1. Introduction
Due to the reasons of global population growth, climate change, air and noise pollution of conventional combustion-engine cars, and the limitation of unrenewable energy, Electric vehicles (EVs) have been widely used. Compared to traditional fuel-guzzling vehicles, EVs can use renewable energy as a power source, which saves on the consumption of limited resources (fossil fuel) and reduces air pollution (SO2, NOX and COX,). As an inevitable part of EVs, batteries play a very crucial role. Lithium-ion battery (LIB) is the most popular battery in the market since it has properties of high specific energy, high energy density, long lifespan, low self-discharge property, and nearly zero-memory effect [1]. However, there are also some aspects that LIBs can improve, such as the charging speed, heavyweight, heat dissipation, etc. Nanotechnology is one of the most promising options to solve these issues.
The capacity to design and investigate structures with critical dimensions of the order from 1 to 100 nanometers is called nanotechnology. This advanced technology has the benefits of a large specific surface area, robustness, and high resistance. Nanoscale particles are classified as 0D, 1D, 2D, and 3D, including metals, metal oxides, carbon-based, metal-nitrides, metal-carbides, and nanocomposites [2]. To speed up the charging speed of LIB in EVs, the performance of electrodes can be enhanced using nanotechnology by doping, nanocoating, and peanut-like Hierarchical nanostructure. The nanotechnique applied in the electrode of LIB not only can stabilize the material structure and prevent the Jahn-Teller distortions but also can increase the reaction rate and conductivity.
LIBs are made up of four essential components, namely the cathode, anode, separator, and electrolyte. As electrodes, cathode, and anode in batteries, both can assist with the flow of electric charge. The lithium in a lithium-ion battery undergoes chemical reactions to produce electricity. The cathode decides the Li-ion battery's voltage and capacity. Reduction (electron gain) takes place at the cathode, which is the positive electrode. By contrast, the anode is the negatively charged electrode where oxidation (electronic loss) takes place. The key to allowing the utilization of electricity in a battery is the movement of Li+ through the electrolyte and electrons via the wire. Only lithium ions can travel between the cathode and the anode through the electrolyte. The separator acts as a barrier to keep the cathode and the anode apart, which carefully prevents the direct passage of electrons while allowing only ions to pass through the interior's small hole. This research will concentrate on the performance of various anode and cathode materials when combined with nanotechnology because the properties of the electrode materials largely impact the functions of LIBs.
2. Cathode
Lithium is employed for cathodes in the form of lithium oxide since lithium in its elemental form is unstable. For the first generation of LIBs, LiCoO2 was used as the cathode material [3]. However, businesses and institutions researched ways to lessen the dependency of cathode on cobalt because of the Co’s scarcity, high price, and toxicity. Therefore, the electrical vehicle battery market is dominated by LIBs made of lithium nickel-manganese-cobalt (NMC) oxide, lithium nickel-cobalt-aluminum (NCA) oxide, lithium-iron phosphate (LFP), and lithium manganese oxide (LMO) [4]. There are considerable differences in the energy density, thermal stability, and affordability of each of these materials.
2.1. NMC
One of the most popular classes of LiBs, especially in EVs, is nickel-manganese-cobalt oxide, which uses LiNixMnyCozO2 (x+y+z=1) as the cathode material. NMC has remarkable specific energy, high voltage, and great structural stability during cycling [4]. Rich-Ni NMC offers enhanced Li-ion utilization at a relatively low voltage window, resulting in higher specific energy to achieve the high theoretical capacity of NMC batteries. LiNixMnyCozO2 with x ≥ 0.8 is called nickel rich. Rich-Ni cathode, however, still has weak cycling performance even at tolerable voltage ranges. This is caused by the lattice oxygen release, which triggers the creation of a rock-salt NiO layer on the cathode surface, indicating nickel loss and lattice shrinkage [4]. The application of nanostructured NMC cathodes is an appropriate way to prevent the production of NiO layer as well as lattice shrinkage. Furthermore, high Ni presence induces the cathode material and non-aqueous electrolyte to react chemically with each other more frequently, which leads to issues that include a significant drop in safety quality, extreme loss of capacity, and reduction of power density [4]. Hence, the nanocoating technique can be used to produce a protective layer to keep the electrolyte and the electrode material from coming into contact [5]. Combining nanostructures and nanocoating might be beneficial for addressing these two issues with NMCs and enhancing their overall effectiveness.
