1. Introduction
Hydroformylation of olefins, particularly propene, is the main homogeneous synergist process in the current industry. However, homogeneous catalytic systems have inherent disadvantages such as intermittent operation and difficulty in separating the catalyst from the reaction mixture. Therefore, it is necessary to reduce energy consumption, minimize the use of fossil fuels, optimize the technological schemes of chemical and energy production taking into account the decreasing availability of raw materials, and significantly reduce the amount of jointly generated waste. Catalysts are universal in all chemical processes and therefore can affect each of these points. Therefore, the development of new catalysts, the improvement of existing ones, and their large-scale use are very important topics of modern research.
2. Hydroformylation
Hydroformylation of olefins, particularly propene, is the main homogeneous synergist process in the current industry. Obtaining pure products from this reaction can be challenging because it often results in a mixture of different isomers and products due to the complex nature of the reaction mechanism and the various parameters that can affect it, such as temperature, pressure, and catalyst type. Additionally, side reactions can occur, leading to further product complexity, and making it difficult to isolate and purify specific products.
3. Introduction of aldehyde
3.1. Classification of aldehyde in industry
In industry, aldehydes are typically classified based on their chemical structure, functional group, and properties. Here are some common classifications of aldehydes in industry:
First, Aliphatic Aldehydes: These are aldehydes that have an aliphatic carbon chain. Examples include formaldehyde (methanal), acetaldehyde (ethanal), and propionaldehyde (propanal). These aldehydes are widely used as solvents, disinfectants, and preservatives.
Second, Aromatic Aldehydes: These are aldehydes that contain an aromatic ring in their structure. One of the most well-known aromatic aldehydes is benzaldehyde, which is used as a flavoring agent, fragrance, and in the production of dyes and pharmaceuticals.
Third, Alpha, Beta-Unsaturated Aldehydes: These aldehydes have a double bond adjacent to the carbonyl group. Examples include crotonaldehyde and acrolein. They are used in the production of resins, and polymers, and as intermediates in the synthesis of various compounds.
Fourth, Heterocyclic Aldehydes: These are aldehydes that contain a heterocyclic ring, such as furfural and pyridine carboxaldehyde. Furfural is used as a solvent and in the production of resins, while pyridine carboxaldehyde is used in the synthesis of pharmaceuticals and agrochemicals. Fifth, Long-Chain Aldehydes: These aldehydes have a long carbon chain, typically more than 10 carbon atoms. Examples include decanal, dodecanal, and octadecanal. They are used as flavoring agents, fragrances, and in the production of surfactants.
It is important to note that the classification of aldehydes may vary depending on the specific.
3.2. Industry and application of aldehyde
These are a portion of the purposes for aldehydes. To make solvents, coatings, plasticizers, alcohols, tars, cleanser alcohols, and as intermediates for the combination of drugs and agrochemicals, aldehydes or oxo compounds were delivered. First, production of resins: Aldehydes such as formaldehyde and acetaldehyde are used to produce resins that have many industrial applications, including adhesives, coatings, and laminates. Second, aldehydes such as glutaraldehyde are used as disinfectants or preservatives in the food and beverage industry in food and beverage industry. Third, Glutaraldehyde is also used as a sterilizing agent for medical equipment, especially in hospitals and clinics in the medical industry. Fourth, Formaldehyde is used as a fumigant and preservative in the agriculture industry in agriculture. Fifth, aldehydes like butyraldehyde are used as textile finishing agents to improve the fabric's durability and wrinkle resistance in the eextile industry.
3.3. Current development of aldehyde production in China.
With a restricted beginning yearly creation limit of around 3 kilotons, it initially began in 1956. The creation of formaldehyde and products containing it extended during the 1990s following thirty years of humble extension, outperforming the US as the world's top maker in 2004. China's genuine creation of formaldehyde has generally paired its assembling limit and crested in 2007 at a bewildering 12,000 kilotons.
4. Production of aldehyde
4.1. Metal
Metal is an urgent part of an impetus. The accompanying homogeneous edifices are common impetuses for the hydroformylation of olefins. [HM(CO)xLy], homogeneous edifices, where L is CO or a natural ligand. Coming up next are the regularly perceived action series of unaltered metals: Rh ≫ Co > Ir, Ru > operating system > Pt > Pd ≫ Fe > Ni.
