1 Literature Review
1.1 The Traditional Chinese Medicine
1.1.1 The History of the TCM
The start of TCM was not founded, but the oldest book recoding TCM founded by archaeologists is Huang Di Nei Jing, which was written in the Han Dynasty, was founded in 202 BC and ended in 220 AC. The TCM has also deeply affected Asian countries including Japan, Korea, Malaysia, and Vietnam. In Japan, their traditional medicine is commonly called 漢方薬, where “漢” refers to “Chinese”. Traditional Chinese Medicine was the majority of medication and therapy until the rise of Western Medicine in China after the Opium Wars. One of the most famous TCM books is The Compendium of Materia Medica, written by Li Shizhen in the Ming Dynasty, which recorded different recipes for a wide range of symptoms. [1]
1.1.2 The Ideas in the TCM
The start of the TCM is from the Chinese occult, which was an old interpretation of the nature of ancient Chinese people in China. In TCM, every part of the human body can be classified as either Yin or Yang. For example, the back can be identified as Yang, while the front is Yin. Qi can be identified as Yang; the body fluid is Yin. However, according to Su Wen, Yin and Yang can be identified infinitely times. For instance, the organs are Yin, but the different activities can be classified again, including Yin liver, Yang kidney, etc. [2] Five elements are also a crucial concept in TCM. TCM identified 5 attributes to all objects in the world: metal, wood, water, fire, and earth. They also correspond to five organs: lung, liver, kidney, heart, and spleen. Each of the five elements has relationships with each other. For example, water is against fire, and with wood. The five elements were used by the ancient Chinese people to explain how the world works. Anatomy also appears in the TCM. The ancient books have recorded several organs in the human body, and TCM emphasises the Qi, blood and body fluid. In addition, the meridians also called the channels, are found in the TCM. The meridians, and the acupoints, made the pavement for acupuncture, one of the most common therapies in TCM. Acupuncture will be discussed in the next part.
1.1.3 Acupuncture
Acupuncture is one of the most famous physical treatments. It is a type of alternative therapy. It means the treatment uses a fine needle to punctuate some specific points -- called acupoints, to reduce the pain of the body parts. The archaeologists founded a statuette made in the Song Dynasty (960-1279 AC) made of brass. The statuette indicates the acupoints on the human body, to help the doctors to practice. In TCM concepts, as the needles punctuate into the skin, some responses in the body are activated, to enhance the circulation of the body fluid, thus helping to the recovery. An experiment conducted by Goldman et.al. indicated that acupuncture can stimulate the synthesis of the adenosine A1 receptor agonist, which is responsible for reducing pain. The experiment showed that the injection of the agonist directly can replicate the effects of acupuncture. [3] The advantage of acupuncture is obvious: it is easy to conduct, and this vital property makes it possible to be used in some special circumstances. In addition, the total therapy is short, compared to intake of medicine, or moxibustion, which may need to be conducted for a long time.
1.1.4 Herbal Medicine
Herbal medicine refers to the drugs that use herbs or processed herbs as ingredients without additional chemical modification. Herbal medicine is one type of TCM, different from alternative therapy. Although it is called herbal medicine, there are several ingredients made from animals and minerals occasionally. There are several types of herbal medicine: drugs made from boiling the prescribed ingredients, made from soaking in alcohol for a prolonged time, made from brewing with hot water, made from steaming with water, made from adding honey or batter, to make ball-like drugs, and so on. Different ways to process the drugs can provide different effects on the human body.
1.2 The introduction of COVID-19
1.2.1 A Brief Overview
The first case of COVID-19 was reported in Wuhan, China in 2019. In January 2020, the first case of death was reported in China. The virus was not identified as SARS-CoV-2 until 14/Jan/2020 by WHO. After that, South Korea and the USA found patients who were affected by COVID-19. On 24/Jan/2020, the Chinese government took the lockdown measurement to Wuhan to control the spread of the pandemic. After that, an increasing number of cases appeared worldwide, and several countries took the lockdown policy as well. By 5/May/2023, WHO ended the global health emergency declaration for COVID-19. There are still some cases around the world, but with less extent. It reported that the patients have symptoms like cough, fever, and pulmonary fibrosis in more clinical cases.
1.2.2 The Structure and Mechanism of SARS-CoV-2
A virus highly related to COVID-19 is called SARS-CoV-2 and has a similar structure to other coronavirus, which are made of spike protein(S), membrane protein(M), envelope protein(E), and RNA.
The infection of COVID-19 can be divided into 2 parts: Spike protein attaches to the receptor, named ACE-2 (angiotensin-converting enzyme 2), which is the protein embedded in the cell of the host, having a complementary shape with the spike protein. Identification of proteins then allows SARS-CoC-2 enter the cell.
The replication stage can be divided into 3 parts: First, the enzyme in the cell breaks down the E protein and releases RNA. Then, mRNA is produced to manufacture the viral proteins and viral nucleic acids. After that, the virion assembles itself in the host’s cell. Finally, the next generation of viruses is matured and released outside of the cell. In conclusion, it can be said that the replication depends on the host. (Fig. 1) illustrates the infection process of the virus.
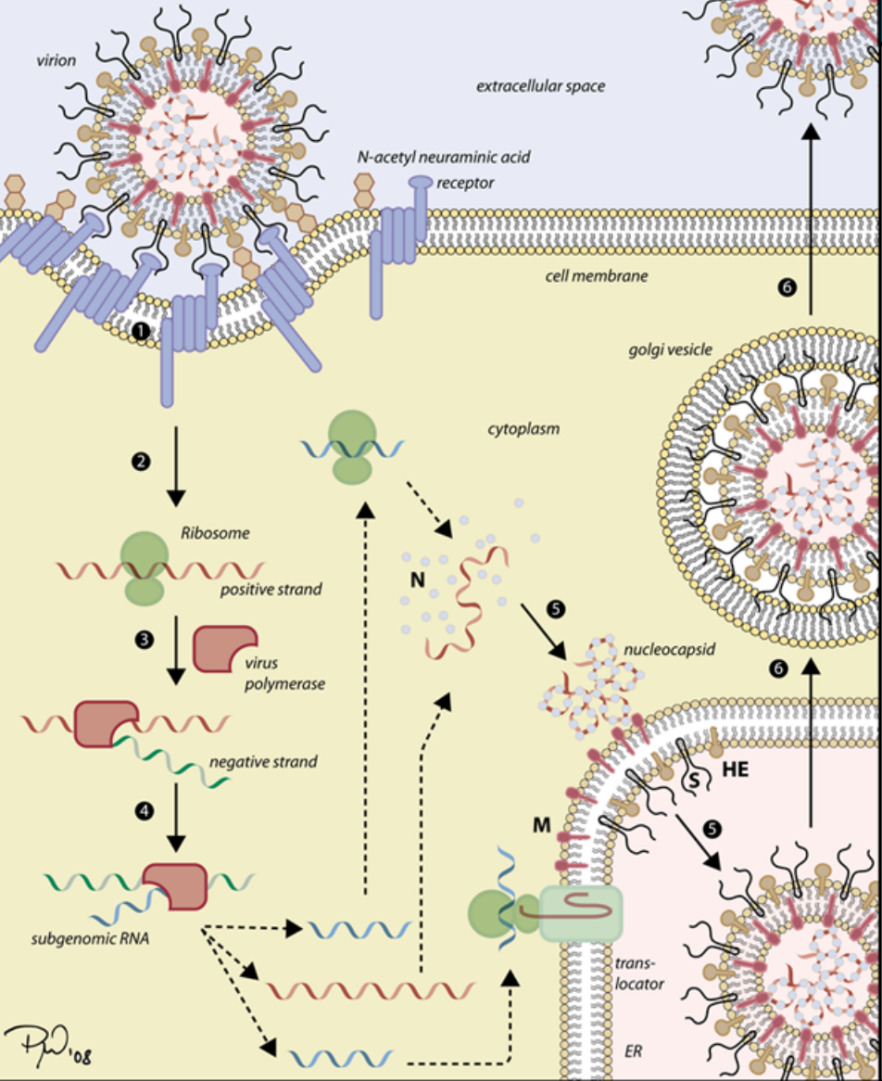
Figure 1. The process of infecting of virus [4]
1.2.3 The Effects on Patients
