1 Introduction
Myocardial ischemia-reperfusion injury is the interruption of myocardial blood supply within a short period of time, and after the blood supply is restored for a period of time, the degree of myocardial damage is further aggravated than in ischemia and produces a series of adverse reactions, even irreversible damage occurs [1]. Due to the high number of patients with MIRI, the high mortality rate and the economic burden, there is an urgent need to develop current potential drugs to reduce MIRI. According to the findings, the pathogenesis of MIRI is complex, with many pathways and pathophysiological processes associated with oxidative stress, inflammatory response, intracellular Ca2+ overload, rapid restoration of physiological pH during reperfusion, and mitochondrial dysfunction [2,3,4,5,6]. Thus, treatment strategies of reduction of oxidative stress, inhibition of calcium overload and mTOR are available for attenuate MIRI [7]. Traditional Chinese medicine (TCM) has certain effects on MIRI in the integral medication of multi-channel, multi-target action, et al. The use of TCM in the delivery system has a lengthy history, but its complexity increases the difficulty in elucidating the mechanism of MIRI. In traditional Chinese medicine theory, MIRI was similar to “pectoralgia and heart pains,” which were treated with Gualou Xiebai Baijiu decoction (GLXB). GLXB is a classical prescription of TCM from Synopsis of the Golden Chamber (Jin Kui Yao Lue) for thousands of years from the Han Dynasty of China (150 CE). GLXB has been widely used in clinics because of its slight side effects and specific therapeutic effects on myocardial ischemic diseases [8,9]. So far, clinical practice has proved that GLXB has significant effects on the prevention and treatment of coronary heart disease, angina pectoris, and MIRI [6,10,11]. A classic GLXB formulation includes Snakegourd Fruit (gualou) Onion Bulb (xiebai), and liquor. Modern pharmacological studies have also shown that this formula has the effect of dilating coronary blood vessels, increasing myocardial blood supply, and reducing the rate of myocardial infarction [12]. Although GLXB, a well-known traditional Chinese recipe, has been used to treat cardiovascular disorders for approximately two thousand years [13], more reports need to be on MIRI and its underlying mechanism. It has been verified that GLXB can significantly improve the myocardial tissue lesions of rats after myocardial ischemia and reperfusion [14]? The whole blood viscosity and the content of CK and LDH in the blood plasma were significantly decreased after myocardial ischemia and reperfusion [14]. we need to explore the potential mechanisms of GLXB for the treatment of MIRI and provide scientific guidance and a theoretical basis for the clinical application of MIRI. In 2007, Hopkins came up with the idea of network pharmacology, an approach that analyzes the intervention of a drug and the underlying therapeutic target of the disease. This “drug-compound-target” model offers an improved strategy for exploring associations between various drugs and diseases [15,16]. Therefore, we aim to reveal the active ingredient of GLXB for the treatment of MIRI and predict the underlying mechanism of GLXB using a network pharmacological approach.
2 Materials and methods
2.1 Searching for Components and Targets of GLXB
TCMSP (https://www.tcmsp-e.com/tcmsp.php) is an analysis platform for Comprehensive Study of Traditional Chinese Medicine, including 499 Chinese herbal medicines, 29384 chemical components and 3311 targets Based on Traditional Chinese Medicine Pharmacology Database System [17]. The components of snake gourd fruit and onion bulb were retrieved (=screen) by TCMSP database (https: //tcmspw.com/tcmsp.php), according to the condition of Drug-likeness (DL) ≥ 0.18 and Oral bioavailability (OB ≥ 30%). The protein targets of these components were then collected using TCMSP. UniProt is the world’s leading high-quality, comprehensive, and freely accessible resource of protein sequence and functional information [18]. The target protein information was entered into the UniProt website (https://www.uniprot.org/), and the gene name corresponding to the drug target protein was retrieved by selecting the species as Homo Sapiens.
2.2 Constructing protein-protein interaction (PPI) core network.
The protein-protein interaction core network (PPICN) refers to the correlation between compounds and disease-related protein molecules, taking into account biochemistry, signal transduction, and genetic networks. We imported the drug-disease intersection targets into STRING database [19] (https://cn.string-db.org/) and set the species as Homo sapiens. The data was saved as tab-separated value (TSV) format. We imported the TSV file into Cytoscape 3.7.1, selected AnalyzeNetwork, and adjusted the node size based on the Degree value in the analysis result. Degree value refers to the number of nodes connected to other nodes in the network. The larger the Degree is, the more critical the node is.
2.3 Gene Ontology (GO) function and Genome Encyclopedia (KEGG) pathway enrichment analysis
David database (https://david.ncifcrf.gov/) [20] is an online gene annotation enrichment analysis tool. GO function and KEGG enrichment analysis were performed on the David database for the intersecting target proteins, and the species was set as "Homo sapiens". The resulting data was imported into the Microbiology website to create enrichment bar plots and bubble plots.
