1. Introduction
The rising demand for electronic devices substantially boosts lithium-ion battery (LIBs) utilization [1]. The high demand will result in substantial use of LIB resources and produce a large number of spent lithium-ion batteries (S-LIBs), leading to severe environmental issues [2]. Consequently, determining how to safely recycle used lithium-ion batteries and manage the pollutants from the recycling process has become a crucial scientific concern. Recycling these batteries offers considerable economic value [3]. The intensified extraction of key battery materials from natural ores has led to resource depletion, prompting the recycling of S-LIBs [2]. Advancing the recycling market will further encourage sustainable growth and industrial enhancement in the lithium battery sector. Yet, by 2018, only 8.86% of spent lithium-ion batteries had been recycled [3]. Studies demonstrate that processing 4000 t of S-LIBs recovers over 1100 t of metals and 200 t of electrolyte compounds [4]. Disposing of S-LIBs in landfills could lead to toxic heavy metals seeping into groundwater, resulting in significant environmental contamination. Similarly, burning these S-LIBs as regular solid waste will produce a substantial amount of toxic gas, leading to atmospheric pollution. Addressing S-LIBs safely is essential, and the reagents and procedures employed must also be non-toxic and harmless.
LIBs comprise five primary recyclable components: cathode, anode, electrolyte, current collector, and separator (Fig. 1) [5]. Typically, most LIBs employ standardized elements such as the electrolyte, separator, and casing. Their key variations arise from the diverse lithium metal oxides used in cathode fabrication [6]. The cathode consists of an aluminum metal conductive foil that is coated with active materials embedded electrochemically [7]. Lithium metal oxides primarily consist of LCO (LiCoO2), LMO (LiMnO4), NCM (LiNiMnCoO2), NCA (LiNiAlO2), and LFP (LiFePO4), with Table 1 outlining their structures, uses, and features. Made from copper foil and graphite, the anode uses graphite due to its high coulombic efficiency, extensive applications, cost-effectiveness in manufacturing, and long-lasting cycle life [8]. LIB electrolytes usually consist of a blend of lithium salts such as LiPF6, LiClO4, LiAsF6, Li(SO2CF3)2, LiCF3SO3, and LiBF4, combined with organic solvents like dimethyl carbonate, ethyl carbonate, and diethyl carbonate [8]. To avoid a short circuit caused by direct electrode contact, a separator is necessary. Conventional LIB separators, typically composed of polypropylene or polyethylene, critically influence overall cell performance. These separators function as a physical barrier between electrodes to prevent short circuits while