1. Introduction
Although fluid foods such as colas, juices, coffees, honey and soups are frequently used in daily life, the problem of adhesion of fluid food residues to containers containing fluid foods inevitably occurs due to the very complex nature of their composition. In addition, contact between the human mouth or saliva and the container may also contaminate the vessel wall, because saliva is rich in proteases and other nutrients that are easily contaminated by bacteria. These residues not only severely contaminate containers, but even promote the formation and growth of bacterial biofilms, necessitating application of chemical detergents for cleaning instead of simply rinsing with water. However, the use of chemical detergents causes serious environmental pollution and can be able to damage human skin and physiological functions [1]. For this reason, developing liquid food resistant surfaces for a wide range of applications is need, to reduce the problems of liquid food adhesion, biofilm formation and detergent use.
A superhydrophobic surface inspired by lotus leaves shows outstanding properties, such as strong hydrophobicity. However, despite the many excellent properties of superhydrophobic surfaces, they can still suffer from some drawbacks, including structural instability and poor durability, all of which are difficult to improve. Later, inspired by piggybacking, it was found that these problems can be solved by a new surface-lubricant infused surface (LIS), which shows strong hydrophobic properties as well as stable structure. However, most of the materials used to prepare hydrophobic coatings are hazardous to humans and cannot be used in food packaging [2]. However, LIS made from oleogels such as wormwood and Brazilian carnauba wax can not only store lubricants well and have good durability, but also this oleogel is edible, so there is no need to worry about harm to the human body. As a result, this research will explore ELIS edible lubricant (edible lubricant) immersed in edible surface porous structure constituting edible waxes. This oleogel is called ELIS, and it consists of an edible lubricant (edible lubricant) impregnated with a porous structure constituting edible waxes on edible surfaces. The lubricant can enter the tiny structures-between wax particles and nanostructures. The advantages and disadvantages of carnauba wax oil gel and beeswax oil gel in terms of characterisation, performance and other aspects will be discussed later in the main text.
Residues in food packaging after consumption have been a major food waste problem and hygiene issue. Generally liquid foods are packaged in containers made of plastic. After consumption, the liquids adhere to the inner surfaces of the bottles, requiring the use of detergents to remove them, which can lead to significant food waste. Although previously discovered superhydrophobic modification can be used to resist liquids adhesion, their need for specific structures and lubricants precludes for food applications. An effective way to prevent liquid residues is to utilize the properties of superhydrophobic or superoleophobic surfaces to repel a variety of liquids, such as aqueous or oil solutions. Although superhydrophobic coatings (e.g., polytetrafluoroethylene) are promising for the repulsion of aqueous food products, there are still challenges in achieving good results. Superoleophobic coatings can prevent oil adhesion, but usually require specific nanostructures to have this effect, and such structured surfaces are easily damaged. Although the recently reported mushroom-like columnar structured surfaces can be prevented from becoming wetted surfaces after being damaged, it is difficult to use in food container materials. And superoleophobic coatings are often associated with fluorocarbon chemistry, where possible degradation by-products include perfluorooctanoic acid (PFOA) contaminants [1].
Oleogels are soft matter systems, which can form different types of three-dimensional supramolecular networks, such as crystalline particles, with the final structural and tectonic processes depending on the gelling agent and the oil phase. The compounds used as oil gelling agents need to satisfy the following physicochemical properties, including good affinity, surface-active and self-assembling properties, higher structural arrangements and thermally reversible properties [3]. The main gel factors currently used for oleogel preparation are fatty acids, fatty alcohols, mixtures of lecithin and sugar alcohol esters (sorbitan tristearate), mixtures of lecithin and carotenoids, phytochemicals (sterols and gluten), biowaxes and wax esters, monoethylene glycol esters, ethyl cellulose, and cinnamic acid. These gel factors have been widely used in food, cosmetics and pharmaceuticals. For example: phytosterols (esters) can be added to margarine as a functional ingredient for food; monoglycerides can be used as emulsifiers for skin care products and hair care milks; and ethylcellulose can be used as a film-coating material to wrap medicines [4]. Cinnamic acid is a phenylacrylic acid produced by the deamination of phenylalanine in plants, which can be applied to food, flavours and fragrances and organic synthesis, to serve as a food additive with a wide range of applications and good functionality. Among the various gelling agent molecules, vegetable waxes have great potential for development due to their high availability, excellent structural properties, ability to bind oil and low cost [5].