Due to the reason of Jahn-teller distortion, the NMC cathode continues to have poor rate capability and fast declining capacity. Hierarchical nanostructure is a useful way to strengthen the electrochemical efficiency of NMC, which enables quick rates of lithium-ion intercalation and deintercalation as well as quick electron transfer [6]. This is because larger surface areas ultimately result in enhanced rate capability. In addition, the hierarchical nanostructure provides superior cycle effectiveness while simultaneously providing outstanding structural stability and a decrease in side reactions, particularly by avoiding the Jahn-Teller distortions. One beneficial nanostructure is the Peanut-like Hierarchical nanostructure, which has been successfully created using an easy solvothermal approach in conjunction with a calcination procedure [7]. With remarkable capacity retention of 94.2% over 100 cycles, it offers a discharge capacity of 229.9 mAhg-1 at a current density of 200 mAhg-1 between 2.0 and 4.8 V [7].
As mentioned before, to prevent side effects and prevent immediate contact of the cathode with electrolyte, the nanocoating layer serves as a separation layer. Glasses, lithium-ion conductors, metal fluorides, and electrochemically inert metal oxides with poor electronic conductivity are some of the materials used to change the surface structure of active particles [8]. Each of these coating materials can also function as an HF scavenger, enhancing cycle performance and preventing the build-up of impedance. One remarkable metal, silver, may also be employed as a coating layer due to its stable chemical and physical characteristics, including excellent thermal conductivity, electrical conductivity, and rich ductility for speeding electron transmission [8]. Additionally, Ag is also less reactive than HF, which can substitute the hydrogen. A simple, affordable, scalable, and practical approach is used to create silver-coated nickel-manganese-cobalt oxide cathode material (Ag-NCM811) [8]. The cycling characteristics of NCM811 and Ag-NCM811 are investigated at a rate of 0.1 C. At the 200th cycle, the Ag-NCM811 and NCM811 can discharge 90 mAhg-1 and 55 mAhg-1 in the voltage range of 2.7-4.3 V, respectively [8]. The Ag nanocoating significantly improves the cycling performance of the NMC cathode.
2.2. NCA
NCA(LiNi0.8Co0.15Al0.05O2) is another kind of widely used cathode material, meanwhile, all models sold by Tesla on the European market were based on the system of NCA. NCA has similar benefits as NMC, but NCA is limited by its relatively short life cycle [4]. This is because the long-term cycling efficiency of NCA cathode materials is significantly affected by the interfacial side reactions [9]. Capacity retention and rate capacity in cycling are the two elements of NCA that should be improved. According to an earlier study, there are two reasons for capacity fading and rate-reducing mechanisms: a) The impedance of the battery would rise as the inactive phase (e.g., HF) transformed more quickly, resulting in a proportional decrease in capacity [10]. b) The active Ni3+ engaged in the redox reaction will undergo significant oxidation to Ni4+ in a continuous charge/discharge operation [9]. Additionally, Ni4+ will hasten electrolyte breakdown and create a solid electrolyte interface (SEI) coating on the outer layer of active particles, blocking the path of Li+ diffusion and boosting polarization [9].