So yet, only rhodium and cobalt have seen modern use. One of the essential benefits of cobalt impetuses is their capacity to endure poisons contained in unrefined substances. The Shell Higher Olefin Cycle (SHOP) and enormous scope Fischer-Tropsch combination, specifically, give incredible change combinations of long-chain inside alkenes and extended alkenes. In view of the extreme hydrogenation action of cobalt impetuses, the subsequent aldehydes are immediately decreased to the comparing alcohols; the basic disservice of co-frameworks is the cruel response conditions, which require significant speculation uses. Subsequently, in spite of late investigations [1], a rebound of imaginative cobalt-based oxo processes isn't possible.
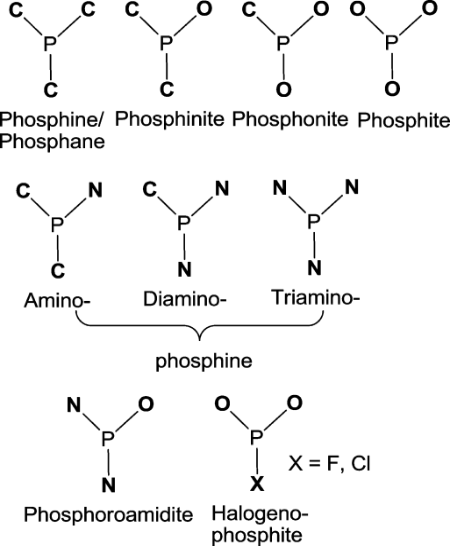
4.2. Ligand
Likewise, ligands are basic to the proficiency of the impetus. Just trivalent phosphorus compounds are utilized as helper ligands in modern settings, paying little mind to metal (Co, Rh): Licenses have been petitioned for the trivalent mixtures As, Sb, and Bi, however, no other potential ligand intensifies in view of components in the fifth section of the occasional table have been utilized. In light of their hydroformylation movement, the relating Rh impetuses are assembled in the accompanying order: Ph3P > >Ph3N > Ph3As, Ph3Sb > Ph3Bi.
|
Figure 1. Classification of trivalent P-ligands based on the nature of the α-atom next to the phosphorus [1]. |
Amines are less chemoselective than phosphines, less dynamic as ligands, and produce the two alkanes and alkanols. Nonetheless, the S ligand is probably not going to stay composed in the dynamic impetus. In specific cases, connecting thiolate ligands have been utilized in binuclear Rh edifices with the expectation of a helpful impact between the two metal habitats. In phosphine ligands, otherwise called phosphanes, a focal phosphorus molecule is regularly encircled by three carbon iotas. The special cases incorporate unsaturated p-heterocycles and essential or auxiliary phosphines[1].
4.3. Catalytic cycle
The reactant cycle incorporates consecutive coordination of alkenes, relocation of hydrides to shape
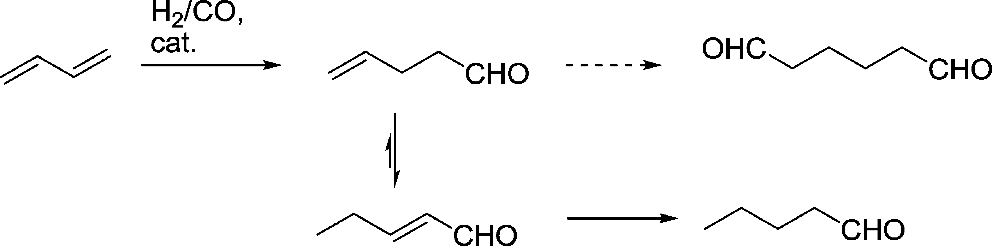
Figure 2. Mechanism of the cobalt catalyzed hydroformylation of alkenes [2].
Straight alkyls (counting isomeric extended alkyls), coordination of CO and presentation of portable CO, oxidative expansion of H2, and reductive end of aldehydes are a few instances of such reality shocks [2]. Make the stride in the upper right corner of the delineation as the most important phase in the main cycle. The following stages are ligand affiliation, hydride inclusion, ligand affiliation, oxidative expansion, and reductive disposal.
5. Application of specific cases
5.1. Specific case 1
In view of huge worries with the breakdown of Rh impetuses during the division of high-bubbling items, co-impetuses are utilized in most business offices for the development of long-chain aldehydes (>C10). These techniques depend on the utilization of impetuses that poor person has been changed at extreme circumstances (30 MPa, 200°C). By changing the co-impetus with phosphine, which upgrades the selectivity of the straight alcohols, the strain might be brought down (by about 10 MPa). Higher olefin beginning materials (up to C20) might be created utilizing Fischer-Tropsch natural substances (accessible from Sasol) or the SHOP cycle. The eventual outcome is broadly used to make surfactant alcohols.