facilitating ion transport [9]. The connection between the collector and the electrode is made with a viscous polymer adhesive, which should exhibit good mechanical strength and chemical properties [10]. The cathode is bonded to aluminum foil using polyvinylidene fluoride (PVDF), whereas polytetrafluoroethylene (PTFE) is employed to attach the anode material to Cu foil [11]. LIBs operate in a reactive and thermal environment, which means that resistive materials like PVDF perform better than alternatives. As a summary, LIBs are rechargeable electrochemical devices comprising electrodes, current collectors, separators, and electrolytes.
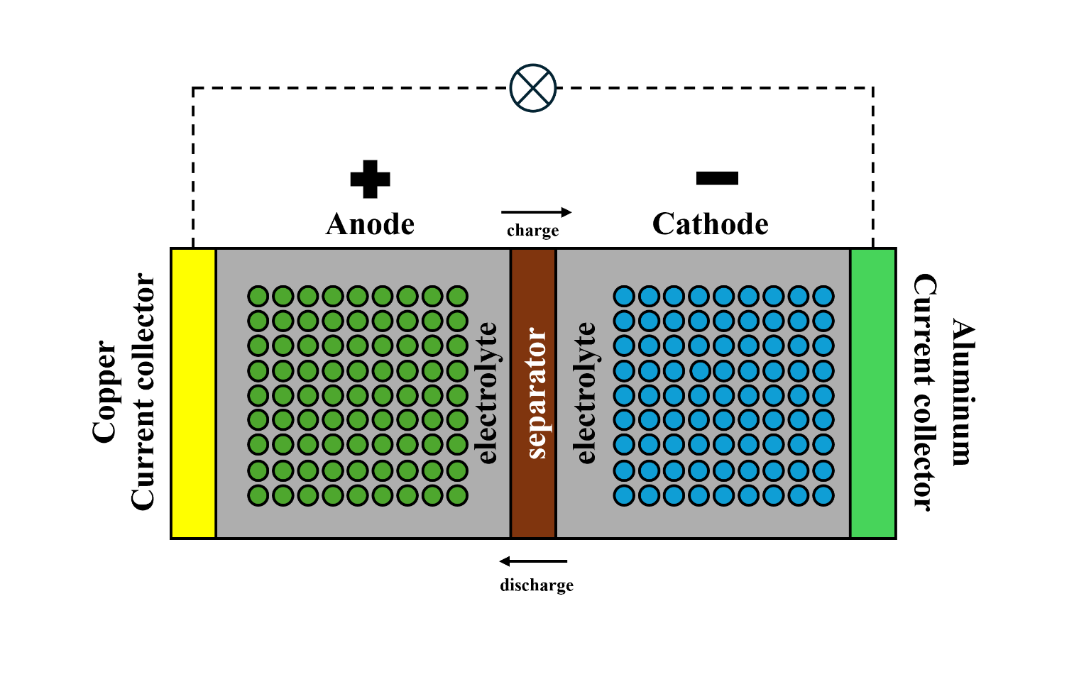
|
Types |
Characteristics |
|
LCO (LiCoO2) |
Poor safety, expensive, medium performance |
|
LMO (LiMnO4) |
Moderate safety, cost-effective, moderate energy density, low lifetime |
|
NCM (LiNiMnCoO2) |
Moderate safety, cost-effective, high energy density, high lifetime |
|
NCA (LiNiAlO2) |
Moderate safety, cost-effective, high energy density |
|
LFP (LiFePO4) |
Good safety, cost-effective, high thermal stability, moderate energy density |
Currently, primary recycling approaches for S-LIBs comprise pyrometallurgical, hydrometallurgical, and direct regeneration methods, as detailed in Fig. 2 [12, 13]. In the industry, the technology that is used most often is a pyrometallurgical process [4, 14]. It consumes a lot of energy, demands significant equipment investment, and leads to severe pollution. To address these issues, numerous companies have created hydrometallurgical processes. Hydrometallurgical methods can extract Li and Al with minimal energy use [2].
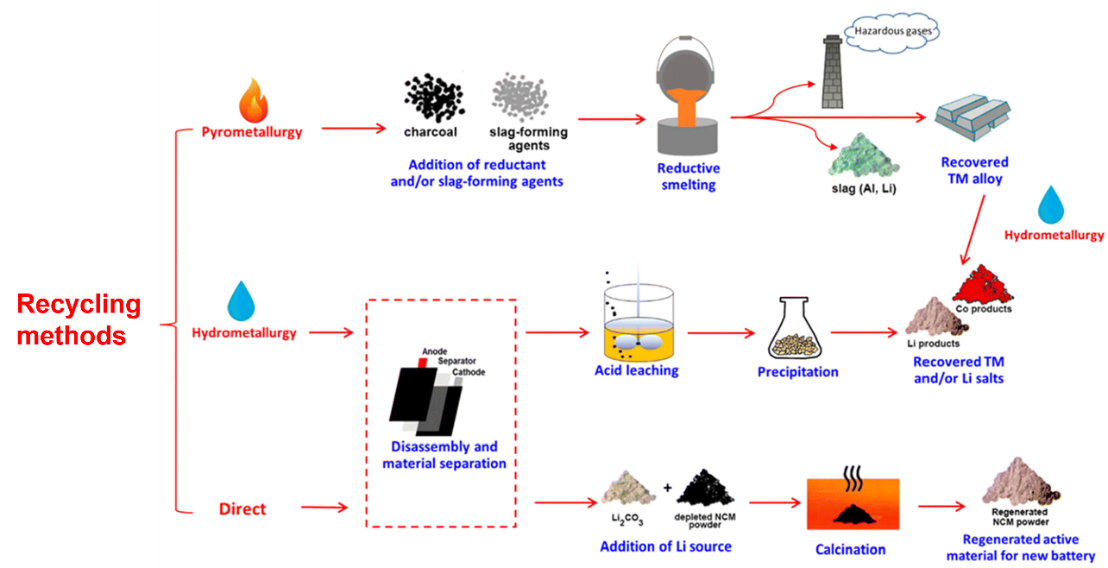
The S-LIBs recycling industry’s healthy growth will be limited by pollutant emissions [12]. Current research efforts primarily focus on recovering valuable materials from batteries, while the environmental impacts of recycling processes remain understudied. Further investigation is needed into the pollution process and its treatment methods [12]. Therefore, this review outlines the waste generated during S-LIB recycling and corresponding treatment methods, guiding for improving their recycling processes and advancing the environmentally sustainable development of this industry.
2. Environmental hazards of improper S-SLIBs recycling
Disposing of S-SLIBs in landfills or through unregulated dumping can result in heavy metals seeping into the soil, which may lead to the leaching of harmful substances into groundwater and could eventually affect crops grown nearby [15]. Soil contamination by heavy metals increases the likelihood of crops absorbing these pollutants, potentially causing various human health issues and decreasing agricultural productivity (Fig. 3) [15].