2.4 Molecular docking
Molecular docking was performed to further investigate possible interactions between the central target protein and the primary active ingredient. The crystal structures of the proteins were downloaded from the RCSB Protein Data Bank (http://www.pdb.org/). The conformations of the proteins were modified using Pymol and AutoDock 1.5.6 software, including ligand and water removal, hydrogen addition, amino acid optimization and charge calculation. The structure files of the vital components were downloaded from TCMSP, and the energy of the components was minimized using Chem3D software. The original format for proteins and components was converted to the PDBQT format. Molecular docking was performed by running AutoGrid and AutoDock software. The results were visualized using the Discovery Studio 2019 software.
3 Results
3.1 Active ingredients and related targets of GLXB
A total of 167 active components of GLXB and 203 related targets were obtained from the TCMSP database, including 22 components with OB≥ 30% and DL ≥ 0.18. There are 22 components, 11 from Snakegourd fruit and 11 from Onion bulb. After a comprehensive analysis of the target components, one component with no target and no gene was eliminated, leaving 20 compounds that met the screening requirements and had a target. According to the screening results, the active ingredients of each drug are listed in Figure 1.
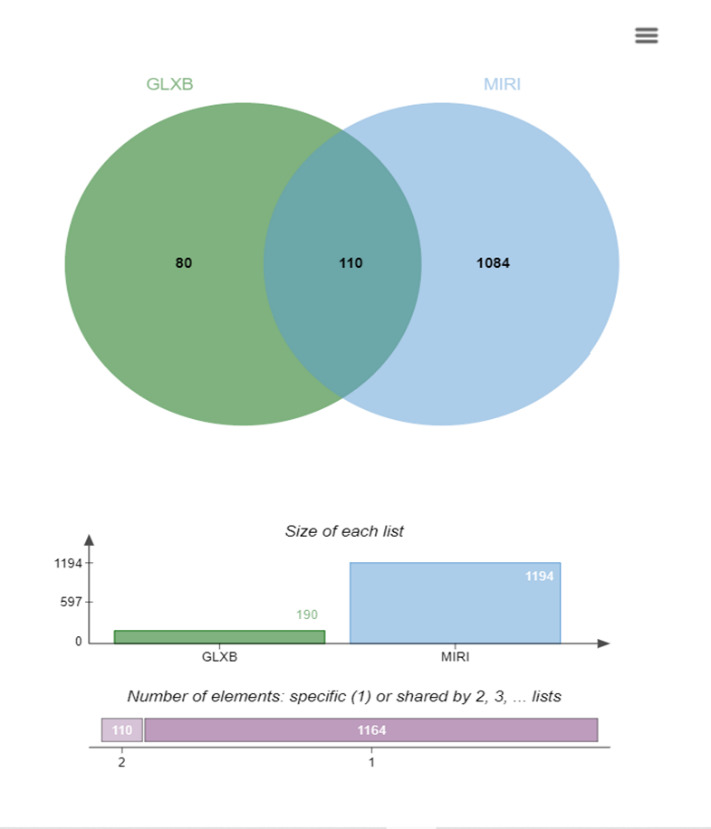
Figure 1. Information of active ingredients of GLXB
3.2 Targets related to MIRI
A total of 190 target genes in 20 chemical components of GLXB, and 1194 targets of MIRI were obtained from NCBI database, Genecards database, OMIM database, and CTD database. Finally, 110 targets of GLXB in treating MIRI were obtained as shown in Figure 2.
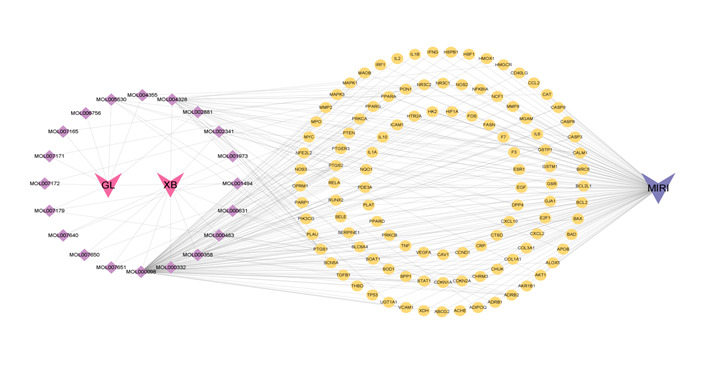
Figure 2. Venn diagram of targets related to Gualou Xiebai and MIRI; a total of 110 common targets were obtained
3.3 Drug-active ingredient-target network analysis
A total of 167 active components of GLXB were obtained from the TCMSP database, including 22 components with OB≥30% and DL≥0.18, but 2 did not find targets and gene names. Finally, 20 components were collected from the TCMSP database removing, with red nodes representing drugs, purple nodes representing active ingredients and yellow nodes representing potential targets. The edges indicate the correlation between the active component and the target, fully reflecting the multi-component and multi-target characteristics of GLXB (Figure 3)
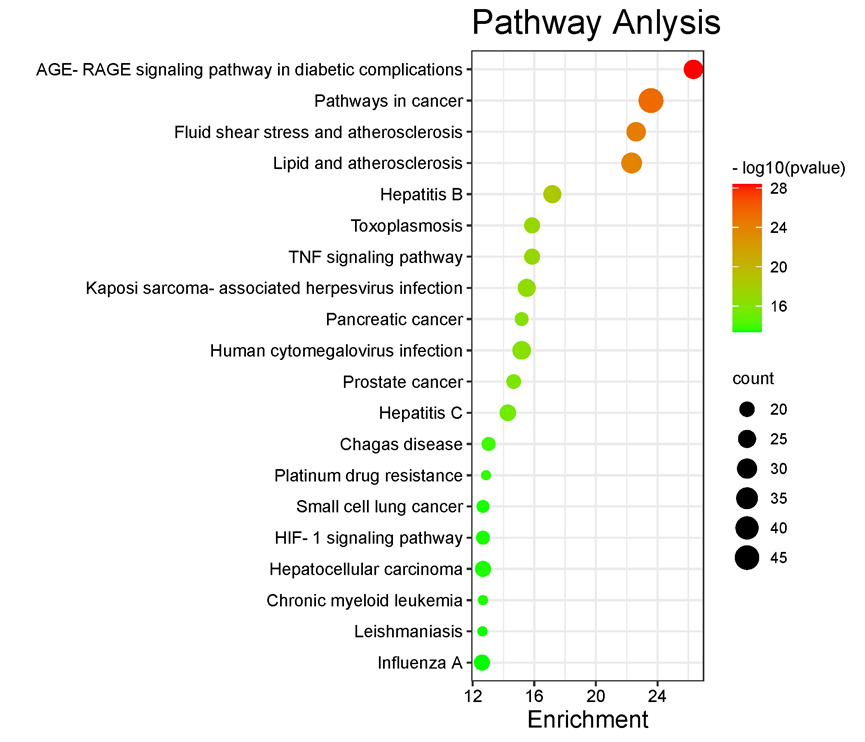