Efforts have been made to discover effective approaches to prevent side effects. The coating layer isolates the active material and electrolyte, which can reduce the probability of side reactions. A variety of materials, including metal oxides, metal fluorides, metal phosphates, multiple oxides, and carbon materials have been employed as surface coatings [9]. However, the diffusion of Li+ is unaffected by a good coating, and many surface-modifying agents are inefficient in removing the HF that significantly corrodes the surface of NCA [9]. Widespread research has focused on NCA cathode materials modified with nanoparticles of oxide, which can lessen immediate contact between the electrode and electrolyte. Nevertheless, the surface-modified coatings prohibit the interfacial charge transfer of the electrode as an insulating layer and lengthen the diffusion length of Li+, which has a negative impact on the conductive capacity of these composites in real application [10].
Hence, to improve the electrochemical performance, NCA-based composite has been utilized as an effective strategy. Using an easy template self-assembly method, a three-dimensional nanostructured NCA/graphene composite (G-NCA) was created [10]. The three-dimensional network's broadened surface area and strengthened synergistic effect promote the advancement of electrical conductivity and stable nanostructure, leading to a much higher rate capability and cycle stability. Experimental results show that, at a discharge rate of 5 C, 1G-NCA showed a reversible capacity of 153.6 mAhg-1 with a retention of 82.1%, whereas immaculate NCA only displayed 125.3 mAhg-1 with a retention of 77% [10]. Obviously, 3D G-NCA has better performance than NCA.
2.3. LMO
Lithium manganese oxide (LMO), LiMn2O4, is an alternative that would be a superior replacement because of its low cost, eco-friendliness, cobalt/nickel free, high operating voltage, and rate performance. The cycle performance of the LMO battery, however, has been negatively impacted by structural instability (Jahn-Teller distortion), which has slowed down further development [6]. Utilization in nanostructured cathodes has shown effectiveness in improving Li+ transport because of the larger electrode-to-electrolyte contact area [11]. However, high surface area-to-volume ratios at the nanoscale have caused a few intrinsic issues that have prevented the widespread use of nanostructured electrode materials in commercial applications [11]. Side reactions are caused by the larger contacting area since the interactions between the active material and electrolytes are increased. This may reduce the cycling life of LIBs, while volumetric energy density is constrained by weak packing density and high additive content [12].
A proposed method to increase the efficiency of LMO cathodes is to use conformal graphene coatings as a conductive addition in high-performance nanostructured LMO spinel cathodes [12]. The resultant nanostructured composite cathodes simultaneously address several issues with nanoparticle-based LIB electrodes, such as poor packing density, weak cycle performance, and high additive content. The n-LG cathodes display good power effectiveness throughout both cycles of charging and discharging up to 20C at room temperature owing to the reduced size of nano-LMO and the particularly conductive GNFs [12], as shown in Figure 1. Additionally, the composite cathode can charge to and then discharge 96% of its room temperature specific capacity while maintaining high-rate capability at 0 °C in a severe environment of -20 °C [12].