For instance, when 1-dodecane was hydroformylation with a co-impetus at 8.5 MPa (CO/H2=1/2), 120°C, and a Co/P proportion of 1/2, roughly 55% of the isomeric C13 liquor was generated.[3] In additional muddled frameworks, changed Rh impetuses can be utilized in two-stage frameworks with surfactant compounds [1].
5.2. Specific case 2
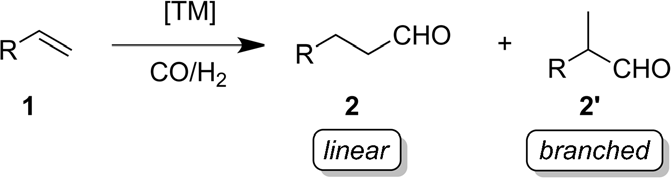
Figure 3. Transition-metal-catalyzed hydroformylation. TM=transition metal [4].
At the point when progress metals are available, hydroformylation, otherwise called oxosynthesis, is the carbonylation of alkenes using carbon monoxide and hydrogen. This cycle is proficient and valuable for shaping C bonds and delivering more aldehydes, and it is utilized in a great many modern applications. Alongside synergist movement and chemoselectivity, regioselectivity is a significant part of hydroformylation responses. For instance, butyraldehyde is utilized to make phthalate esters [5]. The chance of stereoisomer creation, then again, makes the combination of 2' spread aldehydes engaging for the assembling of drugs and fine synthetics. In 1938, Otto Rohlen, a German modern scientist, found hydroformylation responses while concentrating on the side effects of the cobalt-catalyzed Fischer-Tropsch reaction [6].
H2. The revelation of the strain subordinate change of ethene to propanal and diethyl ketone within the sight of CO and H2 was a huge leap forward in manufactured science. The synthetic that led to hydroformylation science was a significant leap forward in homogeneous catalysis. The underlying age of cobalt-catalyzed hydroformylation processes was performed at very high temperatures (150-1808 °C) and pressures (200-350 bar), which was a severe shock. It is still being used today [4].
6. Outlook-- Challenge & Solution
6.1. Challenge
Obtaining pure products from this reaction can be challenging because it often results in a mixture of different isomers or products due to the complex nature of the reaction mechanism and the various parameters that can affect it, such as temperature, pressure, and catalyst type. Additionally, side reactions can occur, leading to further product complexity, and making it difficult to isolate and purify specific products. Despite the enormous advancements achieved since the discovery of hydroformylation in 1938, this reaction is still the focus of various investigations targeted at enhancing chemoselectivity for aldehydes and regioselectivity for linear or branched aldehydes in order to decrease the generation of by-products. These research findings have been quite encouraging. The development of gentler and more ecologically friendly reaction conditions, economical product separation from active catalysts (including catalyst recycling), and practical continuous batch recycling are other areas on which industry and academics are concentrating. Many homogeneous hydroformylation catalyst systems, however, have not been put on the market since it is difficult to separate the catalyst from the reaction products. The reliability of the technology and the total cost of the process both heavily rely on this limiting factor. Unfortunately, during ordinary distillation separation procedures, rhodium hydroformylation catalysts—which generally function at mild settings of 80–100 °C and 10–25 bar—decompose. The costly catalysts are lost when processes like chromatography are utilized. Reduced catalytic activity and metal contamination of downstream processes are also negative effects of this metal loss, both of which are desired. It's important to find alternatives to methods like chromatography [7].
6.2. Solution
First, extraction is better than distillation when separating Cobalt in hydroformylation because Cobalt complexes are often heavy and unstable, making them difficult to distill without decomposition. Additionally, the metal-to-ligand ratios can be difficult to maintain during distillation, leading to impurities in the final product. Extraction techniques, on the other hand, can selectively remove the Cobalt complex from the reaction mixture using a solvent that is immiscible with the reaction mixture. This enables the Cobalt complex to be separated and purified efficiently. Second, the catalyst is heterogenized by immobilizing it on a stable substrate, such as polymers, mesoporous materials, metal oxides, and various forms of carbon. The third alternative is to use biphasic homogeneous catalytic systems with organic immiscible phases such as supercritical fluids, ionic liquids, fluorous solvents, and aqueous solvents. Fourth, hybrid systems employ both biphasic and heterogeneous approaches. Recently, the use of solid-supported catalysts, ionic liquids, supercritical fluids, fluorous/organic systems, and organic systems has been thoroughly investigated.