Figure 3. GO enrichment analysis histogram
3.4 PPI network analysis
Using the STRING database, an initial PPI network was established based on 118 candidate genes. There were 110 nodes and 1932 edges, with an average node degree of 35.1 and a local clustering coefficient of 0.741. The size of the nodes indicates the degree value of the target: the larger the node, the larger the degree value. The thickness of the edge indicates the combination score, the thicker the edge, the larger the combination score value. Nodes represent proteins and edges represent protein interaction relationships. The proteins with large degree play a crucial role in the whole network, and the top 5 proteins in degree are AKT1, IL6, TNF, IL1B and TP53. Data for the degree of each target was shown in Table 1.
Table 1. Information about intersection genes for GLXB and MIRI
name |
degree |
name |
degree |
name |
degree |
name |
degree |
name |
degree |
AKT1 |
90 |
MMP2 |
56 |
CDKN1A |
36 |
AKR1B1 |
21 |
BAD |
10 |
TNF |
87 |
PTEN |
55 |
NR3C1 |
34 |
ALOX5 |
21 |
UGT1A1 |
9 |
IL6 |
87 |
VCAM1 |
54 |
CHUK |
32 |
FASN |
19 |
PTGER3 |
9 |
IL1B |
80 |
SERPINE1 |
51 |
CAV1 |
42 |
PRKCB |
19 |
F7 |
9 |
TP53 |
78 |
CASP8 |
50 |
RUNX2 |
31 |
CTSD |
19 |
PPARD |
8 |
VEGFA |
77 |
RELA |
50 |
PRKCA |
31 |
PLAT |
19 |
NR3C2 |
7 |
CASP3 |
75 |
IL2 |
49 |
GJA1 |
29 |
BCL2 |
18 |
OPRM1 |
6 |
PTGS2 |
72 |
IFNG |
49 |
HSPB1 |
29 |
PON1 |
17 |
MAOB |
5 |
MAPK3 |
70 |
BCL2L1 |
48 |
NCF1 |
29 |
PTGS1 |
17 |
HTR2A |
5 |
PPARG |
68 |
PPARA |
48 |
COL1A1 |
25 |
BIRC5 |
16 |
ADRB1 |
3 |
HIF1A |
67 |
TGFB1 |
47 |
SOD1 |
32 |
ABCG2 |
15 |
SCN5A |
3 |
MMP9 |
66 |
STAT1 |
47 |
GSR |
25 |
HK2 |
15 |
PDE3A |
2 |
CAT |
65 |
MPO |
45 |
NQO1 |
25 |
GSTM1 |
15 |
SOAT1 |
2 |
CCL2 |
65 |
ADIPOQ |
44 |
CD40LG |
33 |
ACHE |
14 |
CHRM3 |
1 |
EGF |
62 |
CRP |
44 |
PARP1 |
30 |
SLC6A4 |
12 |
||
MYC |
62 |
MAPK1 |
43 |
IRF1 |
28 |
E2F1 |
12 |
||
ESR1 |
60 |
CASP9 |
42 |
F3 |
28 |
COL3A1 |
12 |
||
NOS3 |
60 |
NFE2L2 |
42 |
PLAU |
28 |
MGAM |
11 |
||
IL10 |
60 |
IL1A |
41 |
DPP4 |
27 |
HMGCR |
11 |
||
HMOX1 |
58 |
NOS2 |
40 |
CXCL2 |
27 |
ADRB2 |
11 |
||
FOS |
57 |
CDKN2A |
40 |
APOB |
26 |
XDH |
11 |
||
ICAM1 |
57 |
SPP1 |
39 |
BAX |
25 |
PIK3CG |
11 |
||
NFKBIA |
57 |
SELE |
38 |
GSTP1 |
21 |
HSF1 |
11 |
||
CCND1 |
56 |
CXCL10 |
36 |
THBD |
21 |
CALM1 |
10 |
3.5 Cluster analysis