The Three stages and five levels of illness are shown in the graph to illustrate the different symptoms. (Fig.2) During the Medical observation period, the virus is just invading and replicating. So, there are few symptoms in the patients. But as the amount of virus increases, the immune system starts affecting the body, initiating inflammation and cytokine storm. When it goes to the severe and critical stages, the virus can damage the lungs, intestines, brains, lungs, kidneys, and hearts severely. However, different patients have different extents of illness. The statistics showed that, among all patients investigated, the symptoms include: fever (81.2); dyspnoea (26.1%); cough (58.5%); and sputum (25.8%). According to the John Hopkins University study, by September 19, 2023, 676 million people had been affected by COVID-19 (has been positive), and 6.8 million died affected by COVID-19. Older people, have pulmonary problems like asthma, heart diseases, brain and neuro problems, diabetes, obesity, cancer, some special blood diseases, weakness of the immune system, chronic renal or hepatic disease, mental problems and Down syndrome are more easily to be infected by COVID-19. [5]
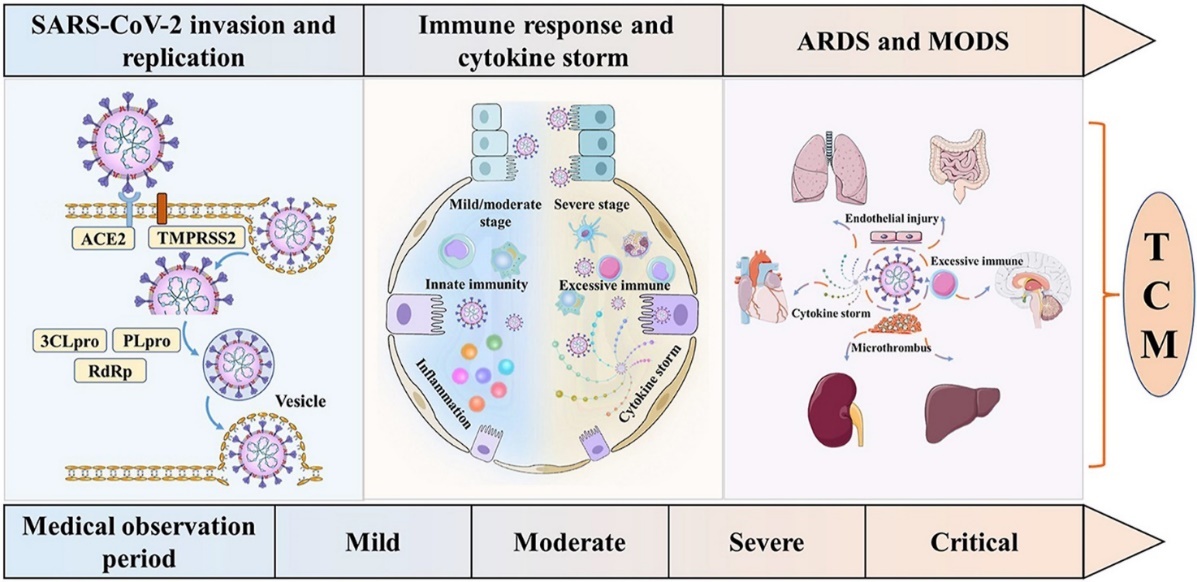
Figure 2. Three stages and five levels of illness5
1.2.4 The Interpretation of COVID-19 from the TCM Perspective
There is no concept for microorganisms in TCM. TCM emphasises the “balance of the Qi”, where Qi is the special term in TCM, which can control the functions of the human body. COVID-19 is a breakage of the balance of Qi. In TCM, COVID-19 can be considered as Qi of plague, which mainly attacks the lungs. The identity of COVID-19 is “dampness”, “heat”, “toxin”, and “blood accumulation”. Clinical symptoms including cough, fever, lack of appetite, irritability and cold limbs are summarised in TCM, according to the National Health Commission of the PRC. [6]
1.3 Recovery by taking herbal medicine prescribed by TCM
The herbal medicine can help the patients to recover in different ways. In total, four prescriptions are found to be effective for COVID-19 which will be shown below.
1.3.1 Jinhua Qinggan (JHQG)
JHQG is a type of granule which can disperse the Qi of the lung and clear heat-toxin. It is useful in treating most kinds of influenzas including H1N1. The ingredients of JHQG are Lonicera japonica Thunb (Flower), and Fritillaria thunbergii Miq. (Hemispheric stem), Scutellaria baicalensis Georgi (root), Arctium lappa L. (fruit), Artemisia caruifolia Buch.-Ham. ex Roxb. (leaves), etc.
A study group at Integrated Traditional Chinese and Western Medicine Center, Beijing YouAn Hospital, Capital Medical University, Beijing 100069, China [7] found that 46% of the male and 54% of female patients were infected with COVID-19, among 80 the total, had a negative nucleic acid test result after intaking JHQG. Compared to the control group, who did not intake JHQG or intake for less than two days, and used 10 days to turn negative in nucleic acid test (p=0.010), the varial group used 7 days to turn negative in nucleic acid (p=0.009) In other words, JHQG can shorten the time for patients being positive result of nucleic acid. In addition, the pneumonia recovery time indicated by chest CT was shortened (from 10 days to 8 days, p=0.021). No adverse reactions appeared in the experiment. It can significantly alleviate clinical symptoms of mild COVID patients, such as fever, cough, fatigue, and expectoration, and can relieve anxiety of the patients. The critical molecules in the prescription can combine with 3CLpro (3-Chymotrypsin-like protease) and ACE-2. Thus, the possibility of the virus attaching to the receptor decreases, reducing the possibility of infection.
1.3.2 Lianhua Qingwen (LHQW)
LHQW is a type of capsule which is useful for dispersing the Qi of the lung and detoxication. The ingredients are Forsythia suspensa (Thunb.)Vahl (flower), Lonicera japonica Thunb (flower), Ephedra (stem), heated Semen Armeniacae Amarum (nut), gypsum, dried of IsatisindigoticaFort. (root), Male Fern Rhizome (stem), Houttuynia cordata Thunb. (stem), Pogostemon cablin (Blanco) Benth. (leaves), Rheum palmatum L. (root and stem), Menthol (derivatives of mint), Glycyrrhiza uralensis Fisch. (root), accompanied with starch.
A study showed that LHQW can shorten the recovery time, which is shown in the line graph (Fig. 3). The definition of recovery made by them covers 3 criteria: recovery of body temperature for 3 days; no symptoms; significant improvement in CT scan of chest; and twice negative nucleic acid result (at least one day as interval)
Little evidence showed that LHQW will lead to side effects. A study in Shantou, Guangzhou, China did an experiment of LHQW about recovery of fever, and 128 children participated in this experiment. The result showed that both the control group and the observation group had 2 children who had slight vomiting, but the symptom was not severe, and all of them recovered by themselves. [8]
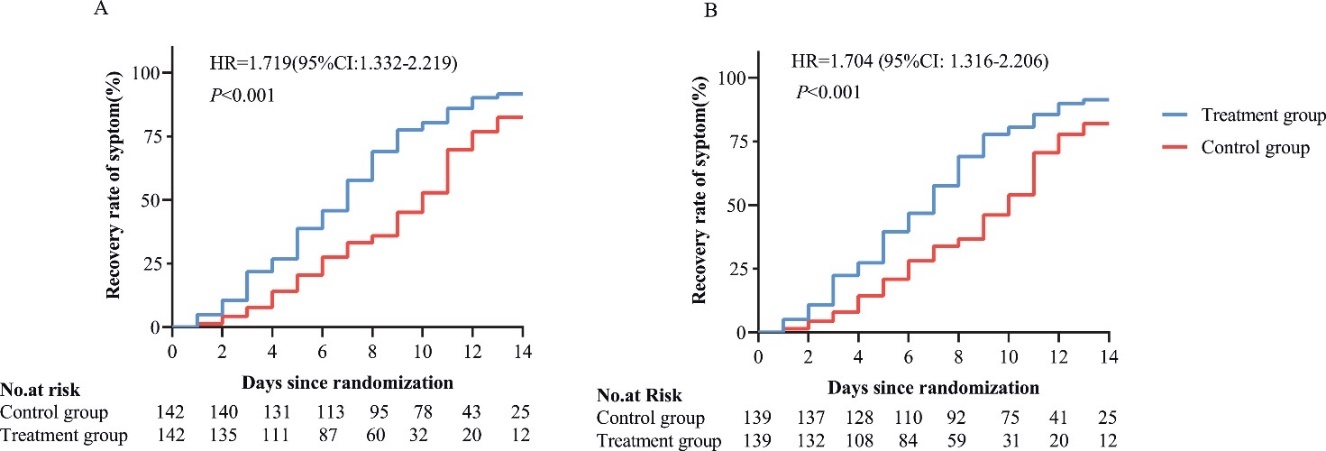
Figure 3. the recovery time compared to the control group [9]
In addition, the research indicated that LHQW with Arbidol can contribute to the recovery of mild symptoms. [10] The antibiotics in LHQW are effective in reducing the inflammatory response in the body, although they cannot kill the virus directly. [11]
1.3.3 Xuanfei Baidu (XFBD)
XFBD exerts anti-COVID-19 effects in different ways. XFBD contains molecules which can bind strongly to 3CLpro, and ACE-2, the receptor protein embedding on the cell membrane, indicating that XFBD can directly suppress COVID-19 virus by blocking its entry into host cells and inhibiting its replication. A study proved that 326 out of 1224 putative XFBD targets were associated with the disease target of COVID-19, of which 109 targets were enriched in the disease pathways of viral infection and lung injury. Some molecules in this herbal medicine may contribute to exhibiting the potential activity of the virus. [12] The chart below (Fig.4) illustrates the mechanism of XFBD treating COVID-19. [13] Studies showed that XFBD was used to regulate the primary biological pathways involving viral infection, parasites and bacterial infections.