7. Conclusion
From the information given, we learned about the importance of hydroformylation for the organic synthesis of specialty chemicals, fragrances, and pharmaceuticals. The application of these chemical products is also crucial to our daily life. The different metals and ligands affect the production of the reactants, and at the same time, after looking at the catalytic cycle and two notable examples, we can also understand some of the chemical reactions. Although people have made outstanding achievements in the hydroformylation industry, there are still some problems that deserve our attention and consideration.
References
[1]. Robert Franke, Detlef Selent, and Armin Börner Applied Hydroformylation Chemical Reviews2012112(11), 5675-5732 DOI: 10.1021/cr3001803
[2]. Frédéric Hebrard and Philippe Kalck ]Cobalt-Catalyzed Hydroformylation of Alkenes: Generation and Recycling of the Carbonyl Species, and Catalytic Cycle Chemical Reviews2009109(9), 4272-4282 DOI: 10.1021/cr8002533
[3]. Steynberg, J. P.; van Rensburg, H.; Cronje, C. J.; Otto, S.; ́ Crause, C. (to Sasol Technology Ltd.) WO Patent 2003068719, 2003; Chem. Abstr. 2003, 139, 199087.
[4]. Pospech, J., Fleischer, I., Franke, R., Buchholz, S., & Beller, M. Alternative metals for homogeneous catalyzed hydroformylation reactions. Angewandte Chemie International Edition,2013. 52(10), 2852-2872.
[5]. P. M. Lorz, F. K. Towae, W. Enke, R. J_x0007_ckh, N. Bhargava, W. Hillesheim in Ullmanns Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2007, DOI: 10.1002/ 14356007.a20_181.pub2.
[6]. For selected reviews on branched-selective hydroformylations, see a) J. Klosin, C. R. Landin, Acc. Chem. Res. 2007, 40, 1251 – 1259; b) M. L. Clarke, Curr. Org. Chem. 2005, 9, 701 – 718; c) B. Breit, Acc. Chem. Res. 2003, 36, 264 – 275; d) F. Agbossou, J.-F. Carpentier, A. Mortreux, Chem. Rev. 1995, 95, 2485 – 2506.
[7]. Matsinha, L. C., Siangwata, S., Smith, G. S., & Makhubela, B. C. (2019). Aqueous biphasic hydroformylation of olefins: From classical phosphine-containing systems to emerging strategies based on water-soluble nonphosphine ligands.Catalysis Reviews,61(1), 111-133.
Cite this article
Qian,K. (2024). A review of hydroformylation to produce aldehyde. Applied and Computational Engineering,56,86-91.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Robert Franke, Detlef Selent, and Armin Börner Applied Hydroformylation Chemical Reviews2012112(11), 5675-5732 DOI: 10.1021/cr3001803
[2]. Frédéric Hebrard and Philippe Kalck ]Cobalt-Catalyzed Hydroformylation of Alkenes: Generation and Recycling of the Carbonyl Species, and Catalytic Cycle Chemical Reviews2009109(9), 4272-4282 DOI: 10.1021/cr8002533
[3]. Steynberg, J. P.; van Rensburg, H.; Cronje, C. J.; Otto, S.; ́ Crause, C. (to Sasol Technology Ltd.) WO Patent 2003068719, 2003; Chem. Abstr. 2003, 139, 199087.
[4]. Pospech, J., Fleischer, I., Franke, R., Buchholz, S., & Beller, M. Alternative metals for homogeneous catalyzed hydroformylation reactions. Angewandte Chemie International Edition,2013. 52(10), 2852-2872.
[5]. P. M. Lorz, F. K. Towae, W. Enke, R. J_x0007_ckh, N. Bhargava, W. Hillesheim in Ullmanns Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2007, DOI: 10.1002/ 14356007.a20_181.pub2.
[6]. For selected reviews on branched-selective hydroformylations, see a) J. Klosin, C. R. Landin, Acc. Chem. Res. 2007, 40, 1251 – 1259; b) M. L. Clarke, Curr. Org. Chem. 2005, 9, 701 – 718; c) B. Breit, Acc. Chem. Res. 2003, 36, 264 – 275; d) F. Agbossou, J.-F. Carpentier, A. Mortreux, Chem. Rev. 1995, 95, 2485 – 2506.
[7]. Matsinha, L. C., Siangwata, S., Smith, G. S., & Makhubela, B. C. (2019). Aqueous biphasic hydroformylation of olefins: From classical phosphine-containing systems to emerging strategies based on water-soluble nonphosphine ligands.Catalysis Reviews,61(1), 111-133.