The MCODE network analysis revealed three clusters. The scores were 41.28,3.667 and 3.000. The highest scoring cluster, cluster 1, contained 51 nodes AKT1、TNF、IL6、IL1B、TP53、VEGFA、CASP3、PTGS2、MAPK3、PPARG、HIF1A、MMP9、CAT、CCL2、EGF、MYC、ESR1、NOS3、IL10、HMOX1、FOS、ICAM1、NFKBIA、CCND1、MMP2、PTEN、VCAM1、SERPINE1、CASP8、RELA、IL2、IFNG、BCL2L1、PPARA、TGFB1、STAT1、MPO、ADIPOQ、CRP、MAPK1、CASP9、NFE2L2、IL1A、NOS2、CDKN2A、SPP1、SELE、CXCL10、CDKN1A、NR3C1、CHUK) and 1032 edges. Cluster 2 contained 7 nodes (CAV1、RUNX2、PRKCA、GJA1、HSPB1、NCF1、COL1A1) and 11 edges. Cluster 3 contained 5 nodes (APOB, F2, LDLR, THBD, PLAT) and 6 edges. Cluster 4 contained 3 nodes (SOD1、GSR、NQO1) and 3 edges.
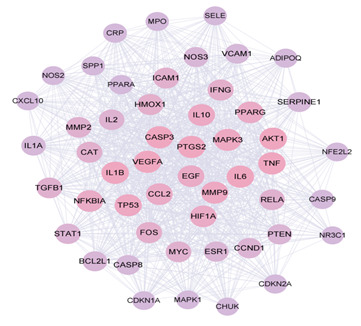
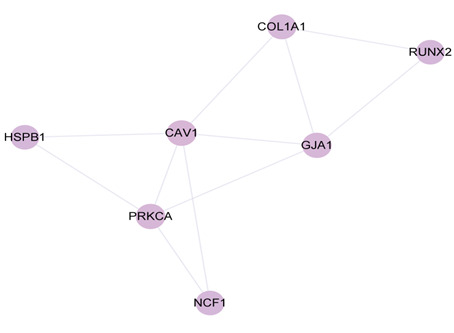
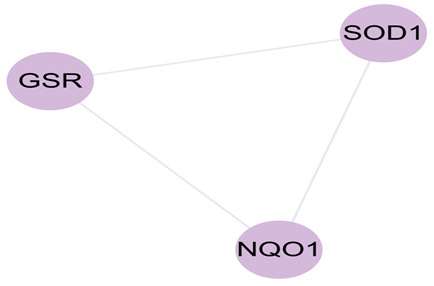
Figure 4. Cluster 1, 2 and 3
3.6 GO function and KEGG pathway enrichment analysis
A total of 848 GO items were obtained from GO enrichment analysis, including 677 biological process (BP) items, 59 cellular component (CC) items and 112 molecular function (MF) items. The GO analysis was enriched and screened to obtain the 10 most enriched signal pathways (P < 0.05). The results showed that the biological processes of myocardial ischemia-reperfusion injury treated by GLXB mainly involved response to drug, positive regulation of gene expression, response to hypoxia, estradiol, response to lipopolysaccharide, aging, response to ethanol, inflammatory response, response to xenobiotic stimulus, apoptotic processed. The cellular components mainly include extracellular space, macromolecular complex, extracellular region, caveola, membrane raft, cytosol, mitochondrion, nucleoplasm, cell surface, plasma membrane. The molecular functions mainly include identical protein binding, enzyme binding, protein kinase binding, protein binding, protein homodimerization activity, transcription factor binding, cytokine activity, transcription factor activity, protease binding, sequence-specific DNA binding. A total of 166 pathways were obtained by KEGG pathway enrichment analysis. The KEGG analysis were enriched and screened to obtain the 20 most enriched signal pathways (P<0.05). This signaling pathway is mainly involved in AGE-RAGE signaling pathway in diabetic complications(P=3.97E-29,molecular amount: 28), Pathways in cancer(P=4.54E-26,molecular amount:46), Fluid shear stress and atherosclerosis (P=6.28E-23,molecular amount:28), Lipid and atherosclerosis (P=3.62E-26,molecular amount:32), Hepatitis B (P=2.87E-19,amount:25), TNF signaling pathway (P=8.31E-18,molecular:21), Toxoplasmosis (P=8.31E-18,molecular:21), Kaposi sarcoma-associated herpesvirus infection (P=2.12E-17,molecular amount:25), Pancreatic cancer (P=5.22E-17,molecular amount:18), Human cytomegalovirus infection (P=5.65E-17,molecular:26), HIF-1 signaling pathway (P=3.27E-14,molecular:18).
