]. Sturgeon, K. M., et al. (2019). A population-based study of cardiovascular disease mortality risk in US cancer patients. European Heart Journal, 40, 3889–3897.
[99]. Tong, X., et al. (2021). Generative models for de novo drug design. Journal of Medicinal Chemistry, 64, 14011–14027.
[100]. Torre, L. A., Siegel, R. L., Ward, E. M., & Jemal, A. (2016). Global cancer incidence and mortality rates and trends: An update. Cancer Epidemiology, Biomarkers & Prevention, 25, 16–27.
[101]. Waddell, I. S., & Orfila, C. (2023). Dietary fiber in the prevention of obesity and obesity-related chronic diseases: From epidemiological evidence to potential molecular mechanisms. Critical Reviews in Food Science and Nutrition, 63.
[102]. Wang, J., et al. (2022). Enterobacterial LPS-inducible LINC00152 is regulated by histone lactylation and promotes cancer cells invasion and migration. Frontiers in Cellular and Infection Microbiology, 12.
[103]. Yang, J., et al. (2022). A positive feedback loop between inactive VHL-triggered histone lactylation and PDGFRβ signaling drives clear cell renal cell carcinoma progression. International Journal of Biological Sciences, 18, 3470–3483.
[104]. Yoon, S., et al. (2021). Circulation, 143, 1912–1925.
[105]. Yu, J., et al. (2021). Histone lactylation drives oncogenesis by facilitating m6A reader protein YTHDF2 expression in ocular melanoma. Genome Biology, 22.