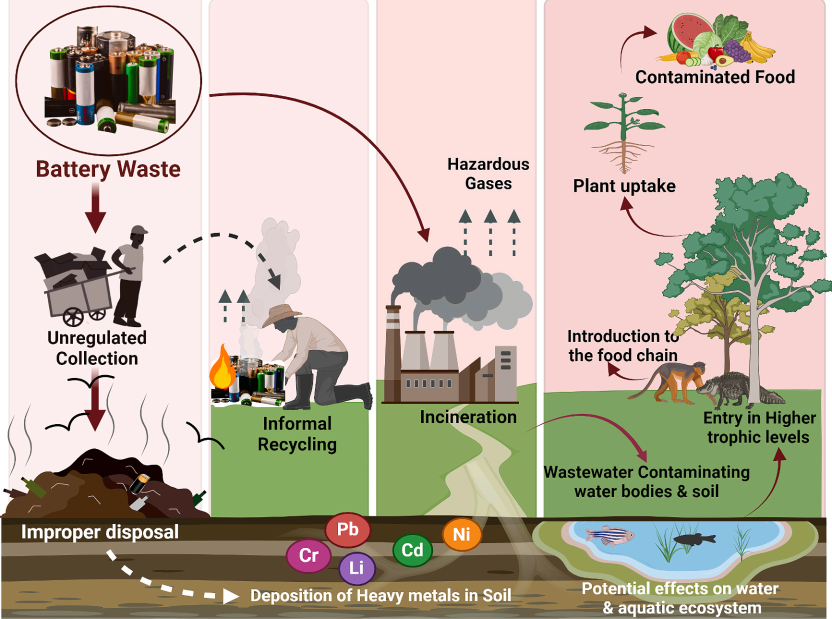
Informal disposal methods of S-LIBs, including dismantling, shredding, or incineration, can contribute to atmospheric pollution through the release of toxic compounds such as dioxins, adversely impacting respiratory health. In regions lacking recycling infrastructure, valuable materials are extracted from S-LIBs using acids, desoldering techniques, and other chemicals that release toxic fumes. Over time, pollution in the air can negatively impact water quality, soil, and vegetation, potentially causing lasting harm to ecosystems and resulting in severe neurological effects on humans, wildlife, and larger animals [15].
The environmental impact of discarded batteries is documented in soils, aquatic ecosystems, and water bodies, where heavy metals induce genetic damage in diverse plant species and soil bacteria [15]. Exposure to these metallic pollutants (e.g., Co, Cu, Li, Ni) causes oxidative stress in organisms critical to biogeochemical cycles, including primary producers and microorganisms. Crustaceans, essential for nutrient cycling, are also vulnerable. These contaminants exhibit significant synergistic toxicity, infiltrate the food chain, and pose risks to public health.
3. Environmental hazards of S-LIBs recycling methods
3.1 Pretreatment of S-LIBs
By removing extraneous parts such as shells and adhesives, the pretreatment process helps in obtaining the target negative electrode material, which simplifies the later recycling process. Table 2 illustrates the possible pollution sources during the pretreatment process [2]. Conventional mechanical treatment processes are not completely effective in removing the principal residual components, comprising the electrolyte, separator, and PVDF binder [2].
|
Factor |
Main components |
Potential environmental risk |
|
Cathode |
Lithium metal oxide |
Heavy metal pollution |
|
Anode |
Graphite |
Dust pollution |
|
Solute salt |
LiPF6 |
Fluoride and phosphorus pollution |
|
Solvent |
Carbonate ester |
Organic pollution |
|
Separator |
Polypropylene, Polyethylene |
Organic and white pollution |
|
Binder |
Polyvinylidene fluoride |
Organic and fluoride pollution |
Before recycling battery materials in an eco-friendly and efficient way, pretreatment is necessary. During preprocessing, mechanical crushing and heating emit substantial quantities of pollutants, such as particulate matter, heavy metals, and VOCs. Moreover, the pretreatment process will produce a significant amount of microplastic pollution because of components like battery separators and plastic packaging [12]. There are three main steps in the pretreatment process: discharge, disassembly, and separation [12, 16]. During the discharge process, using a chemical solution can lead to the production of wastewater that contains heavy metal ions. For instance, after discharging with a solution that contains heavy metal ions, elements like Li, Co, and Ni will still be present in the solution [17]. At the same time, discharge can emit numerous organic substances such as hydrocarbons, CH3OCH3, and CH3OCOOCH3, leading to symptoms like dizziness, headaches, sleep disturbances, irritability, fatigue, general discomfort, and memory impairment [17]. During disassembly, dust might be generated, mainly due to the crushing of battery shells and electrode materials. Simultaneously, the electrolyte might leak, containing organic solvents like dimethyl carbonate, vinyl carbonate, along with lithium salts and other dangerous substances [8]. During the separation process, heating induces the decomposition and hydrolysis of LiPF6, releasing HF, phosphorus-containing compounds, and other gaseous species, thereby contributing to fluoride and phosphorus pollution. Concurrently, organic carbonate solvents decompose readily into volatile organic compounds (VOCs), resulting in organic contamination. Additionally, the toxic gases produced during the thermal breakdown of PVDF must be properly managed to prevent environmental contamination, including inorganic gases, gaseous hydrocarbons, and gases containing fluoride [18].