3.7 Molecular docking
To further validate the predicted results of network pharmacology and to elaborate the mechanism of action and scientific basis of GLXB as a classical formula for the treatment of MIRI, the following key active ingredients docked with AKT1, IL1B, IL-6, TNF and TP53 molecules were selected: Diosmetin, beta-sitosterol, baicalein, Naringenin, Quereetin. The binding energy between ligand and receptor is an important index to evaluate the binding ability. It is generally accepted that the lower the binding energy of ligand to receptor, the more stable the conformation. Molecular docking validation was performed for key active ingredients and pivotal targets. A binding energy x≤-5.0 kcal/mol is used as a standard. It was suggested that the key active components of GXBD were well combined with the key targets. It also demonstrated that the prediction of this study was reliable. Furthermore, top five stable conformations are as follows: AKT-1, IL1βwith beta-sitosterol, Tp53 with Hydroxygenkwanin and TNF, IL-6 with Diosmetin, The main forms of interaction. For example, the hydroxyl group of baicalein forms a hydrogen bond with the protein, and the methyl group of Diosmetin also forms van der Waals forces with the protein.
4 Discussion
MIRI is one of the difficulties to be faced in ischemic heart diseases after the restoration of blood supply. So far, there are not affect drugs to prevention or treatment. CTM is the best advantageous characteristic in Chinese natural science, which has certain effects on MIRI in the integral medication of multi-factorial, multi-target action, et al. As a classical prescription in China, GLXB promotes Qi relieving chest stuffiness, eliminating phlegm. Snakegourd fruit (gualou) has the effect of relieving pain, clearing heat and relieving chest stiffness to dissipate mass. Onion bulb (xiebai) can warm yang and expels qi. The combination of the two drugs, one to remove the stagnant phlegm, the other is Qi stagnation. Experimental studies have shown that GLXB has a therapeutic effect on rats with chronic myocardial ischemia [21], reduce significantly whole blood viscosity and plasma CK and LDH levels, and have a protective effect on experimental myocardial ischemia-reperfusion injury in rats [22]. As far as we know, our study is the first to demonstrate the mechanism of GLXB for the treatment of MIRI through system pharmacology exploration and experimental validation. In this study, 167 active components and 190 potential targets were obtained in GLXB. MIRI has a total of 1194 targets, of which GLXB and MIRI share 110 targets. In addition, 848 GO functional enrichment terms and 166 KEGG-related pathways were obtained, indicating that GLXB has a "multi-component, multi-target, multi-functional, and multi-channel" hierarchical network against MIRI. We also found that Diosmetin, Hydroxygenkwanin, beta-sitosterol, Naringenin, Quereetin, and other active ingredients play an essential role in the treatment of MIRI with GLXB. Molecular docking results showed that Diosmetin, Hydroxygenkwanin, beta-sitosterol, Naringenin, Quereetin bound well to AKT1, IL-6, TNF, TP53 and IL1β. Finally, and this is highly crucial, we find that GLXB protects H9c2 cells from MIRI damage by inhibiting myocardial apoptosis, suggesting that there may be therapeutic effects on MIRI in this scenario by modulating these protein targets [23].
5 Conclusion
In summary, this study is the first to explore the potential molecular mechanisms of GLXB for MIRI treatment using network-based pharmacological analysis. The results suggest that the multiple components, targets and pathways of GLXB have synergistic effects in the treatment of MIRI. Our study also serves to guide future research into the molecular mechanisms of alternative herbal remedies for the treatment of MIRI, and provides a current perspective on the potential mechanisms of herbal remedies for the treatment of different forms of ischemia-reperfusion using a network pharmacological approach. However, there are some limitations to this study. This study outlines the mechanism of GLXB for MIRI based on existing databases. It must be confirmed by advanced in vivo and in vitro exploration to ensure the quality and plausibility of the results.