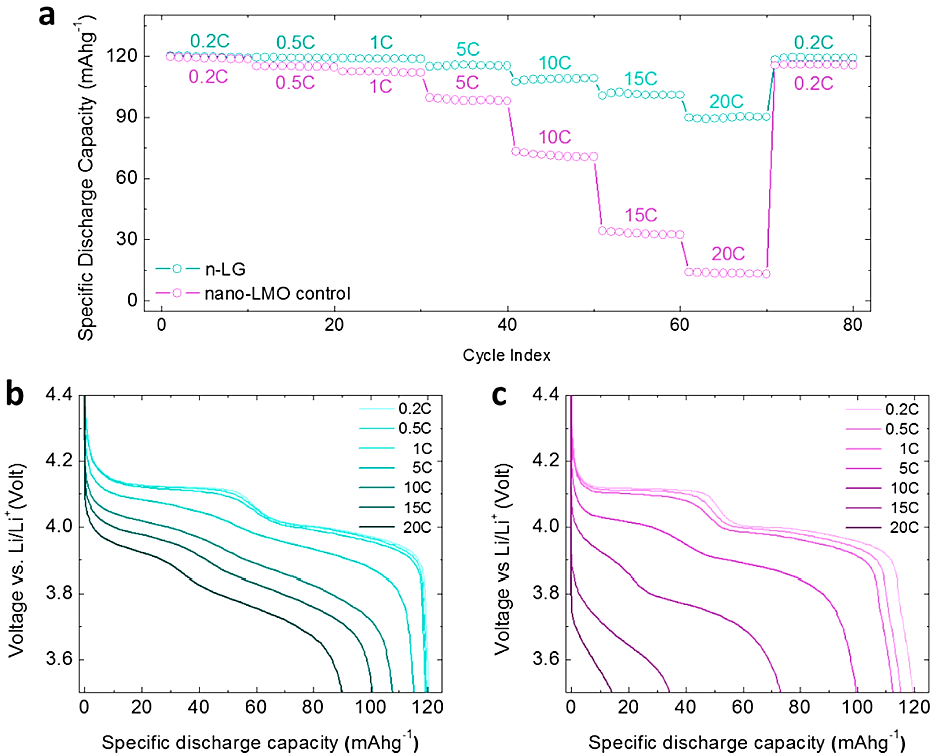
Figure 1. Rate capability comparison [11].
2.4. LFP
LiFePO4 (LFP)-based LIB cells, while having a lower energy density, are quickly gaining favor for electric vehicles because of their unmatched safety, long cycle life, and low price (cobalt-free nature) [13]. However, the LFP battery's poor rate performance, which is connected to the slow electron movement in the LFP crystal structure during the charging and discharging process, is one of the key problems it has faced [13]. Although nano structured LFP and hierarchical nanostructures can decrease the transport length of electrons, the significant increase in surface area destroys the excellent stability of LFP batteries. Nano-coating LiFePO4 nanoparticles with conductive media, such as oxide, carbon, and conductive polymer, is one practical approach to both issues [13].
Reducing particle size, improving morphology, painting the surface with electrically conducting chemicals, and doping the host framework with prevalent cations have all been used to tackle the electronic and ionic transport constraints of LFP [13]. To increase the material efficiency and rate performance of LFP cathode, it is an optimal approach to combine nanosized particles with carbon coating. With a shorter diffusing path and more area on the surface for charge transporters due to particle size reduction, the ionic diffusion constant increases. Additionally, the carbon coating minimizes both the oxidation and migration of the iron as well as increases the surface electrical conductivity, which reduces electrode polarization. The LiFePO4 graphene composite, for instance, exhibits exceptional rate performance, with a discharge capacity of 94.3 mAhg-1 at 100C rate and significantly enhanced cycle life, as well as a high reversible specific capacity of 167.7 mAhg-1 at 0.1C rate [13].
3. Anode
The anode, one of the primary elements of a LIB, is essential to the battery's cycle and electrochemical performance. The anode materials in LIBs act as the host, enabling reversible intercalation and deintercalation of lithium-ion during charge and discharge cycles. Graphite powder is the most common type of anode material used in LIBs. Graphite anodes are reasonably cost-effective, incredibly light, porous, and robust. They also satisfy the voltage requirements of the most popular Li-ion cathodes. However, the need to create anode materials that might potentially replace traditional graphite anodes, whose capacities have fallen short of the specifications for future high-performance LIBs, is currently receiving awareness.
Silica (SiO2)-based material is a promising contender for the anode material of LIBs since it has a large capacity (1965 mAhg-1) and low discharge potential [14]. SiO2 is plentiful on Earth and is commonly present in sand and soil. Silicon-based material has similar benefits as SiO2-based material, silicon-based material even has a much higher capacity (4200 mAhg-1) [14]. However, due to the extreme expansion volume of Si (300%), SiO2 with 100% expansion volume is chosen to be the alternative to Si. Because of the unstable solid electrolyte that is possibly pulverized during the cycle, the high expansion volume of Si results in a low coulombic efficiency and fades the capacity. SiO2 also faces the challenge of being electrochemically active when used as a lithium-ion battery anode including its initial poor coulombic efficiency (52.38%) and electrical conductivity brought on by the strong Si-O bonding, according to preliminary studies [14]. Since lithium can reversibly react with nano-SiO2 at low potentials, research on silica-based anode material combined with nanotechnology is studied.