3.2 Methods for recycling materials
3.2.1 Hydrometallurgy recovery
The hydrometallurgical approach employs solutions like acids and bases to dissolve metals in S-LIBs, followed by separation and recovery through precipitation and extraction [19]. The conventional hydrometallurgical approach for spent lithium-ion battery (S-LIB) recycling entails leaching all valuable metals, thereby dissolving metal ions from solid electrodes into aqueous solution. Subsequently, these metals are separately recovered as purified products from the solution using separation and purification processes. Inorganic acids such as HCl, H2SO4, and HNO3 are commonly employed as leaching agents in complete leaching processes. Protons react with metal oxides from cathode active materials (e.g., LCO, NCM, LFP) as well as with current collectors, including Al and Cu. Nonetheless, the hydrometallurgical process generates substantial quantities of wastewater containing heavy metal ions (such as Ni2+, Co2+, Mn2+, and Cu2+) and Li+. The acids (such as H2SO4, HCl, etc.) employed in the leaching process can also cause wastewater to become more acidic if they are not adequately treated. Moreover, the use of organic extractants can lead to the presence of organic pollutants like tributyl phosphate in the wastewater, which are challenging to degrade and can contaminate the aquatic environment. The enrichment of these elements through the food chain eventually endangers human health, including risks like neurotoxicity and cancer [8].
3.2.2 Pyrometallurgy recovery
Pyrometallurgy involves processing S-LIBs at elevated temperatures to vaporize or create alloys for metal recovery [20]. The process of pyrometallurgical recovery consumes a lot of energy and releases large quantities of harmful gases like CO2, NOx, SO2, and those containing fluorine and sulfur, which significantly pollute the environment. Heavy metals such as Pb, Hg, Cd, Co, and Ni in the batteries will be converted into heavy metal aerosols by volatilizing into the exhaust gas at high temperatures. For instance, metals like Co and Ni, along with their compounds, might be released into the air as gases during the high-temperature smelting of S-LIBs. Metal vapor also condenses into tiny particles, such as cobalt oxide and nickel oxide, which are hard to completely capture with dust removal systems, thus becoming a source of air pollution [20-23].
3.2.3 Direct regeneration
Through physicochemical processes, direct regeneration technology reintroduces lithium into depleted cathode materials to renew their electrochemical properties. Conventional metallurgical recycling of spent cathode materials typically requires significant quantities of acids, bases, or other chemical reagents to generate high-purity raw materials, including metal sulfates and lithium carbonate [24]. Simultaneously, these processes may generate residue and wastewater containing heavy metals, resulting in secondary pollution that fails to meet green chemistry standards [25].
4. Pollution control in the recycling of S-LIBs
Effective LIB recycling necessitates a dual emphasis on valuable metal recovery and pollution control. Particular attention should be directed to hazardous components such as spent graphite anodes and leaching solution contaminants, including fluoride and phosphorus ions.
4.1 Electrolyte pollution emission control
The electrolyte used in LIBs is characterized by volatility, flammability, explosiveness, corrosiveness, and high toxicity. LiPF6 is the primary component of the electrolyte, and it can interact with water or acid to produce harmful gases like HF or other toxic materials, leading to fluorine contamination [18]. Electrolyte solvents are typically combined with other solvents that are flammable and explosive, and their leakage can cause organic pollution. The reaction between fluoride and NaOH creates stable NaF, suggesting that NaOH solution is a practical approach to mitigate fluorine pollution.
4.2 Exhaust gas pollution distribution and pollution control measures
The recycling process emits exhaust gases primarily consisting of roasting flue gas, leaching acid mist, and VOCs. Below is an outline of the main pollution sources and their respective treatment methods:
Roasting flue gas: during the roasting of dismantled S-LIB materials, larger uncrushed components undergo thermal treatment. Concurrently, residual organic matter and electrolytes decompose and combust. The flue gas treatment system typically comprises: secondary combustion, a cyclone dust collector, a waste heat boiler, an emergency cooling tower, a bag filter, an alkaline spray tower, and a demister.
Leaching acid mist: the acid leaching reaction is carried out at a temperature of 85℃, generating leaching acid mist. The leaching tank adopts a suction hood to collect the leaching exhaust gas, and the leaching fog acid exhaust gas is treated by two-stage absorption. The first section is absorbed by a dilute alkaline water spraying device, based on the principle of neutralization reaction to cool and neutralize the sulfuric acid in the exhaust gas; the second section is absorbed by a water spraying device to purify the exhaust gas.