References
[1]. Schulze, C. J., Wang, W., Suarez-Pinzon, W. L., Sawicka, J., Sawicki, G., & Schulz, R. (2003). Imbalance between tissue inhibitor of metalloproteinase-4 and matrix metalloproteinases during acute myocardial ischemia-reperfusion injury. Circulation, 107(19), 2487–2492. https://doi.org/10.1161/01.CIR.0000065603.09430.58
[2]. Hernandez-Resendiz, S., Chinda, K., Ong, S. B., Cabrera-Fuentes, H., Zazueta, C., & Hausenloy, D. J. (2018). The role of redox dysregulation in the inflammatory response to acute myocardial ischaemia-reperfusion injury - Adding fuel to the fire. Current Medicinal Chemistry, 25(11), 1275–1293. https://doi.org/10.2174/0929867324666170329100619
[3]. Zhou, H., Wang, J., Zhu, P., Hu, S., & Ren, J. (2018). Ripk3 regulates cardiac microvascular reperfusion injury: The role of IP3R-dependent calcium overload, XO-mediated oxidative stress and F-action/filopodia-based cellular migration. Cellular Signalling, 45, 12–22. https://doi.org/10.1016/j.cellsig.2018.01.020
[4]. Ashraf, M. I., Ebner, M., Wallner, C., Haller, M., Khalid, S., Schwelberger, H., Koziel, K., Enthammer, M., Hermann, M., Sickinger, S., Soleiman, A., Steger, C., Vallant, S., Sucher, R., Brandacher, G., Santer, P., Dragun, D., & Troppmair, J. (2014). A p38MAPK/MK2 signaling pathway leading to redox stress, cell death and ischemia/reperfusion injury. Cell Communication and Signaling, 12, 6. https://doi.org/10.1186/1478-811X-12-6
[5]. Hausenloy, D. J., & Yellon, D. M. (2013). Myocardial ischemia-reperfusion injury: A neglected therapeutic target. The Journal of Clinical Investigation, 123(1), 92–100. https://doi.org/10.1172/JCI62874
[6]. Yang, M., Linn, B. S., Zhang, Y., & Ren, J. (2019). Mitophagy and mitochondrial integrity in cardiac ischemia-reperfusion injury. Biochimica et Biophysica Acta - Molecular Basis of Disease, 1865(9), 2293–2302. https://doi.org/10.1016/j.bbadis.2019.05.007
[7]. Li, C. Y., Yang, P., Jiang, Y. L., Lin, Z., Pu, Y. W., Xie, L. Q., Sun, L., & Lu, D. (2020). Ginsenoside Rb1 attenuates cardiomyocyte apoptosis induced by myocardial ischemia-reperfusion injury through mTOR signal pathway. Biomedicine & Pharmacotherapy, 125, 109913. https://doi.org/10.1016/j.biopha.2020.109913
[8]. Gao, F., Jing, Y., Li, J., & Guo, J. (2018). Therapeutic efficacy of Gualou Xiebai Banxia decoction in the treatment of angina pectoris: A systematic review. Journal of Integrated Cardiovascular and Cerebrovascular Diseases of Chinese and Western Medicine. https://doi.org/10.12102/j.issn.1672-1349.2018.23.003
[9]. Zou, C., Wel, M., Yan, H., Zheng, C., & Xu, X. (2020). Effects of Gualou Xiebai dropping pills against myocardial ischemia reperfusion injury. Journal of International Pharmaceutical Research, 05, 926-930. https://doi.org/10.13220/j.cnki.jipr.2016.05.022
[10]. Du, Y. (2020). Effect of Gualou Xiebai Banxia decoction on the efficacy and immune function of patients with hypertension and coronary heart disease. Inner Mongolia Traditional Chinese Medicine, 39(09), 24-25. https://doi.org/10.16040/j.cnki.cn15-1101.2020.09.014
[11]. Gualou Xiebai Banxia Decoction in the treatment of patients with hypertension combined with coronary heart disease and the effect on immune function. (2020). Cardiovascular Disease Electronic Journal of Integrated Traditional Chinese and Western Medicine, 35, 161+182. https://doi.org/10.16282/j.cnki.cn11-9336/r.2020.35.119
[12]. Xu, L., Li, H., Gao, Z., & Zhao, Q. (2014). Experimental study of blood lipid-regulating and myocardium-protecting function of Gualou Xiebai Banxia decoction. Journal of Shandong University of Traditional Chinese Medicine, 38(06), 593-595. https://doi.org/10.16294/j.cnki.1007-659x.2014.06.030
[13]. Ding, Y. F., Peng, Y. R., Shen, H., Shu, L., & Wei, Y. J. (2016). Gualou Xiebai decoction inhibits cardiac dysfunction and inflammation in cardiac fibrosis rats. BMC Complementary and Alternative Medicine, 16, 49. https://doi.org/10.1186/s12906-016-1012-5
[14]. Yan, L. L., Zhang, W. Y., Wei, X. H., Yan, L., Pan, C. S., Yu, Y., Fan, J. Y., Liu, Y. Y., Zhou, H., Han, J. Y., & Yao, X. S. (2018). Gualou Xiebai decoction, a traditional Chinese medicine, prevents cardiac reperfusion injury of hyperlipidemia rat via energy modulation. Frontiers in Physiology, 9, 296. https://doi.org/10.3389/fphys.2018.00296
[15]. Hopkins, A. L. (2007). Network pharmacology. Nature Biotechnology, 25(10), 1110–1111. https://doi.org/10.1038/nbt1007-1110