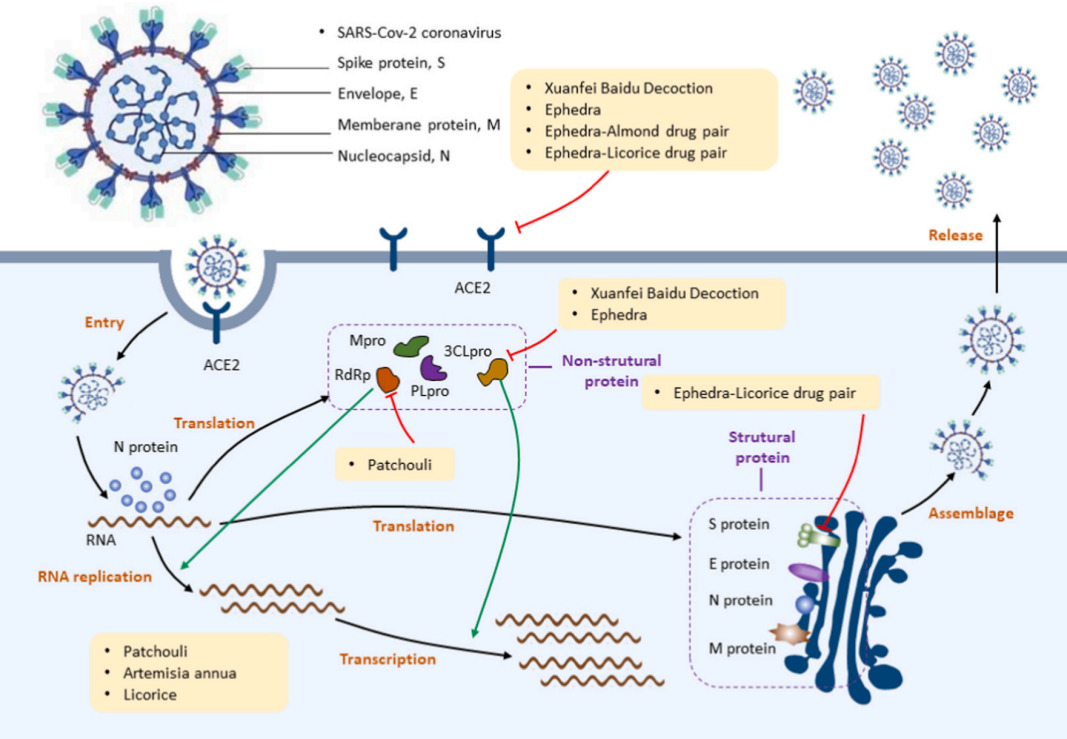
Figure 4. Anti-COVID-19 Mechanisms of Xuanfei Baidu Decoction18
1.3.4 Huashi Baidu (HSBD)
The ingredients of HSBD are Houpoea officinalis (Rehder & E. H. Wilson) N. H. Xia & C. Y. Wu, Astragalus membranaceus (Fisch.) Bunge, Atractylodes Lancea (Thunb.) DC., Agastache rugosa (Fisch. & C. A. Mey.) Kuntze, Amomum tsaoko Crevost & Lem., Glycyrrhiza uralensis Fisch., Pinellia ternata (Thunb.) Ten. ex Breitenb., Draba nemorosa L., dried Paeonia lactiflora Pall., dried Poria cocos(Schw.)Wolf, Ephedra sinica Stapf, Gypsum. [14]
It is effective in significantly shortening the average length of hospitalization and improving the clinical symptoms and pulmonary CT findings. HSBD is likely to inhibit the mediated signalling pathways and reduce the cytokine storm that occurs. [15]
2 Discussion/ Development
2.1 Specific Discussion of 4 Drugs in the Literature Review
2.1.1 Jinghua Qinggan
A meta-analysis conducted by Medicine (Baltimore) included several studies with a total of 528 patients and divided them into an experimental group (347) and a control group (235). JHQG can help to increase the rate of improvement for fever, increase the negative rate of nucleic acid in patients with COVID-19 and reduce the aggravation rate of pneumonia. However, no significant difference was shown in the improvement of cough, nausea, vomiting, and fatigue. [16]
Researchers in Karachi, Pakistan, and Beijing and Hong Kong, China, experimented with a double-blind test. The experiment included 300 patients, with all randomly selected control groups and experimental groups equally into 150 patients each with placebo-controlled trials. On the tenth day of the experiment, the JHQG group showed greater clinical efficacy compared with the placebo group. The percentage of patients with a negative PCR after treatment decreased by 4.67%, however, the decrease rate is not significant. The median time to recovery of COVID-19-related symptoms including cough, sputum, sore throat, dyspnoea, headache, nasal obstruction, fatigue, and myalgia was shorter in the JHQG group compared to the placebo group. [17]
2.1.2 Lianhua Qingwen
A study investigated the effectiveness of LHQW by selecting 2760 as the experimental group and 2158 patients as the control group. The result showed that the control group has a rate of 14.0 in acute liver injury, while there is 11.5 in the experimental group. However, the significance is not shown, as the 95% confidence interval overlaps. However, a significant decrease in rage of acute kidney injury was shown in the experimental group, which decreased from 5.3 to 3.0, compared to the control group. However, as a drug for severe and critical patients, LHQW did not show a significant reduction in the in-hospital mortality rate. [18]
Department of Emergency, Longhua Hospital, Shanghai University of Traditional Chinese Medicine conducted another trial including 692 patients, 346 of them received basic supporting care, and the remaining 346 patients received extra LHQW granules. The rate of recovery for the experiment group is higher than the experimental group, especially from day 2 to day 6. The line graph is shown below. (Fig. 5)
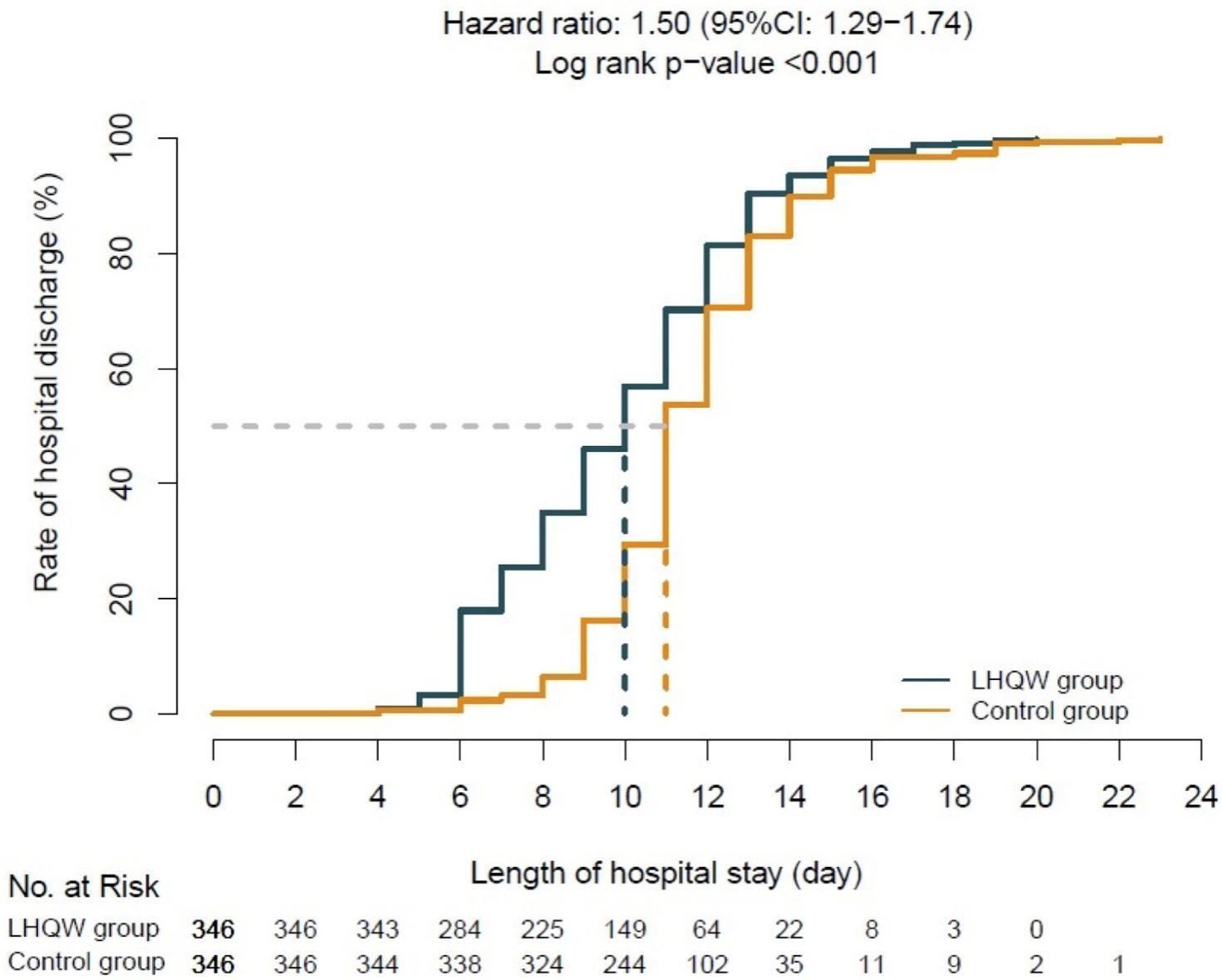
Figure 5. the rate of recovery of the control group and the experiment group (LHQW group) [19]
2.1.3 Xuanfei Baidu
A meta-analysis of the effectiveness is shown by studies in Beijing, China. 107 patients were selected out of 4658 patients in this investigation. XFBD can significantly reduce the length of stay in the hospital time and reduce the symptoms of COVID-19 (cough, fever, fatigue, etc.), The results showed that the rate of clinical symptom disappearance was significantly higher in the XFBD group than in the control group, such as fever (90.0% vs. 72.3%, P = 0.043), cough (76.5% vs. 38.9%, P = 0.028), fatigue (78.9% vs. 42.9%, P = 0.039), and poor appetite (75.0% vs. 22.2%, P = 0.024). XFBD is especially effective in severe or critical patients [20], and it can reduce the mortality rate. [21]
2.1.4 Huashi Baidu
According to the Chinese Journal of Integrative Medicine, an experiment was conducted with 992 people, with 496 each in the treatment group and the control group respectively. The result showed that compared with the control group, HSBD reduced the negative conversion time of the nucleic acid, and the length of hospitalisation (p<0.01) [22]
