1. Introduction
Cancer remains one of the leading causes of mortality in China, with pancreatic cancer—often termed the “king of cancer,”— exhibiting a particular poor prognosis and ranking as the seventh leading cause of cancer-related death worldwide [1]. Due to its asymptomatic nature and aggresive progression, pancreatic cancer is notoriously challenging to detect early, often only being identified in advanced stages. Consequently, it has a substantially higher postoperative mortality rate and lower survival rate compared to most other cancers.
The emergence of nanomaterials and nanotechnologies has opened promising new avenues for treating pancreatic cancer. Nanomaterials, defined as naturally occurring or engineered materials with particle sizes between 1 and 100 nanometers in one or more dimensions, possess unique properties at the nanoscale. Nanotechnology itself operates at the atomic, molecular, and macromolecular levels, making it possible to engineer materials and systems with a precision that can drive advances in pancreatic cancer therapy. Given the scale and unique properties of nanotechnology, it holds the potential to address the critical challenges in treating this disease, including difficulty in early diagnosis, poor prognosis, high recurrence rates post-surgery, and a lack of effective targeted therapies.
One of the notable breakthroughs in this field has been the development of the nanoknife, which has emerged as a powerful tool for treating advanced pancreatic cancer. This paper explores the current advancements and potential of three key nanotechnology-based approaches: the nanoknife, nanoparticle drug delivery systems, and nanoprobes. By examining the underlying principles and application methods of each technology, this paper aims to provide a comprehensive overview of how these innovations are reshaping the therapeutic landscape for pancreatic cancer, synthesizing current insights to inspire future research and clinical insights to inspire future research and clinical applications.
2. Overview of pancreatic cancer
Pancreatic cancer is a highly malignant tumor of the digestive tract, characterized by strong invasiveness and a high matastatic potential. Globally, its five-year survival rate remains dismally low at round 10%. Due to its asymptomatic progression and aggressive nature, pancreatic cancer is typically diagnosed at advanced stages, with over 80% of patients ineligible for surgical resection. Conventional therapies, including radiotherapy and chemotherapy, often prove ineffective due to the cancer’s resistance, and patients are generally insensitive to immunotherapy, resulting in a poor prognosis. Numerous risk factors contribute to pancreatic cancer, such as precancerous lesions, genetic abnormalities, hereditary predispositions, and long-term lifestyle and dietary habits.
The most prominent clinical manifestations include jaundice, abdominal pain and weight loss. Pathological analysis often reveals abnormalities in biomarkers like bilirubin. As shown in Figure 1, Abdominal pain, frequently the initial symptom, often begins as mild discomfort in the upper abdomen. Patients and their families commonly mistake this pain for gastric issues and resort to self-treatment based on common sense or online information, often without seeking further medical evaluation. This may lead to progressive and severe upper abdominal pain as the disease advances.
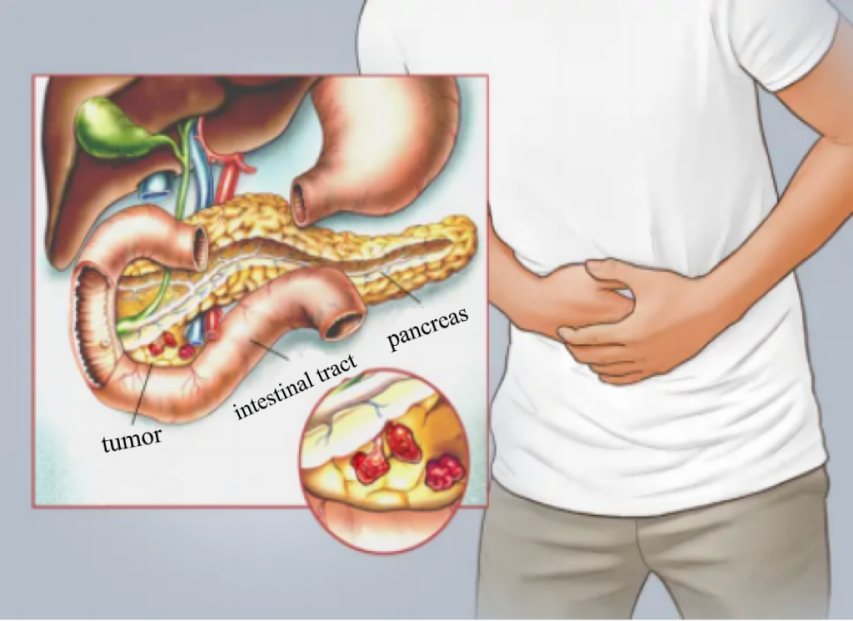
Figure 1. Schematic diagram of pancreatic cancer difficult to find [5]
Jaundice, another key symptom, is marked by yellowing of the skin and sclera, along with darkened urine and stool, often accompanied by persistent itching. Patients usually seek medical help only upon noticing significant jaundice, by which time the disease is often at an advanced stage. When bilirubin levels rise beyond a certain extent, it is difficult to restore them to a level suitable for surgery, often resulting in missed opportunities for optimal surgical intervention.