[16]. Hopkins, A. L. (2008). Network pharmacology: The next paradigm in drug discovery. Nature Chemical Biology, 4(11), 682–690. https://doi.org/10.1038/nchembio.118
[17]. Ru, J., Li, P., Wang, J., Zhou, W., Li, B., Huang, C., Li, P., Guo, Z., Tao, W., Yang, Y., Xu, X., Li, Y., Wang, Y., & Yang, L. (2014). TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. Journal of Cheminformatics, 6, 13. https://doi.org/10.1186/1758-2946-6-13
[18]. UniProt Consortium. (2021). UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Research, 49(D1), D480–D489. https://doi.org/10.1093/nar/gkaa1100
[19]. von Mering, C., Jensen, L. J., Snel, B., Hooper, S. D., Krupp, M., Foglierini, M., Jouffre, N., Huynen, M. A., & Bork, P. (2005). STRING: Known and predicted protein-protein associations, integrated and transferred across organisms. Nucleic Acids Research, 33(Database issue), D433–D437. https://doi.org/10.1093/nar/gki005
[20]. Huang, daW., Sherman, B. T., & Lempicki, R. A. (2009). Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protocols, 4(1), 44–57. https://doi.org/10.1038/nprot.2008.211
[21]. Zhang, F., Duan, B., Zhou, Z., Han, L., Huang, P., Ye, Y., Wang, Q., Huang, F., & Li, J. (2022). Integration of metabolomics and transcriptomics to reveal anti-chronic myocardial ischemia mechanism of Gualou Xiebai decoction. Journal of Ethnopharmacology, 297, 115530. https://doi.org/10.1016/j.jep.2022.115530
[22]. Huang, S., Guo, Y., Zhao, Y., Liu, Y., Ying, G., Liu, Y., & Tang, Y. (2021). Exploration of the adjuvant effect of white liquor in Gualou Xiebai Baijiu decoction on protecting ischemic myocardium in mice based on NO3--NO2--NO pathway. Journal of Liaoning University of Traditional Chinese Medicine, 23(04), 23-27. https://doi.org/10.13194/j.issn.1673-842x.2021.04.007
[23]. Donghai, C., & Zhenqiu, Z. (2019). Study on protective effects of Pericarpium Trichosanthis extract on H9c2 myocardial cells injured by hypoxia/reoxygenation and its mechanism. China Pharmacy, 30(8), 1072-1078. https://doi.org/10.6039/j.issn.1001-0408.2019.08.12
Cite this article
Wu,L.;Fan,J. (2024). The mechanism of Gualou Xiebai Baijiu decoction in the treatment of myocardial reperfusion injury based on network pharmacology and molecular docking technology. Journal of Food Science, Nutrition and Health,2,54-60.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Journal:Journal of Food Science, Nutrition and Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Schulze, C. J., Wang, W., Suarez-Pinzon, W. L., Sawicka, J., Sawicki, G., & Schulz, R. (2003). Imbalance between tissue inhibitor of metalloproteinase-4 and matrix metalloproteinases during acute myocardial ischemia-reperfusion injury. Circulation, 107(19), 2487–2492. https://doi.org/10.1161/01.CIR.0000065603.09430.58
[2]. Hernandez-Resendiz, S., Chinda, K., Ong, S. B., Cabrera-Fuentes, H., Zazueta, C., & Hausenloy, D. J. (2018). The role of redox dysregulation in the inflammatory response to acute myocardial ischaemia-reperfusion injury - Adding fuel to the fire. Current Medicinal Chemistry, 25(11), 1275–1293. https://doi.org/10.2174/0929867324666170329100619
[3]. Zhou, H., Wang, J., Zhu, P., Hu, S., & Ren, J. (2018). Ripk3 regulates cardiac microvascular reperfusion injury: The role of IP3R-dependent calcium overload, XO-mediated oxidative stress and F-action/filopodia-based cellular migration. Cellular Signalling, 45, 12–22. https://doi.org/10.1016/j.cellsig.2018.01.020
[4]. Ashraf, M. I., Ebner, M., Wallner, C., Haller, M., Khalid, S., Schwelberger, H., Koziel, K., Enthammer, M., Hermann, M., Sickinger, S., Soleiman, A., Steger, C., Vallant, S., Sucher, R., Brandacher, G., Santer, P., Dragun, D., & Troppmair, J. (2014). A p38MAPK/MK2 signaling pathway leading to redox stress, cell death and ischemia/reperfusion injury. Cell Communication and Signaling, 12, 6. https://doi.org/10.1186/1478-811X-12-6
[5]. Hausenloy, D. J., & Yellon, D. M. (2013). Myocardial ischemia-reperfusion injury: A neglected therapeutic target. The Journal of Clinical Investigation, 123(1), 92–100. https://doi.org/10.1172/JCI62874
[6]. Yang, M., Linn, B. S., Zhang, Y., & Ren, J. (2019). Mitophagy and mitochondrial integrity in cardiac ischemia-reperfusion injury. Biochimica et Biophysica Acta - Molecular Basis of Disease, 1865(9), 2293–2302. https://doi.org/10.1016/j.bbadis.2019.05.007