2. Preparation process
For the preparation of ELIS surfaces, there is no difference between the two surfaces, and the basic preparation process for preparing ELIS is described uniformly below, as shown in Figure 1. For step 1, it is formation of a layered oleogel on a polyolefin base by cooling a boiling solution of beeswax or carnauba wax and edible oil under certain conditions. An oleogel is a gel of edible oil with an uneven surface, consisting mainly of a small amount of lipid material. Due to the strong adsorption on the surface of the oleogel, when the lipid material forms a fibrous mesh structure through self-assembly or crystallisation, the surrounding oil can be aggregated to form an oleogel. For step 2, spray edible oil on the surface of the oleogel and fix it on the surface of the oleogel to form a smooth coating with a double-layer structure [6].
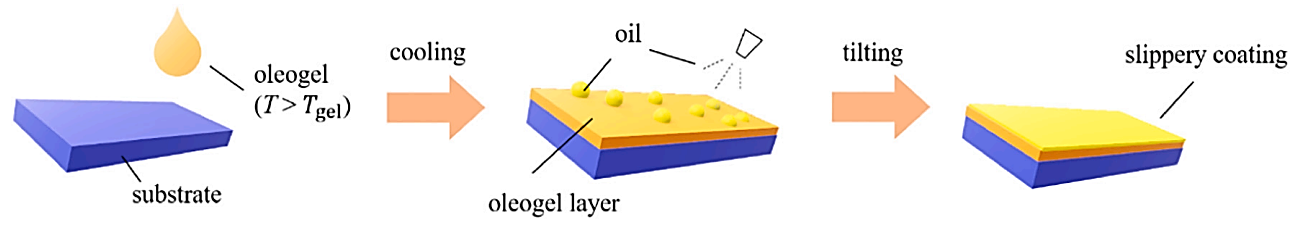
Figure 1. Description of preparation of elis using beeswax oil gel [6].
3. Structure and properties of oleogel
Since solution to gel conversion occurs at lower temperatures, when the coating is removed from the oven, the oleogel forms naturally. ELIS uses beeswax dissolved in ethanol at high temperatures and precipitated at low temperatures to form a porous structure as a porous hydrophobic material on the surface. For mechanical properties and rheology of oleogels, the viscosity decreases while the shear rate becomes higher, which shows that the system has a shear reduction property. Below 47 ℃, the energy storage modulus is greater than the energy dissipation modulus indicating that the system is a viscous gel. Above 47 ℃, the energy dissipation modulus is greater than the energy storage modulus surfaces. Surface oil gels are fragile and susceptible to high temperature reactions. In contrast, the porous hydrophobic surface of ELIS has high durability (high bending resistance) and remains highly hydrophobic at high temperatures.
The microstructure of the oleogel is an interconnected fibrous structure with fibrous crystals. The microstructure of the porous hydrophobic surface of ELIS, beeswax is in the form of layers. At a temperature drop from 100 ℃ to 25 ℃, the straight-chain molecules of the aliphatic hydrocarbons in beeswax fold, resulting in the formation of fibrous crystals. Edible oils if unsaturated fatty acids glycerol. At a temperature drop from 100 ℃ to 25 ℃, the crystallisation of the fatty chains undergoes gelatinisation and the gel is formed. When the two form an oleogel, the fibrous mesh structure formed by cooling the beeswax after heating induces molecules to enter the mesh structure through weak intermolecular forces, thus linking to form an oleogel. It can be seen that the oil molecules and beeswax molecules together form the fiber structure of the oleogel, while the porous and hydrophobic surface of ELIS is purely the structure of beeswax.