The challenges in detecting and treating pancreatic cancer are substantial. Professor He, in Family Health·New Health, highlights five major obstacles: late diagnosis, inoperability, limited effectiveness of radiotherapy and chemotherapy, severe abdominal pain as a core symptom, and obstructive jaundice that rarely meets surgical criteria. Pancreatic cancer also invades and metastasizes to nearby critical organs; once metastasis occurs, there are minimal options for effective treatment. The disease’s deep anatomical location complicates early detection, limiting the efficacy of surgical interventions. While recent advancements have improved surgical safety and resection rates, patient prognosis has not seen significant improvement. Surgical resection remains the only potentially curative option for many, underscoring the urgent need for breakthroughs in pancreatic cancer treatment modalities.
Improving the prognosis of pancreatic cancer patients fundamentally depends on a strategy combining early diagnosis, surgery, sensitive chemotherapy and targeted drug regimens. Future research should increasingly focus on understanding tumor biology, and translating findings between clinical and basic research, fostering personalized diagnosis, treatment measures, and program selection [6].
Most treatments for pancreatic cancer are traditional. In early stages, medication and surgery may be viable options, while in advanced stages, palliative care, radiotherapy, chemotherapy, and ablation are used. Gemcitabine (GEM), an antimetabolic chemotherapeutic agent, is the FDA-approved standard for pancreatic cancer treatment. Common radiotherapy techniques include X-knife and precision radiation, through these are generally limited to early-stage cases. Interventional therapies, such as vascular therapy, percutaneous puncture, and endoscopic operation, are also used to delay disease progression temporarily, though they do not provide curative outcomes.
Therapies in treating Given the limited success of these conventional pancreatic cancer, there is an urgent need to explore the potential of nanotechnology and nanomaterials for more effective treatment options.
3. Principles and methods of nanotechnology in the treatment of pancreatic cancer
3.1. Nano drug delivery system
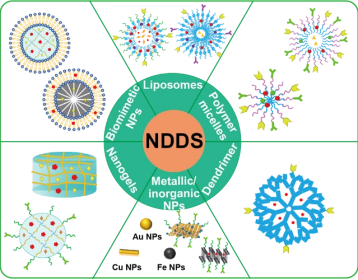

Figure 2. Research progress of nanomedicine delivery system for tumor chemoimmunotherapy combination therapy [7]
Nano drug delivery system (NDDS) leverage nanotechnology to enhance drug solubility, stability, and targeted delivery to tumors, while reducing toxic side effects. Current advancements in nanotechnology focus extensively on cancer therapy, with NDDS using organic and inorganic nanomaterials to serve as anti-cancer carriers drug-carrier interactions. Functional nanomaterials have shown significant breakthroughs in cancer research, particularly when targeting ligands are conjugated to NDDS. These ligands enables selective recognition of tumor cell-specific receptors or transporters, reducing drug exposure to healthy cells and maximizing toxicity to tumor cells, thereby achieving static targeted drug delivery [8].
One of the major obstacles in treating pancreatic cancer is the lack of highly specific targeted drugs, compounded by toxic side effects on non-cancerous tissues. Studies indicate that the poor prognosis of pancreatic cancer is linked to chemotherapy resistance, making NDDS an important avenue for improving therapeutic outcomes.