VOCs: volatile organic compounds (VOCs) pose significant hazards to human health and the environment due to their toxic, mutagenic, and carcinogenic properties. To date, a variety of VOC treatment technologies have been developed, including incineration, condensation, biological degradation, absorption, adsorption, and catalytic oxidation, among others. Among these methods, adsorption stands out as an efficient and economical approach, enabling the recovery and reuse of both the adsorbent and adsorbate [26]. Catalytic oxidation is also acknowledged as one of the most effective methods for removing VOCs due to its low energy use, high efficiency, and minimal secondary pollution [27].
4.3 Distribution of wastewater pollution sources and pollution control measures
Production wastewater from the process primarily consists of: wastewater from aluminum removal in the pretreatment section, extraction and separation wastewater, and wastewater from roasting flue gas treatment. The production wastewater is first pretreated by neutralization and precipitation, and then treated by grease traps, oxidation, biochemical (hydrolysis and acidification, aeration biofilter), coagulation and air flotation, activated carbon filtration process, and discharged into the biochemical section of the production wastewater treatment system.
4.4 Solid waste pollution control measures
The majority of solid waste produced during recycling consists of solid slag, along with some sludge and dismantled lithium battery shells, most of which are hazardous and need special handling. The main solid wastes are: leaching slag, rare earth re-salting precipitation slag, ferromanganese slag, grease trap slag, and dismantled shells of S-LIBs.
Waste residues are first processed for secondary recycling, and the valuable metals in them are further extracted by physical or chemical methods to improve the utilization of resources. For instance, heavy metals in the waste residue are dissolved via acid leaching, followed by separation and purification through extraction and precipitation. For the waste residue that still contains heavy metals after treatment, it can be cured by mixing it with cement, lime and other curing agents, so that the heavy metals are fixed in the curing body to reduce their mobility needs to be safely landfilled, selecting landfills that meet environmental standards and taking measures such as seepage and leakage prevention to prevent the leakage of heavy metals.
5. Analysis of economic and environmental benefits
5.1 Analysis of economic benefits
The recycling of S-LIBs not only mitigates resource constraints but also offers economic advantages through the recovery of valuable materials. By establishing an economic assessment model to analyze the revenue of the recycling of S-LIBs and expressing it in the form of a numerical model, it is easy to propose the management and quantitative analysis of the economy. The profitability model for used lithium batteries is derived from cost analysis and represented by the following formula:
where P is the profit of recycling S-LIBs; PT is the total revenue of recycling S-LIBs; CU is the cost of recycling S-LIBs.
The cost of recycling used lithium batteries includes: raw material cost, auxiliary material cost, energy cost, equipment cost, environmental treatment cost, labor cost, etc. The expression is shown below:
where CM is the cost of raw materials, CN is the cost of auxiliary materials, CP is the cost of energy, CS is the cost of equipment, CE is the cost of environmental treatment, and CH is the cost of labor.
5.2 Analysis of environmental benefits
The typical service life of electronic products is 2-3 years; however, the proliferation of smart devices is accelerating replacement cycles. Consequently, a significant volume of lithium-ion batteries is being decommissioned within shorter timeframes. Unlike conventional household waste, S-LIBs exhibit a complex composition comprising significant concentrations of heavy metals (e.g., Li, Co, Mn, etc.), fluoride, organic compounds, plastics, and electrolytes, posing substantial environmental risks if improperly managed [15]. Traditional incineration and landfill disposal of S-LIBs pose significant environmental hazards.
S-LIBs are of various types and complex compositions. The incineration process may produce a large number of toxic substances. The electrolyte primarily comprises organic solvents, lithium salt electrolytes (e.g., LiPF6), and conductive agents. Upon exposure to moisture, it can generate fluorine-containing contaminants. During thermal treatment processes such as pyrolysis or incineration, the electrolyte decomposes, releasing hazardous gases including HF, LiF, and organic pollutants from the incomplete combustion of solvents [18]. Additionally, the combustion of polymeric separators yields hydrogen halides. Uncontrolled release of these atmospheric pollutants results in significant air pollution.
Due to inadequate public awareness and management systems for S-LIBs in China, most are landfilled indiscriminately alongside other S-LIBs. This practice leads to environmental contamination: metal ions leached from disintegrating battery casings pollute soil and water; acidic electrolytes alter soil pH and, upon volatilization, release fluoride compounds detrimental to plant growth. Furthermore, heavy metal ions absorbed by plants can undergo bioaccumulation within the food chain, posing significant health risks upon human ingestion. These toxic substances may also migrate into the atmosphere, surface water, and groundwater, causing widespread ecological pollution [15].