The stability of the coating is determined by the absorption of the oil layer, the more stable the absorption of the oil layer is. The stability of ELIS is more in favour of the stability of the porous hydrophobic surface (when the surface lubricant fails first). When the beeswax content is 5%, a small part of the solution cannot grow gel, suggesting that it is the absorption state of saturated oil at this time; when the beeswax content is above 5%, it can form a complete and stable oil gel, and it is the absorption state of unsaturated oil at this time. When the beeswax is above 5%, the edible oil sprayed on the oleogel layer can be fully absorbed and fix the oleogel layer. Thus, when the beeswax content increases, the oleogel shows stronger oil absorption ability [6].
4. Description of preparation of elis using carnauba wax oil gel
Brazilian carnauba wax melts at 69.60 ℃ (5% wax) and 74.30 ℃ (15% wax). Except for the difference in melting temperature, the process and principle of formation of the oleogel and the ELIS surface are the same as above for beeswax as raw material. For mechanical properties and rheology of oleogels, at different temperatures, the viscosity remains constant, however, the viscosity increases as the wax concentration increases. And the shear rate increases, the viscosity starts to decrease as in the case of beeswax oil gels. In case of low concentration of waxes, temperature has little effect on the hardness of the oleogel, however when in case of high concentration of waxes, the hardness of the oleogel increases with increase in temperature and remains unchanged when a certain temperature is reached. This is due to the formation of new hydrogen bonding sites with increase in heating temperature.
The microstructure of the porous hydrophobic surface of carnauba wax ELIS exhibits a blocky structure. Beeswax and carnauba wax are chemically approximately the same, as measured by infrared spectroscopy, except for the presence of hydrogen bonds formed by amino and hydroxyl groups between the carnauba waxes. The oil binding ability of palm wax oil gel also increases with wax content. However, the ability to bind to oil is poor at lower temperatures, while when the melting point of palm wax is reached, the hydrogen bonding sites increase and it is easier to form an oleogel network structure. Therefore, lower temperatures, the weak network of oleogel cannot be used to capture the oil. In addition, the smaller temperature gradient in the fabrication process induces a slower cooling rate, leading to a lower oil capacity of the network structure. This may be due to the development of more disordered networks or crystals of different sizes or shapes [7].
5. Comparison of EILS formed by carnauba wax oil gel and beeswax oil gel
As shown in Figure 2, the results of FT-IR show ν-CH2 aliphatic and δ-CH2 aliphatic groups in Brazilian carnauba wax and beeswax, where the hydrophobicity is attributed to the long-chain aliphatic groups. Brazilian carnauba wax with broad peaks reflect the stretching vibrations of hydroxyl and amino groups. Brazilian carnauba wax has hydroxyl and amino groups. It can be hypothesized that the ELIS formed from Brazilian palm wax oil gel has higher stability and durability compared to beeswax oil gel, with a longer life cycle [6].
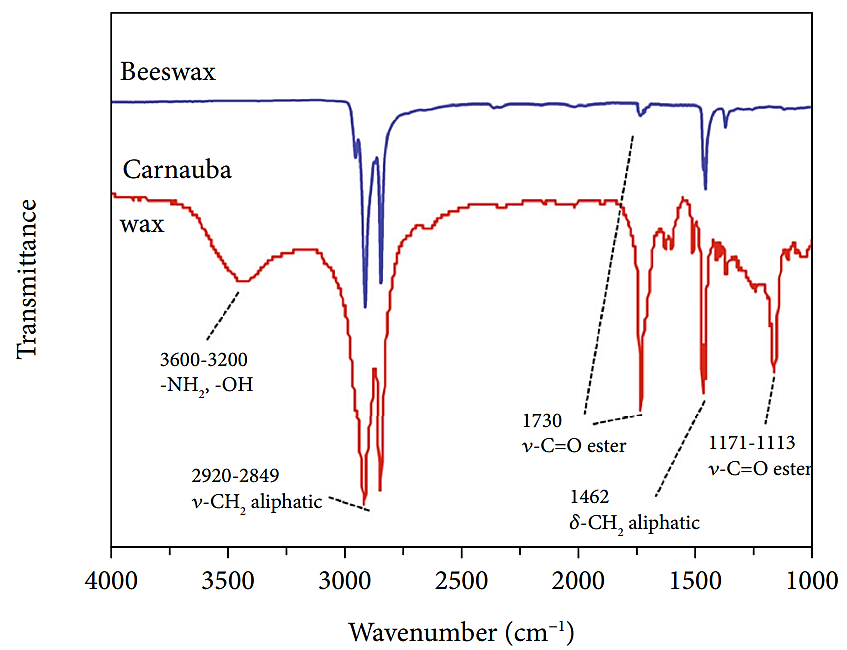
Figure 2. Comparison of infrared spectra of beeswax and carnauba wax [8].