Research and clinical reports highlight NDDS as a common delivery platform for anti-cancer agents, with paclitaxel and gemcitabine as two of the most frequently used drugs in treating pancreatic cancer. Paclitaxel demonstrates substantial anti-tumor activity but may induce side effects such as hypersensitivity reactions and neurotoxicity. Furthermore, its low water solubility, poor delivery efficiency, and low bioavailability present challenges. Gemcitabine, the standard chemotherapy drug for advanced pancreatic cancer, suffers from a short half-life and limited membrane permeability, rendering it effective for only a subset of patients. NDDS can address these limitations by improving drug stability, bioavailability, and targeted delivery.
Despite promising results, NDDS faces challenges due to the complexity of tumor biology, uncertainties surrounding nano-bio interactions, and stringent requirements for nanoparticle composition, manufacturing, and delivery methods. As a result, only a few nanomedicines have been approved for clinical application. However, with ongoing research, NDDS holds great potential to become a transformative solution in cancer therapy, providing an advanced tool for addressing not only pancreatic cancer but also a wide range of tumors through precise, effective, and safer treatment options.
3.2. Nano knife
The NanoKnife is an innovative tumor ablation technology that utilizes high-voltage electrical pulses to create permanent nanoscale pores in tumor cell membranes, resulting in rapid apoptosis. In China, nearly 100 NanoKnife procedures have been performed, with approximately 90% of these procedures conducted on pancreatic cancer patients. The combination of NanoKnife ablation with chemotherapy has demonstrated significant progress in the treatment of locally advanced pancreatic cancer. Case studies indicate that NanoKnife ablation is highly safe, with mild adverse effects and a low incidence of severe complications. Compared to traditional chemotherapy, NanoKnife ablation combined with systemic chemotherapy has shown efficacy in extending progression-free survival (PFS) and overall survival (OS) while improving pain management in patients. For patients who are ineligible for surgical resection and have not experienced distant metastasis, NanoKnife ablation provides a safe and effective therapeutic option [9].
3.3. Nano probes
Nanoprobes are specialized biological probes that consist of nucleic acid and/or protein molecules assembled on the surface of nanomaterials. These probes come in various forms, each offering unique potential for treating pancreatic cancer. For instance, bTiO2@mSiO2@Gd/R848 nanoprobes exhibit superior photothermal properties and magnetic resonance imaging (MRI) capabilities, which have been investigated for combined photothermal and immunotherapy applications at the cellular level [11]. This technology holds promise for advancing early detection and treatment of pancreatic cancer through enhanced imaging and therapeutic precision.
A targeted nanoprobe based on IGF-1R, designed for precise photothermal therapy guided by optical imaging, has demonstrated significant therapeutic efficacy in both molecular imaging and photothermal therapy for pancreatic cancer, displaying strong active targeting capabilities [12].
In summary, various types of nanoprobes enable precise targeting of pancreatic cancer, offering diverse avenues for addressing the challenges associated with pancreatic cancer treatment in future research.
4. Conclusion
Pancreatic cancer, often termed the “king of cancers” due to its high malignancy and poor prognosis, has long posed substantial challenges to clinicians. Yet, the advent of nanotechnology and nanomaterials offers a new horizon of hope in the battle against this aggressive disease. Nano-based drug delivery systems, NanoKnife ablation, and nanoprobe technologies have demonstrated promising roles across a range of applications, including targeted drug delivery, early detection, diagnostic imaging, and hyperthermia treatments. These advances represent critical steps forward in making treatment approaches for pancreatic cancer more precise and effective.
Despite these advancements, considerable obstacles remain. Current nano-based interventions still face challenges related to drug targeting specificity, bioavailability, and seamless clinical translation, which limit their therapeutic efficacy. Future research must focus on refining these nano-delivery methods to achieve more effective targeting, improve drug bioavailability, and expand their applications in clinical settings. Developing novel nanoscale drugs and integrating nanotechnology with emerging fields, such as immunotherapy and artificial intelligence, may offer more sophisticated and individualized treatment strategies. These combined efforts could lead to more effective and comprehensive solutions for pancreatic cancer, potentially overcoming some of the most persistent challenges in the field.