Another data is found by selecting 108 patients and randomised into a control group and an experiment group. The statistics showed that the intervention group (received HSBD) had a shorter length of time to turn negative in the nucleic acid test of SARS-CoV-2. (Fig.6) The median negative conversion time of SARS-CoV-2 nucleic acid was significantly shorter in the intervention group than in the control group (median days [interquartile range (IQR)]: 3 [3–5] vs. 5 [3–6]; p = 0.047). [23]
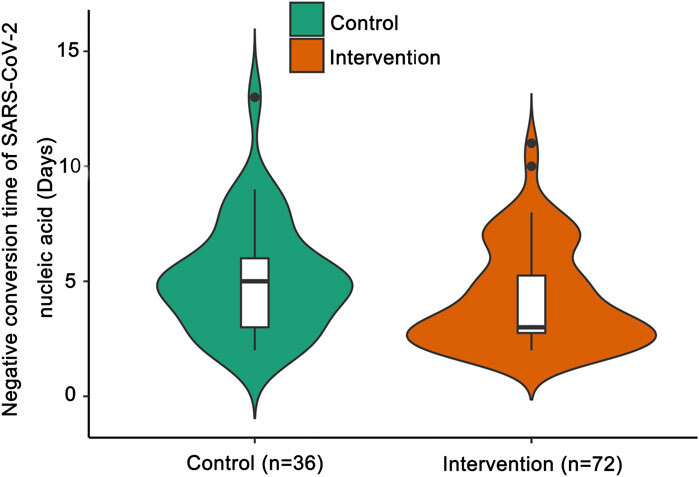
Figure 6. the negative conversion time of the NA test against the number of patients26
2.2 Recovery by Taking Medicine Prescribed by WM
Several prescriptions are also effectively achieved by Western medicine (WM, “western” is added to distinguish it from the TCM). Some of them have the same mechanism as the drugs which are talked about above and some act in a different way.
2.2.1 Chloroquine and Hydroxychloroquine
Chloroquine and Hydroxychloroquine are medicine which is against malaria. From April to
June, the medicine was an emergency use authorization for their use in the USA. [24]
An Indian study experimented with the effectiveness of chloroquine and inflammatory cytokine response in patients with musculoskeletal pain and Chikungunya virus infection. The result showed that chloroquine had no difference in the effectiveness with meloxicam, an injection opposing inframammary response, but there were few side effects. [25] However, when it comes to treating COVID-19, a study stated that chloroquine is competent to treat COVID-19. [26]
The mechanism of Chloroquine for the treatment is quite similar to TCM. It can bind to ACE-2 to block the virus from infecting the cell. [27] In addition, it can raise the vesicular pH value, to provide a difficult environment for the virus to survive. [28] However, some results are obtained from SARS-CoV, another pandemic that happened in China, in 2003, which has similar genetic similarities with SARS-CoV2, tOVID-19. At the same time, several side effects were revealed in the statistics. Some had problems with impaired hearing, confusion, and abnormal nerve conduction, after taking Chloroquine. [29]
Because of the serious side effects, the FDA revoked its emergency use of authorization, claiming that it was "no longer reasonable to believe" that the drug was effective against COVID-19 or that its benefits outweighed "known and potential risks" on 15 June 2020. [30] The World Health Organization also stated that both Chloroquine and Hydroxychloroquine have no effect in treating SARS-CoV-2. (Fig. 7)
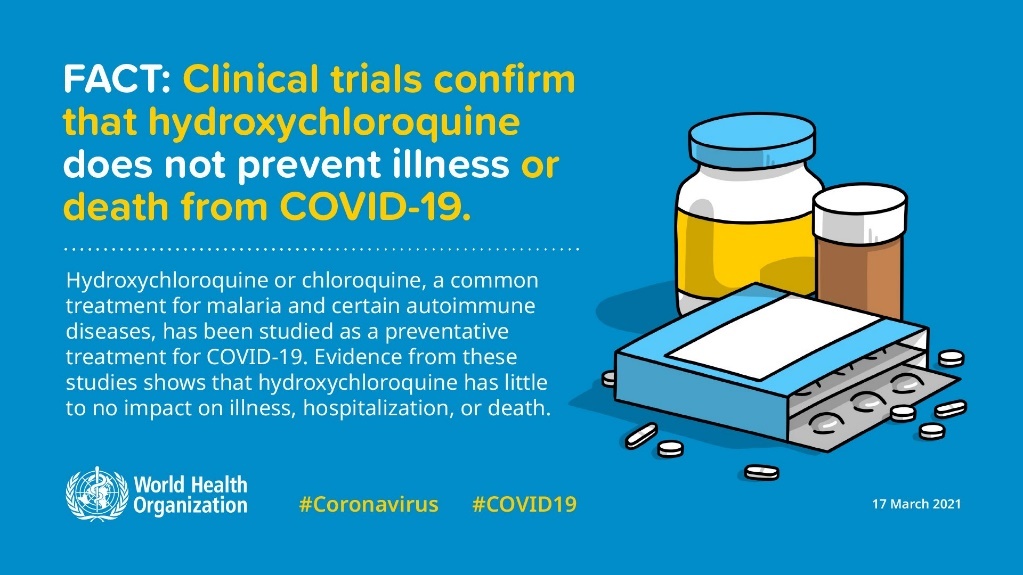
Figure 7. WHO stated that Chloroquine and Hydroxychloroquine do not prevent illness or death from COVID-19 [31]
2.2.2 Azithromycin
Azithromycin is a type of WM which has a different way of impeding the infection of COVID-19. Azithromycin can bind with ribosomes, the organelle responsible for synthesizing proteins in the cells. Thus, the virus cannot synthesise its protein to replicate and mature. However, the side effects seem more serious than CQ, which are headache, dizziness, sleep disturbances, seizures, vertigo, choreoathetosis, psychosis, and delirium. It is easy to find that the side effects are mostly related to mental problems, but the reason is still in the research. It is necessary to state that the evidence of this drug is still in shortage.
Although Azithromycin is a type of antibiotic, research found that Azithromycin has positive effects against Zika and Ebola viruses in test tube experiments. [32] Azithromycin is also effective in preventing severe bacterial respiratory tract infections in children with viral infections. Thus, it is still a potential treatment for the patients.
2.2.3 Lopinavir-ritonavir (Antiviral)
Lopinavir-ritonavir is a drug previously used in curing and protecting against HIV. It is a combination of lopinavir and a small amount of ritonavir. The figure below (Fig. 8) illustrates the skeleton formulae of lopinavir and ritonavir.
Lopinavir-ritonavir can inhibit the enzymes which are crucial for viral replication. The open-label trial showed that lopinavir-ritonavir is effective in SARS-CoV-2.11 Side effects exist when using this drug. Some may have symptoms including diarrhoea, nausea, vomiting, abdominal pain, fatigue, and headache after taking the drugs. [33] A hospital in Rui’an, China, indicated that compared to the control group, the concentration of lymphocytes, and C-reactive protein of the test group could be reduced to some extent. [34] C-reactive protein is a protein made by the liver. It is usually used to be an indicator of inflammation.