Since the contact surface of the liquid to be tested with the ELIS coating is edible oil, the hydrophobicity of the ELIS surface is not affected when the wax content is changed. However, it is different for the surface smoothness of the coating. Neglecting the effects of the self-weight of the droplets themselves and the tilting angle of the coating, the degree of oil control of ELIS itself and the surface structure of the waxes also determine the smoothness of the coating. In particular, the palm wax oil gel has more hydrogen bonding sites and is more capable of absorbing oil at a certain temperature, thus maintaining a smooth surface for a longer period. Not only that, but the surface block structure of carnauba wax is also smoother than the layer structure of beeswax and has less resistance to droplets, which gives the carnauba wax ELIS coating an advantage in terms of surface smoothness [6].
The resistance of surfaces to icing has a profound effect on external systems, and for food packaging surfaces, icing should be prohibited. In the case of carbonated beverages, icing can lead to a rapid release of CO2, due to water solidification reducing the rate of dissolution of CO2 and leading to a loss of flavor in the production. For brittle containers such as glass bottles, freezing of the contents can lead to rupture or damage to the container due to expansion of the frozen contents. The introduction of ELIS can be used to reduce the number of places where ice cores form, thus reducing ice accumulation. A CO-containing Brazilian carnauba wax was chosen as the test surface due to the coating’s better smoothing properties and lower CO freezing point (about -20 ℃). The droplets on the original glass remained transparent up to 279 seconds, while those on the ELIS glass turned into opaque ice at 826 seconds [8]. The significantly longer freezing time of the droplets on the ELIS glass demonstrates the ice delaying capability of the ELIS coated glass.
Through the above comparative analysis, the coating is durable and can be used on a large scale, from the laboratory to the home kitchen [9]. On the other hand, it is suitable for use in situations such as vegetable waste recycling and has excellent liquid-phase storage capabilities, making it an alternative to plastics and packaging that can subjectively slow or release contents [10].
6. Conclusion
This post first describes an easy, green method of making ELIS oil gel coatings. Under the same process, for carnauba wax oleogel and beeswax oleogel, a comparison is made in terms of melting point, mechanical properties, microstructure, group composition, oleogel formation process and oil absorption properties. Finally, the palm wax oil gel is slightly better than the beeswax oil gel in all aspects, but due to the high stability of high melting point, the temperature at which the beeswax oil gel is dissolved in the process is lower than that of the palm wax oil gel. Therefore, the production cost of using beeswax oil gel is lower if it is used at room temperature for the coating of general foodstuffs. However, for the phenomenon of characterization of ELIS coatings, the test of infrared spectroscopy and the comparison of delayed icing properties, the ELIS coatings formed by carnauba wax oleogel with more hydrogen bonding sites have high durability and better sliding properties due to the microscopic manifestation of a blocky structure with a lower coefficient of friction compared to the laminated structure of beeswax. Moreover, for both carnauba wax and beeswax ELIS coatings, the change in wax content only affects the oil absorption properties and thus indirectly affects the contact angle of the droplets, which may still have room for development in the process flow and post-development because there is only the oil and the droplets in contact with each other. Regarding delayed icing properties, Brazilian carnauba wax containing CO was chosen due to the coating’s better smoothing properties and lower CO freezing point.