References
[1]. Liu, Z., Li, Z., Zhang, Y., et al. (2021). Interpretation of the 2020 global cancer statistics report. Electronic Journal of Comprehensive Cancer Therapy, 7(2), 1-13.
[2]. Guo, Y., & Ge, G. (n.d.). EU definition and safety assessment of nanomaterials.
[3]. Huang, Y. (2024). Research progress on the application of nanotechnology in herbicide preparation. Heilongjiang Agricultural Science, 2024(6), 113-118. https://doi.org/10.11942/j.issn1002-2767.2024.06.0113
[4]. Zhao, S., & Zheng, J. (2022). Research progress on etiology and pathogenesis of pancreatic cancer. Chinese Journal of Cancer Prevention and Treatment, 14(3), 253-263.
[5]. 39 Health Network. (2022). Where is pancreatic cancer? Retrieved from https://askar.39.net/a/220831/11660735.html
[6]. Yang, Y. (2015). Hot and difficult points in surgical treatment of pancreatic cancer. Chinese Journal of Digestive Surgery, 14(8), 612-614.
[7]. Mu, W., Chu, Q., Liu, Y., Zhang, N. (2020). A review on nano-based drug delivery system for cancer, chemoimmunotherapy. Micro Letters, 12, 142.
[8]. Zhang, J. (2022). Construction of targeted nano drug delivery systems for enhancing drug delivery and tumor therapy (Doctoral dissertation, Tianjin University).
[9]. Su, D. (2021). The application of nanoknife ablation combined with chemotherapy in the treatment of locally advanced pancreatic cancer (Doctoral dissertation, Zhengzhou University).
[10]. Xu, L. (2022). Preparation of Gd/R848 nano probe and visualization of photothermal immunotherapy for pancreatic cancer cells (Doctoral dissertation, Ningbo University).
[11]. Lu, G. (2019). Experimental study on targeted nanoprobe based on IGF-1R for precise photothermal therapy of pancreatic cancer mediated by optical imaging (Doctoral dissertation, Southern Medical University).
Cite this article
Fu,J. (2024). Advances in nanotechnology for pancreatic cancer therapy: Current status and future prospect. Journal of Food Science, Nutrition and Health,3,23-26.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Journal:Journal of Food Science, Nutrition and Health
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Liu, Z., Li, Z., Zhang, Y., et al. (2021). Interpretation of the 2020 global cancer statistics report. Electronic Journal of Comprehensive Cancer Therapy, 7(2), 1-13.
[2]. Guo, Y., & Ge, G. (n.d.). EU definition and safety assessment of nanomaterials.
[3]. Huang, Y. (2024). Research progress on the application of nanotechnology in herbicide preparation. Heilongjiang Agricultural Science, 2024(6), 113-118. https://doi.org/10.11942/j.issn1002-2767.2024.06.0113
[4]. Zhao, S., & Zheng, J. (2022). Research progress on etiology and pathogenesis of pancreatic cancer. Chinese Journal of Cancer Prevention and Treatment, 14(3), 253-263.
[5]. 39 Health Network. (2022). Where is pancreatic cancer? Retrieved from https://askar.39.net/a/220831/11660735.html
[6]. Yang, Y. (2015). Hot and difficult points in surgical treatment of pancreatic cancer. Chinese Journal of Digestive Surgery, 14(8), 612-614.
[7]. Mu, W., Chu, Q., Liu, Y., Zhang, N. (2020). A review on nano-based drug delivery system for cancer, chemoimmunotherapy. Micro Letters, 12, 142.
[8]. Zhang, J. (2022). Construction of targeted nano drug delivery systems for enhancing drug delivery and tumor therapy (Doctoral dissertation, Tianjin University).
[9]. Su, D. (2021). The application of nanoknife ablation combined with chemotherapy in the treatment of locally advanced pancreatic cancer (Doctoral dissertation, Zhengzhou University).
[10]. Xu, L. (2022). Preparation of Gd/R848 nano probe and visualization of photothermal immunotherapy for pancreatic cancer cells (Doctoral dissertation, Ningbo University).
[11]. Lu, G. (2019). Experimental study on targeted nanoprobe based on IGF-1R for precise photothermal therapy of pancreatic cancer mediated by optical imaging (Doctoral dissertation, Southern Medical University).