6. Conclusion
The rapid proliferation of LIBs in consumer electronics, electric vehicles, and renewable energy storage has resulted in a substantial increase in S-LIBs, posing significant environmental and health hazards if mismanaged. The review highlights the critical need for integrating pollution control into S-LIB recycling processes to achieve sustainable resource recovery. Key findings include: informal recycling (e.g., landfilling or incineration) releases toxic heavy metals (Co, Ni, Li), emits dioxins, volatile organic compounds (VOCs), and particulate matter, contaminating soil, water, and air and endangering human health and ecosystems. Pretreatment (discharge, disassembly, separation) generates wastewater with heavy metals, VOCs, and microplastics. Hydrometallurgy produces acidic wastewater laden with heavy metals (Ni2+, Co2+) and persistent organic pollutants (e.g., tributyl phosphate). Pyrometallurgy emits greenhouse gases (CO₂, SO₂), toxic fumes (HF, NOx), and aerosolized heavy metals. Direct regeneration risks secondary pollution via chemical residues. At the same time, the article gives reasonable control and management methods for roasting flue gas, acid mist, VOCs, wastewater, solid waste and other wastes generated in the recycling process. In summary, sustainable S-LIB recycling requires developing cost-effective, non-toxic leaching agents (e.g., organic acids) and closed-loop processes to minimize waste and a holistic approach that prioritizes pollution control at every stage, balancing economic viability with environmental stewardship to support the circular economy of battery materials.
References
[1]. Zheng Xiaohong, Zhu Zewen, Lin Xiao, et al. A Mini-Review on Metal Recycling from Spent Lithium Ion Batteries [J]. Engineering, 2018, 4(3): 361-370.
[2]. Li Yukun, Lv Weiguang, Huang Hanlin, et al. Recycling of spent lithium-ion batteries in view of green chemistry [J]. Green Chemistry, 2021, 23(17): 6139-6171.
[3]. Mao Jianfeng, Ye Chao, Zhang Shilin, et al. Toward practical lithium-ion battery recycling: adding value, tackling circularity and recycling-oriented design [J]. Energy & Environmental Science, 2022, 15(7): 2732-2752.
[4]. Gu Fu, Guo Jianfeng, Yao Xing, et al. An investigation of the current status of recycling spent lithium-ion batteries from consumer electronics in China [J]. Journal of Cleaner Production, 2017, 161: 765-80.
[5]. Jacoby M. It’s time to recycle lithium-ion batteries [J]. C&EN Global Enterprise, 2019, 97(28): 29-32.
[6]. Jin Shan, Mu Deying, Lu Ziang, et al. A comprehensive review on the recycling of spent lithium-ion batteries: Urgent status and technology advances [J]. Journal of Cleaner Production, 2022, 340: 130535.
[7]. Yao Yonglin, Zhu Meiying, Zhao Zhou, et al. Hydrometallurgical Processes for Recycling Spent Lithium-Ion Batteries: A Critical Review [J]. ACS Sustainable Chemistry & Engineering, 2018, 6(11): 13611-13627.
[8]. Zhang Xun, Zhu Maiyong. Recycling spent lithium-ion battery cathode: an overview [J]. Green Chemistry, 2024, 26(13): 7656-7717.
[9]. Li Hang, Wang Li, Song Youzhi, et al. Significance of Current Collectors for High Performance Conventional Lithium-Ion Batteries: A Review [J]. Advanced Functional Materials, 2023, 33(49): 2305515.
[10]. Lv Wei, Wang Zhonghang, Cao Hongbin, et al. A Critical Review and Analysis on the Recycling of Spent Lithium-Ion Batteries [J]. ACS Sustainable Chemistry & Engineering, 2018, 6(2): 1504-1521.
[11]. Yun Liu, Linh Duy, Shui Li, et al. Metallurgical and mechanical methods for recycling of lithium-ion battery pack for electric vehicles [J]. Resources, Conservation and Recycling, 2018, 136: 198-208.
[12]. Wang Rui, Bulati Akemareli, Zhan Lu, et al. Complicated pollution characteristics (particulate matter, heavy metals, microplastics, VOCs) of spent lithium-ion battery recycling at an industrial level [J]. Science of The Total Environment, 2025, 962: 178406.
[13]. Hu Qiushi, Xu Linghong. An overview on Lithium-ion batteries recycling processes [J]. Journal of Physics: Conference Series, 2021, 1885(3): 032031.
[14]. Yuan Lixia, Wang Zhaohui, Zhang Wuxing, et al. Development and challenges of LiFePO4cathode material for lithium-ion batteries [J]. Energy & Environmental Science, 2011, 4(2): 269-284.
[15]. Singh Shatrupa, Santhiya Deenan, Sharma Jai Gopal. An overview of bioprocess based recovery and recycling techniques for battery waste management: Transitioning from environmental hazard to sustainability [J]. Minerals Engineering, 2025, 233: 109598.
[16]. Du Kaidi, Ang Edison Huixiang, Wu Xinglong, et al. Progresses in Sustainable Recycling Technology of Spent Lithium-Ion Batteries [J]. Energy & Environmental Materials, 2022, 5(4): 1012-1036.