Carbon nanotubes (CNTs) are one-dimensional allotropes of carbon that are excellent candidates for application in LIBs due to their exceptional electrochemical and mechanical characteristics. CNTs can improve the conductivity of SiO2 in composite SiO2/CNT materials, assist in absorbing SiO2's mechanical stress during cycling, and serve as a buffer to withstand volume changes [14]. The wide interior space and conductivity of CNTs make them an excellent matrix component for encasing SiO2 because of their large diameter. The specific morphology of SiO2/CNT material has an interconnected CNT network, which raises the cycle stability of LIBs. To maintain SiO2 particles electrochemically active, CNTs also offer a more effective channel for the transfer of electrons and ions. Therefore, besides promoting quicker cycle rates, the CNT network may greatly increase lithium-ion transport between the electrolyte and anode. The manufacturing process is a key factor in SiO2/CNT composites that contributes to both benefits. The most common technique for creating SiOx/CNT is chemical vapor deposition (CVD), which has few faults, excellent quality, and massive output [14]. With a high capacity (791.4 mAhg-1) and outstanding cycle stability, the SiO2/C/CNT anode demonstrated remarkable electrochemical performance [14].
4. Limitations
Although nanotechnology can enhance the performance of LIBs including electron conductivity, energy density, and capacity, it also has several drawbacks. Nanotechniques help active materials to have larger surface area that faster the redox reactions in Lithium-ion batteries. However, larger surface area of electrodes may cause more side reactions. Hence, sometimes micron designs may be more convenient for improving the efficiency of LIBs. What’s more, nanotechnology is a high-cost technology and may not be the best option for businesses and consumers, which may not be industrialized. In addition, there is not much research on nanotechnology for LIBs electrodes. In the future, people may work on more aspects of research to improve the performance of lithium batteries, and these issues will be refined.
5. Conclusions
Overall, this research summarizes some examples of widely used cathode materials and anode materials combined with nanotechniques of LIBs. Silver was designed to be the nano-isolation layer of NMC to strengthen the electrochemical ability of LIBs. To increase the cycle stability of NCA, a three-dimensional nanostructured NCA/graphene composite (G-NCA) was developed. As the Jahn-teller distortion brings detrimental effects on stability, conformal graphene coatings have been added as conductive additions to nanostructured lithium manganese oxide spinel anodes to enhance cycling stability. An efficient method for enhancing LFP's ionic transport and rate performance also used a mix of nanoparticles and carbon coating. Furthermore, SiO2/CNT composites promote the faster Li+ ion transfer between the anode and electrolyte, which increases the cycle rate. However, there are also some limitations of these nanotechniques, such as high cost, side reactions, and difficulty to be industrialization. More research will be done as a consequence of the desire for lithium batteries to work even better in the future. These issues will be refined, or alternative industrialization strategies will be found.