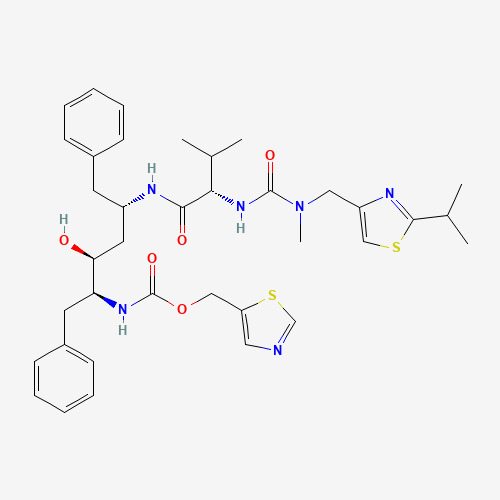
Figure 8. skeleton formulae of lopinavir and ritonavir [35]
2.3 What Are the Main Similarities and Differences Between TCM and WM Approaches?
First of all, it is necessary to acknowledge that both TCM and WM use trials to test the effects of the drugs. So, it is common to use statistical data to show the effectiveness and side effects of the drugs. This methodology is very useful so that it is used globally. However, despite the similarities, there are some differences between TCM and WM.
The difference can be divided into several parts. Firstly, and the most obvious is that TCM uses plants or animals with a process to an extent, while WM prefers to chemically modify the crucial molecules in plants or animals, or even bacteria, and purify them into tablets or capsules. Both ways have their drawbacks and benefits. Herbal medicines can give better effectiveness. In many cases, a wide range of molecules together is more effective than a single molecule, and purification and concentration may leave only a single molecule. The variety of effective molecules can have benefits beyond this. For example, the tumour/cancer cells and several bacteria have rapid spread of drug resistance. If they are not killed in a short time, the drug may fail to be used anymore in one patient. The TCM is like a cocktail treatment, giving more types of molecules to the body at the same time, thus few pathogens have genes which resistant to all kinds of molecules. [36] It can lead to a rapid decline in the amount of pathogens, and it is a way to oppose the spread of drug resistance. In addition, the extraction process in WM may lead to several problems, while it is much easier to process the plants into herbal medicine.
However, there are some limitations in the TCM treatment. The proportion of effective molecules in herbal medicine is pretty low, and even scepticism argues that herbal medicines have placebo effects. The effectiveness of herbal medicine also depends on the location of the plants, the weather, precipitation, etc.. This will lead to the variable in the effectiveness of herbal medicine. In comparison, the production of tablets and capsules is relatively more stable. In conclusion, both advantages and disadvantages are present in the TCM.
Despite the differences in the drug itself, there are some differences in the cultural considerations. TCM seems only to be accepted by the Chinese culture, and other societies prefer to take Western medicine. (And a considerable number of Chinese people prefer WM as well). Even fewer Chinese people would like to choose TCM as the only way to cure themselves when they are ill. However, in China, people would like to use TCM as the daily prevention of illness. The current trend increases public awareness of health, and TCM has become more popular in China. Tongrentang, the famous TCM pharmacy founded in Beijing, created a new brand selling soft drinks with herbal medicine in it. In addition, the function of prevention is fully embodied in the southern part of China. Due to the hot and humid weather in southern China, a lot of people like to drink herbal tea which can reduce the heat in the body.
In comparison, a much smaller proportion of foreign people choose TCM as their treatment in recovery and prevention of illness. However, because of the low cost, and rapid effect of acupuncture, which is talked about above, acupuncture is used in the US military. “They don’t have to wait hours for medications to take maximal effect or endure side effects, like drowsiness or allergic reactions, of common pain medications,” said Air Force Col. Lynda Vu, who recently administered BFA while deployed in Qatar. “This allows personnel to go back to the fight with minimal impact to continuing mission operations.” [37] Although herbal medicine is not used widely in other countries, alternative treatments seem very effective in some special circumstances.
2.4 Protein ACE-2, and Problems of Inhibiting ACE-2
Angiotensin-converting enzyme 2 (ACE-2) is an enzyme which can be found on the cell membrane of the intestines, kidney, testis, gallbladder, and heart. [38] ACE-2 can be also found on the upper bronchial epithelia and nasal epithelium. [39] ACE-2 is a type of enzyme for controlling the blood pressure. The spike protein of SARS-Cov-2 can attach to ACE-2 can result in the endocytosis of both the virus and the enzyme to complete the entry step of infection.
At the same time, ACE-2 is also responsible for other uses. ACE-2 can protect against the increase in blood pressure, and the deficiency of ACE-2 will lead to hypertension [40] by cleaving angiotensin II (Ang II) into angiotensin (1-7). [41] A study suggested that ACE-2 can also interact between pregnant mothers and their fetuses. [42] The multiple uses of ACE-2 contribute to more side effects because the inhibition of ACE-2 will make other irrelevant functions become invalid.
However, is it safe to use ACE-2 inhibitors completely? As mentioned before, ACE-2 is responsible for not only responsible for controlling blood pressure, but it also contributes to entrance of virus. However, most drugs – whether the TCM or the WM, targeting ACE-2, meaning that if a lot of ACE-2 is inhibited by the drug, the lack of concentration may bring several side effects such as hypertension. This is also the reason why chloroquine and hydroxychloroquine were banned by the FDA. However, properties of TCM including multitargeting approach and immunomodulatory effects could reduce side effects by inhibiting ACE-2.
2.5 How Is the Prevention of TCM Different from WM when Encountering COVID-19?
First of all, it is important to state that most countries take some quarantine measures to prevent and limit the spread of COVID-19. However, there are some differences despite the governmental measurement, in the approaches of TCM and WM.
2.5.1 The TCM approach to prevention
The TCM provided two different ways to prevent COVID-19 – medical one and non-medical one. One of the medical prevention the tea with a special recipe. According to the recommendation by the Chinese government, 9 grams of raw Radix Astragali, 5 grams of Lonicera japonica Thunb., and 3 grams of Pogostemon cablin (Blanco) Benth., brewing with boiling water, and drink once a day. The tea can improve the immune system of the human body. Another medical treatment is Yupingfeng granule, which contains Radix Astragali, Saposhnikovia divaricate, and Atractylodes macrocephala mainly, 5 grams of granules per time and 3 times a day. This granule is more suitable for people who catch colds easily. Non-medical treatment includes exercising including playing Taichi, and Baduanjin Qigong. Non-medical treatment also suggests massage spa rub sauna for specific points like Hegu, Yingxiang, and Fengchi. [43]
Another study found that physical treatment including acupuncture treatment and moxibustion treatment tend to prevent the infection of COVID-19. They can regulate the immune response, preventing inflammation and infection. The observation proved that other specific points on the body called Fengmen, Feishu, Pishu, Quchi, Chize, Yuji, Qihai, Zusanli, and Sanyinjiao [44] can be the target of the physical treatments. The research on physical treatment is still in progress. Targeting acupuncture, there is a risk that acupuncture may cause infection of bacteria when using inappropriate methods. More careful treatment and better disinfection like using ethanol can reduce the risk.
Both herbal medicine and alternative therapies illustrated that they are useful for improving the immune system, and making it harder for the virus to cause symptoms in the body. However, the symptoms are also mainly caused by the cytokine storm, the overreaction of the immune system. So, the usefulness of TCM as prevention is still in the research stage.
2.5.2 The WM approach to prevention
Vaccines have the most important role in preventing COVID-19 which was invented after the spread of the pandemic. There are different sorts of vaccines, and their final objectives are activating the B cell in our body to clone themselves to be the memory cell. Thus, the second infection (infection of the virus) can be acted more intensively and more quickly. [2]
The chart below illustrates the development of normal vaccines and SARS-CoV-2 vaccines. (Fig.9). [45] It is obvious that the COVID-19 vaccines have accelerated at an unimaginable speed. The vaccines for treating COVID-19 can be divided into 3 types, which will be listed below. [46]
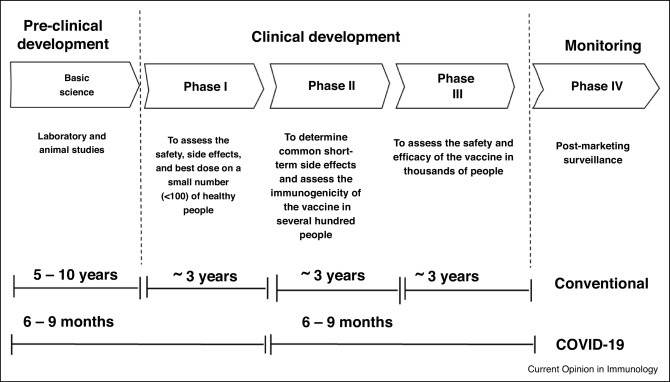
Figure 9. the development of COVID-19 vaccines and conventional vaccines
Whole virus vaccine: It uses the weakened (attenuated) or inactivated form of the severe acute COVID-19 virus. They can still replicate and mature in hosts, and activate the immune response to a regulated extent, but they are unable to cause disease because the harmful RNAs are eroded by heat, chemicals or radiation. However, the drawbacks exist in this type of vaccination. Because the virus will no longer be a threat to the vaccinated people, the virus will be released by faeces, affecting the unvaccinated ones, and causing diseases. This type of vaccination will also accelerate the variation of the virus, impeding the development of vaccination. At least 2 of them are approved to be used in an emergency.