[17]. Yao Lin Peng, Zeng Qi, Qi Ting, et al. An environmentally friendly discharge technology to pretreat spent lithium-ion batteries [J]. Journal of Cleaner Production, 2020, 245: 118820.
[18]. Chen Yongming, Liu Nannan, Jie Yafei, et al. Toxicity Identification and Evolution Mechanism of Thermolysis-Driven Gas Emissions from Cathodes of Spent Lithium-Ion Batteries [J]. ACS Sustainable Chemistry & Engineering, 2019, 7(22): 18228-18235.
[19]. Xiao Jiefeng, Li Jia, Xu Zhenming. Novel Approach for in Situ Recovery of Lithium Carbonate from Spent Lithium Ion Batteries Using Vacuum Metallurgy [J]. Environmental Science & Technology, 2017, 51(20): 11960-11966.
[20]. Zhao Yunze, Liu Bingguo, Zhang Libo, et al. Microwave Pyrolysis of Macadamia Shells for Efficiently Recycling Lithium from Spent Lithium-ion Batteries [J]. Journal of Hazardous Materials, 2020, 396: 122740.
[21]. Cui Ke, Zhao Mingchen, Li Yiran, et al. Recycling of spent lithium iron phosphate batteries: Research progress based on environmental protection and sustainable development technology [J]. Separation and Purification Technology, 2025, 354: 128982.
[22]. Mrozik Wojciech, Rajaeifar Mohammad Ali, Heidrich Oliver, et al. Environmental impacts, pollution sources and pathways of spent lithium-ion batteries [J]. Energy & Environmental Science, 2021, 14(12): 6099-6121.
[23]. Zhou Ming, Li Bang, Li Jia, et al. Pyrometallurgical Technology in the Recycling of a Spent Lithium Ion Battery: Evolution and the Challenge [J]. ACS ES&T Engineering, 2021, 1(10): 1369-1382.
[24]. Natarajan Subramanian, Aravindan Vanchiappan. Burgeoning Prospects of Spent Lithium-Ion Batteries in Multifarious Applications [J]. Advanced Energy Materials, 2018, 8(33): 1802303.
[25]. Meng Xiangqi, Hao Jie, Cao Hongbin, et al. Recycling of LiNi1/3Co1/3Mn1/3O2cathode materials from spent lithium-ion batteries using mechanochemical activation and solid-state sintering [J]. Waste Management, 2019, 84: 54-63.
[26]. Zhang Xueyang, Gao Bin, Creamer Anne Elise, et al. Adsorption of VOCs onto engineered carbon materials: A review [J]. Journal of Hazardous Materials, 2017, 338: 102-123.
[27]. Zhang Honghong, Wang Zhiwei, Wei Lu, et al. Recent progress on VOC pollution control via the catalytic method [J]. Chinese Journal of Catalysis, 2024, 61: 71-96.
Cite this article
Jin,L. (2025). Research on Pollution Control in the Recycling of Spent Lithium-ion Batteries. Applied and Computational Engineering,181,48-57.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of CONF-FMCE 2025 Symposium: Semantic Communication for Media Compression and Transmission
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Zheng Xiaohong, Zhu Zewen, Lin Xiao, et al. A Mini-Review on Metal Recycling from Spent Lithium Ion Batteries [J]. Engineering, 2018, 4(3): 361-370.
[2]. Li Yukun, Lv Weiguang, Huang Hanlin, et al. Recycling of spent lithium-ion batteries in view of green chemistry [J]. Green Chemistry, 2021, 23(17): 6139-6171.
[3]. Mao Jianfeng, Ye Chao, Zhang Shilin, et al. Toward practical lithium-ion battery recycling: adding value, tackling circularity and recycling-oriented design [J]. Energy & Environmental Science, 2022, 15(7): 2732-2752.
[4]. Gu Fu, Guo Jianfeng, Yao Xing, et al. An investigation of the current status of recycling spent lithium-ion batteries from consumer electronics in China [J]. Journal of Cleaner Production, 2017, 161: 765-80.
[5]. Jacoby M. It’s time to recycle lithium-ion batteries [J]. C&EN Global Enterprise, 2019, 97(28): 29-32.
[6]. Jin Shan, Mu Deying, Lu Ziang, et al. A comprehensive review on the recycling of spent lithium-ion batteries: Urgent status and technology advances [J]. Journal of Cleaner Production, 2022, 340: 130535.
[7]. Yao Yonglin, Zhu Meiying, Zhao Zhou, et al. Hydrometallurgical Processes for Recycling Spent Lithium-Ion Batteries: A Critical Review [J]. ACS Sustainable Chemistry & Engineering, 2018, 6(11): 13611-13627.
[8]. Zhang Xun, Zhu Maiyong. Recycling spent lithium-ion battery cathode: an overview [J]. Green Chemistry, 2024, 26(13): 7656-7717.