References
[1]. Kim T, Song W, Son D, Ono L and Qi Y 2019 J. Mater. Chem. A 7 2942-64
[2]. Abdelkareem M, Maghrabie H, Abo-Khalil A, KadhimAdhari O, Sayed E, Radwan A, Elsaid K, Wilberforce T and Olabi A 2022 Journal of Energy Storage 50 104385
[3]. Ellingsen L, Hung C, Majeau-Bettez G, Singh B, Chen Z, Whittingham M and Strømman A 2016 Nature Nanotechnology 11 1039-51
[4]. Qiu Y 2023 Highlights in Science, Engineering and Technology 58 379-86
[5]. Qin H 2023 Highlights in Science, Engineering and Technology 43 563-68
[6]. Wang A 2023 Highlights in Science, Engineering and Technology 32 325-331
[7]. Zhang Y, Li Y, Niu X, Wang D, Zhou D, Wang X, Gu C and Tu J 2015 J. Mater. Chem. A 3
[8]. Li X, Chang K, Abbas S, El-Tawil R, Abdel-Ghany A, Hashem A, Wang H, Coughlin A, Zhang S, Mauger A, Zhu L and Julien C 2023 Micromachines 14 907
[9]. Yang Z and Li Z 2022 International Journal of Electrochemical Science 17
[10]. Luo W, Liu L, Li X, Yu J and Fang C 2019 Journal of Alloys and Compounds 810 151786
[11]. Nakajima K, Souza F, Freitas A, Thron A and Castro R 2021 Chemistry of Materials 33(11) 3915-3925
[12]. Chen K, Xu R, Luu N, Secor E, Hamamoto K, Li Q, Kim S, Sangwan V, Balla I, Guiney L, Seo J, Yu X, Liu W, Wu J, Wolverton C, Dravid V, Barnett S, Lu J, Amine K and Hersam M 2017 Nano Letters 17(4) 2539-2546
[13]. Li J, Zhang L, Zhang L, Hao W, Wang H, Qu Q and Zheng H 2014 Journal of Power Sources 249 311-19
[14]. Ja’farawy M, Hikmah D, Riyadi U, Purwanto A and Widiyandari H 2021 Journal of Electronic Materials 50 6667-87
Cite this article
Sun,B. (2024). Application of nanotechnology in the battery of electric vehicles. Applied and Computational Engineering,59,149-154.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Kim T, Song W, Son D, Ono L and Qi Y 2019 J. Mater. Chem. A 7 2942-64
[2]. Abdelkareem M, Maghrabie H, Abo-Khalil A, KadhimAdhari O, Sayed E, Radwan A, Elsaid K, Wilberforce T and Olabi A 2022 Journal of Energy Storage 50 104385
[3]. Ellingsen L, Hung C, Majeau-Bettez G, Singh B, Chen Z, Whittingham M and Strømman A 2016 Nature Nanotechnology 11 1039-51
[4]. Qiu Y 2023 Highlights in Science, Engineering and Technology 58 379-86
[5]. Qin H 2023 Highlights in Science, Engineering and Technology 43 563-68
[6]. Wang A 2023 Highlights in Science, Engineering and Technology 32 325-331
[7]. Zhang Y, Li Y, Niu X, Wang D, Zhou D, Wang X, Gu C and Tu J 2015 J. Mater. Chem. A 3
[8]. Li X, Chang K, Abbas S, El-Tawil R, Abdel-Ghany A, Hashem A, Wang H, Coughlin A, Zhang S, Mauger A, Zhu L and Julien C 2023 Micromachines 14 907
[9]. Yang Z and Li Z 2022 International Journal of Electrochemical Science 17
[10]. Luo W, Liu L, Li X, Yu J and Fang C 2019 Journal of Alloys and Compounds 810 151786
[11]. Nakajima K, Souza F, Freitas A, Thron A and Castro R 2021 Chemistry of Materials 33(11) 3915-3925
[12]. Chen K, Xu R, Luu N, Secor E, Hamamoto K, Li Q, Kim S, Sangwan V, Balla I, Guiney L, Seo J, Yu X, Liu W, Wu J, Wolverton C, Dravid V, Barnett S, Lu J, Amine K and Hersam M 2017 Nano Letters 17(4) 2539-2546
[13]. Li J, Zhang L, Zhang L, Hao W, Wang H, Qu Q and Zheng H 2014 Journal of Power Sources 249 311-19
[14]. Ja’farawy M, Hikmah D, Riyadi U, Purwanto A and Widiyandari H 2021 Journal of Electronic Materials 50 6667-87