Protein-based vaccines: They include 2 sub-types of vaccine, that is, subunit and virus-like particle vaccinees. The subunits are easy to be produced, and relatively safe and well-tolerated compared to whole virus vaccines. However, the limitation is less immunogenicity. There are currently 33 of them in clinical development. The virus like particle vaccines is the artificial protein which has a virus-like shape without RNA inside. [47]
Virus-vector vaccine: The virus replicates in the host’s body and uses its proteins and organelles to grow. However, the virus in the vaccine contains codes to specific antigens without harmful RNA. The vaccine acts as the delivery system, providing a means to invade the cell and insert the code for COVID-19 antigens. The virus used as a vector is chemically weakened so that it cannot cause disease. In this way, the body can mount an immune response safely, without developing the disease. One drawback with viral vector vaccines is that if people have been previously exposed to the viral vector and have developed an immune response against it this could potentially blunt the vaccine’s effectiveness. There are currently 16 non-replicating and two replicating viral vector candidate COVID-19 vaccines in clinical development.
According to WHO, 183 types of vaccines are in clinical development, and 199 types are in pre-clinical development. Vaccination is also an effective solution to prevent being infected by COVID-19. [15]
WHO also reported that approximately 5.6 billion people around the world have injected vaccines for COVID-19. In China, 239.03 doses of vaccine were injected into each 100 people. The vaccination programme is conducted very well by developed countries and advanced developing countries.15
2.6 How Do We Tackle the Mutation of the Virus?
There was a mutation which made a wide spread of SARS-CoV-2 (delta). The Beijing Daily reported it as the property of the virus changed from “cold-dampness” to “dampness-heat”, where cold dampness and dampness heat are the special terms in the TCM. The dampness can be divided into two types in TCM, the exterior and interior. “Dampness” in these terms indicates the interior one, which is related to digestion. The idea of TCM suggests that the reason patients experience severe symptoms is that the virus brings cold dampness. The cold dampness refers to the extra cold Qi and humidity present in the patients’ bodies. For example, patients will have an experience that they still feel cold although they have been wrapped in warm blankets. The TCM book Su Wen, written in approximately 200 BC, stated that patients will find their weakness in muscles, the feebleness of the knees, and enormous condition of their faeces if they catch the “cold-dampness”. The common symptoms of the “dampness heat” are fatigue, loss of appetite, enormous yellow colour of urine. The different descriptions of the virus can help the doctor design a better prescription for the patients who are affected by COVID-19. In addition, in the TCM term, there is no description of killing the virus, they only refer to how to change the condition of the body, instead of targeting the virus itself. It is useful when the virus mutates, as it doesn’t need to design another type of the drug, they just need to identify the new symptom and use another prescription.
2.7 Which One Is the Proper One for Using on The Approach of Costs?
Considering the total time spent on the treatment, and cost of the medicine, 1680 CNY is used for the average curing cost per person (5~9 days for the curing, and 240 CNY per day). The cost per dose for the Qingfei Baidu (QFPD) dictation is 40 CNY. In comparison, considering different stages of infection have different demands for medical service (e.g., the use of ECMO, ICU, etc.), the weighted average cost for curing COVID-19 is 99560 per person ([1000*89.23% (mild and normal patients) +1500*12.7% (severe patients) +4500*5% (clinical patients)] *20(average curing period)). [48] The difference is considerable that the cost of TCM is low compared with WM. This can help TCM popularize in the middle-class groups, and reduce the spread of the virus.
However, this data cannot significantly illustrate the difference in the cost of WM and TCM. In reality, people would like to take more advanced treatment (ICU, ECMO) if they have extremely severe symptoms. In China, people with mild symptoms prefer TCM treatment. In addition, no medical service appears in TCM, which only contains herbal medicine in these statistics. Thus, this question cannot be replied to by myself as the data found are biased and inappropriate, and more investigation is needed to illustrate the difference in the costs of the two types of medicine.
3 Conclusion
All in all, it is effective for Traditional Chinese Medicine concerning the physical recovery of COVID-19. As shown above, there are 4 types of TCM drugs found to be useful in reducing clinical symptoms to a different extent.
3.1 Jinhua Qinggan
JHQG can help to increase the negative rate of nucleic acid, reduce the aggravation rate of pneumonia, and a higher rate of recovery of clinical symptoms such as fever, cough, sputum, sore throat, dyspnoea, headache, nasal obstruction, fatigue, and myalgia.
3.2 Lianhua Qingwen
LHQW is responsible for reducing the risk of getting acute liver injuries and kidney injuries. This drug can also enhance the rate of hospital discharge, that is, the rate of recovery, including the disappearance of the symptoms, significant improvement in CT scan of the chest, and twice negative nucleic acid result. Little information showed that LHQW has side effects. Considering the effectiveness in lowering the risk of hepatic and renal injuries, LHQW is more useful for patients who are at the critical level of illness. However, the statistics showed that there are no significant changes made by LHQW in reducing the mortality rate.
3.3 Xuanfei Baidu
XFBD is proven to be effective in reducing the length of stay in the hospital time and reducing the symptoms of COVID-19, with fever as the most significant reduction, followed by fatigue, cough, and poor appetite. In addition, XFBD is effective for severe or critical patients. XFBD can also reduce the mortality rate significantly.
3.4 Huashi Baidu
It is already shown that HSBD can reduce the negative conversion time of the nucleic acid, and the length of hospitalisation. In addition, Studies showed that HSBD has little side effect.
3.5 Summary
In conclusion, different types of the drug are useful for different stages of illness, and types of clinical symptoms. Among these four drugs, LHQW is the most useful for patients who get severe symptoms, while XFQD can be used appropriately for severe patients with less risk of getting acute damage. HSBD and JHQG are more useful in reducing hospitalisation time and some mild symptoms for patients who are not so severe.
Speaking of prevention in the consideration of TCM, the two different therapies (alternative therapy and herbal medicine) both aim at the improvement of the immune system, making it harder for the virus to invade and try to kill them before the symptoms start to appear. Nevertheless, the effectiveness of prevention is very hard to quantify, as other measures were also taken during the pandemic, like the quarantine policy. In addition, different cultures, and governments may also change the result. Also, there are some differences in the measurements of effectiveness in the TCM and WM, so it is hard to compare them together.
References
[1]. Cheung, F. (2011). TCM: Made in China. Nature, 480(7378), S82-S83.
[2]. Tian, D. (2005). 黄帝内经素问 [Huangdi Nei Jing Su Wen].
[3]. Goldman, N., et al. (2010). Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nature Neuroscience, 13(7), 883-888.
[4]. Lai, M. M., & Cavanagh, D. (1997). The molecular biology of coronavirus. Advances in Virus Research, 48, 1-100.
[5]. Mayo Clinic. (2023, July 21). Covid-19: Who’s at higher risk of serious symptoms? Mayo Foundation for Medical Education and Research. Retrieved from https://www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/coronavirus-who-is-at-risk/art-20483301
[6]. A new type of coronavirus infection pneumonia diagnosis and treatment schemes (third edition). (2023, January 23). Retrieved from www.gov.cn/zhengce/zhengceku/2020-01/23/5471832/files/106d59e45ac948ceb3cb12d400b8053c.pdf
[7]. Liu, Z., Li, X., Gou, C., Li, L., Luo, X., Zhang, C., Zhang, Y., Zhang, J., Jin, A., Li, H., Zeng, Y., Li, T., & Wang, X. (2020). Effect of Jinhua Qinggan granules on novel coronavirus pneumonia in patients. Journal of Traditional Chinese Medicine, 40(3), 467-472.
[8]. Zheng, X., et al. (2013). 连花清瘟胶囊治疗儿童流行性感冒的临床观察 [Clinical observation of Lianhua Qingwen capsule in the treatment of children with influenza] (Doctoral dissertation).
[9]. Hu, K., et al. (2021). Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: A multicenter, prospective, randomized controlled trial. Phytomedicine, 85, 153242.
[10]. Fang, J., et al. (2020). Efficacy of early combination therapy with Lianhuaqingwen and arbidol in moderate and severe COVID-19 patients: A retrospective cohort study. Frontiers in Pharmacology, 11, 560209.
[11]. Xiao, M., et al. (2020). Efficacy of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules in treatment of COVID-19: A randomized controlled trial. Pharmacological Research, 161, 105126.