[9]. Li Hang, Wang Li, Song Youzhi, et al. Significance of Current Collectors for High Performance Conventional Lithium-Ion Batteries: A Review [J]. Advanced Functional Materials, 2023, 33(49): 2305515.
[10]. Lv Wei, Wang Zhonghang, Cao Hongbin, et al. A Critical Review and Analysis on the Recycling of Spent Lithium-Ion Batteries [J]. ACS Sustainable Chemistry & Engineering, 2018, 6(2): 1504-1521.
[11]. Yun Liu, Linh Duy, Shui Li, et al. Metallurgical and mechanical methods for recycling of lithium-ion battery pack for electric vehicles [J]. Resources, Conservation and Recycling, 2018, 136: 198-208.
[12]. Wang Rui, Bulati Akemareli, Zhan Lu, et al. Complicated pollution characteristics (particulate matter, heavy metals, microplastics, VOCs) of spent lithium-ion battery recycling at an industrial level [J]. Science of The Total Environment, 2025, 962: 178406.
[13]. Hu Qiushi, Xu Linghong. An overview on Lithium-ion batteries recycling processes [J]. Journal of Physics: Conference Series, 2021, 1885(3): 032031.
[14]. Yuan Lixia, Wang Zhaohui, Zhang Wuxing, et al. Development and challenges of LiFePO4cathode material for lithium-ion batteries [J]. Energy & Environmental Science, 2011, 4(2): 269-284.
[15]. Singh Shatrupa, Santhiya Deenan, Sharma Jai Gopal. An overview of bioprocess based recovery and recycling techniques for battery waste management: Transitioning from environmental hazard to sustainability [J]. Minerals Engineering, 2025, 233: 109598.
[16]. Du Kaidi, Ang Edison Huixiang, Wu Xinglong, et al. Progresses in Sustainable Recycling Technology of Spent Lithium-Ion Batteries [J]. Energy & Environmental Materials, 2022, 5(4): 1012-1036.
[17]. Yao Lin Peng, Zeng Qi, Qi Ting, et al. An environmentally friendly discharge technology to pretreat spent lithium-ion batteries [J]. Journal of Cleaner Production, 2020, 245: 118820.
[18]. Chen Yongming, Liu Nannan, Jie Yafei, et al. Toxicity Identification and Evolution Mechanism of Thermolysis-Driven Gas Emissions from Cathodes of Spent Lithium-Ion Batteries [J]. ACS Sustainable Chemistry & Engineering, 2019, 7(22): 18228-18235.
[19]. Xiao Jiefeng, Li Jia, Xu Zhenming. Novel Approach for in Situ Recovery of Lithium Carbonate from Spent Lithium Ion Batteries Using Vacuum Metallurgy [J]. Environmental Science & Technology, 2017, 51(20): 11960-11966.
[20]. Zhao Yunze, Liu Bingguo, Zhang Libo, et al. Microwave Pyrolysis of Macadamia Shells for Efficiently Recycling Lithium from Spent Lithium-ion Batteries [J]. Journal of Hazardous Materials, 2020, 396: 122740.
[21]. Cui Ke, Zhao Mingchen, Li Yiran, et al. Recycling of spent lithium iron phosphate batteries: Research progress based on environmental protection and sustainable development technology [J]. Separation and Purification Technology, 2025, 354: 128982.
[22]. Mrozik Wojciech, Rajaeifar Mohammad Ali, Heidrich Oliver, et al. Environmental impacts, pollution sources and pathways of spent lithium-ion batteries [J]. Energy & Environmental Science, 2021, 14(12): 6099-6121.
[23]. Zhou Ming, Li Bang, Li Jia, et al. Pyrometallurgical Technology in the Recycling of a Spent Lithium Ion Battery: Evolution and the Challenge [J]. ACS ES&T Engineering, 2021, 1(10): 1369-1382.
[24]. Natarajan Subramanian, Aravindan Vanchiappan. Burgeoning Prospects of Spent Lithium-Ion Batteries in Multifarious Applications [J]. Advanced Energy Materials, 2018, 8(33): 1802303.
[25]. Meng Xiangqi, Hao Jie, Cao Hongbin, et al. Recycling of LiNi1/3Co1/3Mn1/3O2cathode materials from spent lithium-ion batteries using mechanochemical activation and solid-state sintering [J]. Waste Management, 2019, 84: 54-63.
[26]. Zhang Xueyang, Gao Bin, Creamer Anne Elise, et al. Adsorption of VOCs onto engineered carbon materials: A review [J]. Journal of Hazardous Materials, 2017, 338: 102-123.
[27]. Zhang Honghong, Wang Zhiwei, Wei Lu, et al. Recent progress on VOC pollution control via the catalytic method [J]. Chinese Journal of Catalysis, 2024, 61: 71-96.