[7]. Li, C. Y., Yang, P., Jiang, Y. L., Lin, Z., Pu, Y. W., Xie, L. Q., Sun, L., & Lu, D. (2020). Ginsenoside Rb1 attenuates cardiomyocyte apoptosis induced by myocardial ischemia-reperfusion injury through mTOR signal pathway. Biomedicine & Pharmacotherapy, 125, 109913. https://doi.org/10.1016/j.biopha.2020.109913
[8]. Gao, F., Jing, Y., Li, J., & Guo, J. (2018). Therapeutic efficacy of Gualou Xiebai Banxia decoction in the treatment of angina pectoris: A systematic review. Journal of Integrated Cardiovascular and Cerebrovascular Diseases of Chinese and Western Medicine. https://doi.org/10.12102/j.issn.1672-1349.2018.23.003
[9]. Zou, C., Wel, M., Yan, H., Zheng, C., & Xu, X. (2020). Effects of Gualou Xiebai dropping pills against myocardial ischemia reperfusion injury. Journal of International Pharmaceutical Research, 05, 926-930. https://doi.org/10.13220/j.cnki.jipr.2016.05.022
[10]. Du, Y. (2020). Effect of Gualou Xiebai Banxia decoction on the efficacy and immune function of patients with hypertension and coronary heart disease. Inner Mongolia Traditional Chinese Medicine, 39(09), 24-25. https://doi.org/10.16040/j.cnki.cn15-1101.2020.09.014
[11]. Gualou Xiebai Banxia Decoction in the treatment of patients with hypertension combined with coronary heart disease and the effect on immune function. (2020). Cardiovascular Disease Electronic Journal of Integrated Traditional Chinese and Western Medicine, 35, 161+182. https://doi.org/10.16282/j.cnki.cn11-9336/r.2020.35.119
[12]. Xu, L., Li, H., Gao, Z., & Zhao, Q. (2014). Experimental study of blood lipid-regulating and myocardium-protecting function of Gualou Xiebai Banxia decoction. Journal of Shandong University of Traditional Chinese Medicine, 38(06), 593-595. https://doi.org/10.16294/j.cnki.1007-659x.2014.06.030
[13]. Ding, Y. F., Peng, Y. R., Shen, H., Shu, L., & Wei, Y. J. (2016). Gualou Xiebai decoction inhibits cardiac dysfunction and inflammation in cardiac fibrosis rats. BMC Complementary and Alternative Medicine, 16, 49. https://doi.org/10.1186/s12906-016-1012-5
[14]. Yan, L. L., Zhang, W. Y., Wei, X. H., Yan, L., Pan, C. S., Yu, Y., Fan, J. Y., Liu, Y. Y., Zhou, H., Han, J. Y., & Yao, X. S. (2018). Gualou Xiebai decoction, a traditional Chinese medicine, prevents cardiac reperfusion injury of hyperlipidemia rat via energy modulation. Frontiers in Physiology, 9, 296. https://doi.org/10.3389/fphys.2018.00296
[15]. Hopkins, A. L. (2007). Network pharmacology. Nature Biotechnology, 25(10), 1110–1111. https://doi.org/10.1038/nbt1007-1110
[16]. Hopkins, A. L. (2008). Network pharmacology: The next paradigm in drug discovery. Nature Chemical Biology, 4(11), 682–690. https://doi.org/10.1038/nchembio.118
[17]. Ru, J., Li, P., Wang, J., Zhou, W., Li, B., Huang, C., Li, P., Guo, Z., Tao, W., Yang, Y., Xu, X., Li, Y., Wang, Y., & Yang, L. (2014). TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. Journal of Cheminformatics, 6, 13. https://doi.org/10.1186/1758-2946-6-13
[18]. UniProt Consortium. (2021). UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Research, 49(D1), D480–D489. https://doi.org/10.1093/nar/gkaa1100
[19]. von Mering, C., Jensen, L. J., Snel, B., Hooper, S. D., Krupp, M., Foglierini, M., Jouffre, N., Huynen, M. A., & Bork, P. (2005). STRING: Known and predicted protein-protein associations, integrated and transferred across organisms. Nucleic Acids Research, 33(Database issue), D433–D437. https://doi.org/10.1093/nar/gki005
[20]. Huang, daW., Sherman, B. T., & Lempicki, R. A. (2009). Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protocols, 4(1), 44–57. https://doi.org/10.1038/nprot.2008.211
[21]. Zhang, F., Duan, B., Zhou, Z., Han, L., Huang, P., Ye, Y., Wang, Q., Huang, F., & Li, J. (2022). Integration of metabolomics and transcriptomics to reveal anti-chronic myocardial ischemia mechanism of Gualou Xiebai decoction. Journal of Ethnopharmacology, 297, 115530. https://doi.org/10.1016/j.jep.2022.115530
[22]. Huang, S., Guo, Y., Zhao, Y., Liu, Y., Ying, G., Liu, Y., & Tang, Y. (2021). Exploration of the adjuvant effect of white liquor in Gualou Xiebai Baijiu decoction on protecting ischemic myocardium in mice based on NO3--NO2--NO pathway. Journal of Liaoning University of Traditional Chinese Medicine, 23(04), 23-27. https://doi.org/10.13194/j.issn.1673-842x.2021.04.007
[23]. Donghai, C., & Zhenqiu, Z. (2019). Study on protective effects of Pericarpium Trichosanthis extract on H9c2 myocardial cells injured by hypoxia/reoxygenation and its mechanism. China Pharmacy, 30(8), 1072-1078. https://doi.org/10.6039/j.issn.1001-0408.2019.08.12