[12]. Wang, Y., et al. (2020). Mechanism of Xuanfei Baidu Tang in treatment of COVID-19 based on network pharmacology. China Journal of Chinese Materia Medica, 45(10), 2249-2256.
[13]. Xiong, W., et al. (2020). Efficacy of herbal medicine (Xuanfei Baidu decoction) combined with conventional drug in treating COVID-19: A pilot randomized clinical trial. Integrative Medicine Research, 9(3), 100489.
[14]. Yang, X. (2020). 抗新型冠状病毒肺炎 (COVID-19) 的化湿败毒颗粒药味物质基础研究 [Basic study of anti-novel coronavirus pneumonia (COVID-19) dampness and septic granules medicinal flavor substances]. 中国现代中药 [Modern Chinese Medicine], 22(5), 672-689.
[15]. Wang, Y., et al. (2021). Efficacy and safety assessment of severe COVID-19 patients with Chinese medicine: A retrospective case series study at early stage of the COVID-19 epidemic in Wuhan, China. Journal of Ethnopharmacology, 277, 113888.
[16]. Si, X., et al. (2023). Efficacy and safety of Jinhua Qinggan granules in the treatment of coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Medicine, 102(15).
[17]. Shah, M. R., et al. (2022). Jinhua Qinggan granules for non-hospitalized COVID-19 patients: A double-blind, placebo-controlled, and randomized controlled trial. Frontiers in Medicine, 9, 928468.
[18]. Lu, Y., et al. (2023). Effectiveness and safety of Lianhua Qingwen capsules for COVID-19: A propensity-score matched cohort study. Evidence-Based Complementary and Alternative Medicine, 2023, 113888.
[19]. Shah, M. R., et al. (2022). Jinhua Qinggan granules for non-hospitalized COVID-19 patients: A double-blind, placebo-controlled, and randomized controlled trial. Frontiers in Medicine, 9, 928468.
[20]. Zhao, J., et al. (2021). Efficacy and safety of Xuanfei Baidu granules for treating COVID-19: A protocol for systematic review and meta-analysis. Medicine, 100(20).
[21]. Li, X.-C., et al. (2022). Clinical observation of Xuanfei Baidu Decoction in treatment of severe coronavirus disease 2019 (COVID-19). Zhongguo Zhongyao Zazhi = China Journal of Chinese Materia Medica, 47(13), 3667-3674.
[22]. Chen, C.-Y., et al. (2023). Efficacy and safety of Huashi Baidu granules in treating patients with SARS-CoV-2 Omicron variant: A single-center retrospective cohort study. Chinese Journal of Integrative Medicine, 1-8.
[23]. Chen, J., et al. (2023). Huashi Baidu granule in the treatment of pediatric patients with mild coronavirus disease 2019: A single-center, open-label, parallel-group randomized controlled clinical trial. Frontiers in Pharmacology, 14, 1092748.
[24]. Centers for Disease Control and Prevention. (2023). Coronavirus disease 2019 (COVID-19). Retrieved October 8, 2023, from https://www.cdc.gov/coronavirus/2019-ncov/index.html
[25]. Chopra, A., Saluja, M., & Venugopalan, A. (2014). Effectiveness of chloroquine and inflammatory cytokine response in patients with early persistent musculoskeletal pain and arthritis following chikungunya virus infection. Arthritis & Rheumatology, 66(2), 319-326.
[26]. Meo, S. A., Klonoff, D. C., & Akram, J. (2020). Efficacy of chloroquine and hydroxychloroquine in the treatment of COVID-19. European Review for Medical and Pharmacological Sciences, 4539-4547.
[27]. Pastick, K. A., et al. (2020). Hydroxychloroquine and chloroquine for treatment of SARS-CoV-2 (COVID-19). Open Forum Infectious Diseases, 7(4).
[28]. Wang, M., et al. (2020). Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Research, 30(3), 269-271.
[29]. Kumar, A., Chattopadhyay, A., & Gupta, S. (2022). Neuropsychiatric manifestation of the drugs used in the treatment of SARS-2-CoV-2019 (COVID-19) infection and their management: An overview and practice implications. Asian Journal of Psychiatry, 73, 103101.
[30]. U.S. Food and Drug Administration. (2023). Coronavirus (COVID-19) update: FDA revokes emergency use authorization for chloroquine and hydroxychloroquine. Retrieved October 8, 2023, from https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and
[31]. World Health Organization. (2023). Covid-19 mythbusters. Retrieved October 8, 2023, from https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public/myth-busters
[32]. Gautret, P., et al. (2020). Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. International Journal of Antimicrobial Agents, 56(1), 105949.
[33]. Drugs.com. (2023). Lopinavir and ritonavir monograph for professionals. Retrieved October 9, 2023, from https://www.drugs.com/monograph/lopinavir-and-ritonavir.html
[34]. Ye, X.-T., et al. (2020). Clinical efficacy of lopinavir/ritonavir in the treatment of coronavirus disease 2019. European Review for Medical and Pharmacological Sciences, 24(6), 3390-3396.
[35]. PubChem. (2023). Lopinavir. PubChem. Retrieved October 9, 2023, from https://pubchem.ncbi.nlm.nih.gov/compound/Lopinavir
[36]. Carmona, F., & Soares Pereira, A. M. (2013). Herbal medicines: Old and new concepts, truths and misunderstandings. Revista Brasileira de Farmacognosia, 23(2), 379-385.
[37]. Bauke, N. (2022, August 18). Battlefield acupuncture? Yes, it exists, and the military is using it to fight troops’ pain. Military Times. Retrieved from https://www.militarytimes.com/news/your-military/2018/02/09/battlefield-acupuncture-yes-it-exists-and-the-military-is-using-it-to-fight-troops-pain/
[38]. Hikmet, F., Méar, L., Edvinsson, Å., Micke, P., Uhlén, M., & Lindskog, C. (2020). The protein expression profile of ACE-2 in human tissues. Molecular Systems Biology, 16(7), e9610.
[39]. Jackson, C. B., et al. (2022). Mechanisms of SARS-CoV-2 entry into cells. Nature Reviews Molecular Cell Biology, 23(1), 3-20.
[40]. Hamming, I., et al. (2007). The emerging role of ACE-2 in physiology and disease. The Journal of Pathology, 212(1), 1-11.
[41]. Dhaundiyal, A., et al. (2021). Is highly expressed ACE 2 in pregnant women “a curse” in times of COVID-19 pandemic? Life Sciences, 264, 118676.
[42]. Valdés, G., et al. (2006). Distribution of angiotensin-(1-7) and ACE-2 in human placentas of normal and pathological pregnancies. Placenta, 27(2-3), 200-207.
[43]. 中国政府网. (2022). 新冠病毒感染者居家中医药干预指引 [Guidelines for home Chinese medicine intervention for persons infected with the novel coronavirus]. Retrieved October 17, 2023, from https://www.gov.cn/xinwen/2022-12/12/content_5731565.htm
[44]. Zhao, Z., et al. (2020). Prevention and treatment of COVID-19 using traditional Chinese medicine: A review. Phytomedicine, 79, 153242.
[45]. Ndwandwe, D., & Wiysonge, C. S. (2021). COVID-19 vaccines. Current Opinion in Immunology, 71, 111-116.
[46]. Ndwandwe, D., et al. (2021). COVID-19 vaccines. Current Opinion in Immunology, 71, 111-116.
[47]. Ndwandwe, D., et al. (2021). COVID-19 vaccines. Current Opinion in Immunology. Retrieved September 26, 2023, from https://www.sciencedirect.com/science/article/pii/S095279152100090X
[48]. Zhu, H., et al. (2022). Efficacy and safety of Chinese herbal medicine for treating mild or moderate COVID-19: A systematic review and meta-analysis of randomized controlled trials and observational studies. Frontiers in Pharmacology, 13, 988237.
Cite this article
Xiang,Y. (2024). A meta-analysis of efficiency of traditional Chinese medicine concerning physical recovery in COVID-19. Journal of Food Science, Nutrition and Health,2,61-75.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Journal:Journal of Food Science, Nutrition and Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Cheung, F. (2011). TCM: Made in China. Nature, 480(7378), S82-S83.
[2]. Tian, D. (2005). 黄帝内经素问 [Huangdi Nei Jing Su Wen].
[3]. Goldman, N., et al. (2010). Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nature Neuroscience, 13(7), 883-888.
[4]. Lai, M. M., & Cavanagh, D. (1997). The molecular biology of coronavirus. Advances in Virus Research, 48, 1-100.
[5]. Mayo Clinic. (2023, July 21). Covid-19: Who’s at higher risk of serious symptoms? Mayo Foundation for Medical Education and Research. Retrieved from https://www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/coronavirus-who-is-at-risk/art-20483301
[6]. A new type of coronavirus infection pneumonia diagnosis and treatment schemes (third edition). (2023, January 23). Retrieved from www.gov.cn/zhengce/zhengceku/2020-01/23/5471832/files/106d59e45ac948ceb3cb12d400b8053c.pdf
[7]. Liu, Z., Li, X., Gou, C., Li, L., Luo, X., Zhang, C., Zhang, Y., Zhang, J., Jin, A., Li, H., Zeng, Y., Li, T., & Wang, X. (2020). Effect of Jinhua Qinggan granules on novel coronavirus pneumonia in patients. Journal of Traditional Chinese Medicine, 40(3), 467-472.