The ELIS coating is in line with the current socio-environmental theme of going green and reducing waste. Although there is a slight performance disadvantage compared to traditional hydrophobic materials, the easy availability of edible raw materials, and the simplicity of the process make ELIS a promising hydrophobic coating for everyday food containers. In addition, the field of research on edible waxes is still expanding. On the one hand, hybrid carnauba-beeswax coatings are obtained by combining carnauba wax on one side and beeswax on the other. Durable mixed edible wax coatings with extensible superhydrophobicity can be used not only for protecting or sealing the interior surfaces of containers, but also for sealing and opening containers containing valuable liquids. The durable edible mixed wax coating obtained retains its superhydrophobic properties, which are retained even after stretching, and prevents abrasion by sandpaper and scratching by knives. On the other hand, edible wax can also be combined with the field of biomimicry, and through a superhydrophobic coating of beeswax and a protein membrane of recycled tomato waste slices, it was possible to prepare a superhydrophobic edible surface, achieving the hydrophobicity of the lotus surface, the water vapor barrier of tomato peel, and some of the chemical characteristics of the two plants. In addition to possessing mechanical strength, edibility, and superhydrophobicity similar to that of tomato peel, it also has good oxygen and water vapor barrier, underwater storage capacity, selective release capacity, and recycling capacity.
References
[1]. Yang C, Wu Q, Zhong L et al. 2021 Journal of Colloid and Interface Science 589 327-335
[2]. Xiang H, Yuan Y, Zhu T et al. 2023 ACS Applied Materials & Interfaces 15(28) 33191-34322
[3]. Patel A R, Schatteman D, De Vos W H et al. 2013 Journal of Colloid and Interface Science 411 114-121
[4]. Wang X, Huang J, Guo Z 2022 Advances in Colloid and Interface Science 301102602
[5]. Thakur D, Singh A, Prabhakar P K et al. 2022 LWT 157 113108
[6]. Hao P, Lou X, Tang L, et al. 2022 Progress in Organic Coatings 162 106590
[7]. Hou J, Liu S, Su M, et al. 2023 Journal of Food Engineering 338 111255
[8]. Wang D, Guo Z, Liu W. 2019 Research 2019 1649427
[9]. Wang D, Huang J, Guo Z, et al. 2021 Journal of Materials Chemistry A 9(3) 1495-1499
[10]. Wang D, Huang J, Guo Z. 2020 Chemical Engineering Journal 400 125883
Cite this article
Zhang,Z. (2024). Comparison and investigation of edible hydrophobic coatings on beeswax and carnauba wax oil gels. Applied and Computational Engineering,58,237-242.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Yang C, Wu Q, Zhong L et al. 2021 Journal of Colloid and Interface Science 589 327-335
[2]. Xiang H, Yuan Y, Zhu T et al. 2023 ACS Applied Materials & Interfaces 15(28) 33191-34322
[3]. Patel A R, Schatteman D, De Vos W H et al. 2013 Journal of Colloid and Interface Science 411 114-121
[4]. Wang X, Huang J, Guo Z 2022 Advances in Colloid and Interface Science 301102602
[5]. Thakur D, Singh A, Prabhakar P K et al. 2022 LWT 157 113108
[6]. Hao P, Lou X, Tang L, et al. 2022 Progress in Organic Coatings 162 106590
[7]. Hou J, Liu S, Su M, et al. 2023 Journal of Food Engineering 338 111255
[8]. Wang D, Guo Z, Liu W. 2019 Research 2019 1649427
[9]. Wang D, Huang J, Guo Z, et al. 2021 Journal of Materials Chemistry A 9(3) 1495-1499
[10]. Wang D, Huang J, Guo Z. 2020 Chemical Engineering Journal 400 125883