[8]. Zheng, X., et al. (2013). 连花清瘟胶囊治疗儿童流行性感冒的临床观察 [Clinical observation of Lianhua Qingwen capsule in the treatment of children with influenza] (Doctoral dissertation).
[9]. Hu, K., et al. (2021). Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: A multicenter, prospective, randomized controlled trial. Phytomedicine, 85, 153242.
[10]. Fang, J., et al. (2020). Efficacy of early combination therapy with Lianhuaqingwen and arbidol in moderate and severe COVID-19 patients: A retrospective cohort study. Frontiers in Pharmacology, 11, 560209.
[11]. Xiao, M., et al. (2020). Efficacy of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules in treatment of COVID-19: A randomized controlled trial. Pharmacological Research, 161, 105126.
[12]. Wang, Y., et al. (2020). Mechanism of Xuanfei Baidu Tang in treatment of COVID-19 based on network pharmacology. China Journal of Chinese Materia Medica, 45(10), 2249-2256.
[13]. Xiong, W., et al. (2020). Efficacy of herbal medicine (Xuanfei Baidu decoction) combined with conventional drug in treating COVID-19: A pilot randomized clinical trial. Integrative Medicine Research, 9(3), 100489.
[14]. Yang, X. (2020). 抗新型冠状病毒肺炎 (COVID-19) 的化湿败毒颗粒药味物质基础研究 [Basic study of anti-novel coronavirus pneumonia (COVID-19) dampness and septic granules medicinal flavor substances]. 中国现代中药 [Modern Chinese Medicine], 22(5), 672-689.
[15]. Wang, Y., et al. (2021). Efficacy and safety assessment of severe COVID-19 patients with Chinese medicine: A retrospective case series study at early stage of the COVID-19 epidemic in Wuhan, China. Journal of Ethnopharmacology, 277, 113888.
[16]. Si, X., et al. (2023). Efficacy and safety of Jinhua Qinggan granules in the treatment of coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Medicine, 102(15).
[17]. Shah, M. R., et al. (2022). Jinhua Qinggan granules for non-hospitalized COVID-19 patients: A double-blind, placebo-controlled, and randomized controlled trial. Frontiers in Medicine, 9, 928468.
[18]. Lu, Y., et al. (2023). Effectiveness and safety of Lianhua Qingwen capsules for COVID-19: A propensity-score matched cohort study. Evidence-Based Complementary and Alternative Medicine, 2023, 113888.
[19]. Shah, M. R., et al. (2022). Jinhua Qinggan granules for non-hospitalized COVID-19 patients: A double-blind, placebo-controlled, and randomized controlled trial. Frontiers in Medicine, 9, 928468.
[20]. Zhao, J., et al. (2021). Efficacy and safety of Xuanfei Baidu granules for treating COVID-19: A protocol for systematic review and meta-analysis. Medicine, 100(20).
[21]. Li, X.-C., et al. (2022). Clinical observation of Xuanfei Baidu Decoction in treatment of severe coronavirus disease 2019 (COVID-19). Zhongguo Zhongyao Zazhi = China Journal of Chinese Materia Medica, 47(13), 3667-3674.
[22]. Chen, C.-Y., et al. (2023). Efficacy and safety of Huashi Baidu granules in treating patients with SARS-CoV-2 Omicron variant: A single-center retrospective cohort study. Chinese Journal of Integrative Medicine, 1-8.
[23]. Chen, J., et al. (2023). Huashi Baidu granule in the treatment of pediatric patients with mild coronavirus disease 2019: A single-center, open-label, parallel-group randomized controlled clinical trial. Frontiers in Pharmacology, 14, 1092748.
[24]. Centers for Disease Control and Prevention. (2023). Coronavirus disease 2019 (COVID-19). Retrieved October 8, 2023, from https://www.cdc.gov/coronavirus/2019-ncov/index.html
[25]. Chopra, A., Saluja, M., & Venugopalan, A. (2014). Effectiveness of chloroquine and inflammatory cytokine response in patients with early persistent musculoskeletal pain and arthritis following chikungunya virus infection. Arthritis & Rheumatology, 66(2), 319-326.
[26]. Meo, S. A., Klonoff, D. C., & Akram, J. (2020). Efficacy of chloroquine and hydroxychloroquine in the treatment of COVID-19. European Review for Medical and Pharmacological Sciences, 4539-4547.
[27]. Pastick, K. A., et al. (2020). Hydroxychloroquine and chloroquine for treatment of SARS-CoV-2 (COVID-19). Open Forum Infectious Diseases, 7(4).
[28]. Wang, M., et al. (2020). Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Research, 30(3), 269-271.
[29]. Kumar, A., Chattopadhyay, A., & Gupta, S. (2022). Neuropsychiatric manifestation of the drugs used in the treatment of SARS-2-CoV-2019 (COVID-19) infection and their management: An overview and practice implications. Asian Journal of Psychiatry, 73, 103101.
[30]. U.S. Food and Drug Administration. (2023). Coronavirus (COVID-19) update: FDA revokes emergency use authorization for chloroquine and hydroxychloroquine. Retrieved October 8, 2023, from https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and
[31]. World Health Organization. (2023). Covid-19 mythbusters. Retrieved October 8, 2023, from https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public/myth-busters
[32]. Gautret, P., et al. (2020). Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. International Journal of Antimicrobial Agents, 56(1), 105949.
[33]. Drugs.com. (2023). Lopinavir and ritonavir monograph for professionals. Retrieved October 9, 2023, from https://www.drugs.com/monograph/lopinavir-and-ritonavir.html
[34]. Ye, X.-T., et al. (2020). Clinical efficacy of lopinavir/ritonavir in the treatment of coronavirus disease 2019. European Review for Medical and Pharmacological Sciences, 24(6), 3390-3396.
[35]. PubChem. (2023). Lopinavir. PubChem. Retrieved October 9, 2023, from https://pubchem.ncbi.nlm.nih.gov/compound/Lopinavir
[36]. Carmona, F., & Soares Pereira, A. M. (2013). Herbal medicines: Old and new concepts, truths and misunderstandings. Revista Brasileira de Farmacognosia, 23(2), 379-385.
[37]. Bauke, N. (2022, August 18). Battlefield acupuncture? Yes, it exists, and the military is using it to fight troops’ pain. Military Times. Retrieved from https://www.militarytimes.com/news/your-military/2018/02/09/battlefield-acupuncture-yes-it-exists-and-the-military-is-using-it-to-fight-troops-pain/
[38]. Hikmet, F., Méar, L., Edvinsson, Å., Micke, P., Uhlén, M., & Lindskog, C. (2020). The protein expression profile of ACE-2 in human tissues. Molecular Systems Biology, 16(7), e9610.
[39]. Jackson, C. B., et al. (2022). Mechanisms of SARS-CoV-2 entry into cells. Nature Reviews Molecular Cell Biology, 23(1), 3-20.
[40]. Hamming, I., et al. (2007). The emerging role of ACE-2 in physiology and disease. The Journal of Pathology, 212(1), 1-11.
[41]. Dhaundiyal, A., et al. (2021). Is highly expressed ACE 2 in pregnant women “a curse” in times of COVID-19 pandemic? Life Sciences, 264, 118676.
[42]. Valdés, G., et al. (2006). Distribution of angiotensin-(1-7) and ACE-2 in human placentas of normal and pathological pregnancies. Placenta, 27(2-3), 200-207.
[43]. 中国政府网. (2022). 新冠病毒感染者居家中医药干预指引 [Guidelines for home Chinese medicine intervention for persons infected with the novel coronavirus]. Retrieved October 17, 2023, from https://www.gov.cn/xinwen/2022-12/12/content_5731565.htm
[44]. Zhao, Z., et al. (2020). Prevention and treatment of COVID-19 using traditional Chinese medicine: A review. Phytomedicine, 79, 153242.
[45]. Ndwandwe, D., & Wiysonge, C. S. (2021). COVID-19 vaccines. Current Opinion in Immunology, 71, 111-116.
[46]. Ndwandwe, D., et al. (2021). COVID-19 vaccines. Current Opinion in Immunology, 71, 111-116.
[47]. Ndwandwe, D., et al. (2021). COVID-19 vaccines. Current Opinion in Immunology. Retrieved September 26, 2023, from https://www.sciencedirect.com/science/article/pii/S095279152100090X
[48]. Zhu, H., et al. (2022). Efficacy and safety of Chinese herbal medicine for treating mild or moderate COVID-19: A systematic review and meta-analysis of randomized controlled trials and observational studies. Frontiers in Pharmacology, 13, 988237.









