1. Introduction
With the obvious trend of global warming and the growing energy crisis, international countries are eager to seek low-carbon and environmentally friendly new energy industries. The market demand for lithium batteries continues increasing in the areas of mobile devices, electric vehicles, and other industries because lithium battery has the advantages of no memory effect, large specific energy, small self-discharge, and mature technology.
The selection of anode materials is a key factor restricting the improvement of battery performance [1]. Therefore, in order to meet the needs of higher performance lithium batteries, modification of anode materials is of great significance. Lithium battery anode materials are mainly divided into graphite, non-graphite carbon, and non-carbon-based anode materials.
Graphite, including natural graphite and artificial graphite, is one of the most commonly used anode materials for its rich resources, low price, and excellent electrochemical performance. Though the application of anode materials accounts for a high proportion, the micro-structure of pristine graphite leads to problems such as poor electrolyte compatibility and high volume expansion rate [2]. Therefore, the modification of graphite anode materials is of great significance to promote the long storage life technology of lithium ion batteries and help lithium batteries achieve a higher performance stage.
Non-graphite carbon includes soft carbon and hard carbon. Soft carbon exhibits significant initial irreversible capacity and a reduced output voltage, and lacks a distinct charge and discharge plateau during its initial charging and discharging cycles. Typically, soft carbon finds application not as an anode material but rather as a coating or an ingredient within a composite. Hard carbon has excellent charge-discharge performance and is becoming a new research hotspot of anode materials.
Non-carbon-based anode materials include silicon-based materials, tin-based materials, titanium-based materials, and lithium metal. Silicon-based materials have a high capacity, but the volume expansion is too high. Tin-based anode materials have high specific capacity, rich natural storage, low price, and are safe and environmentally friendly, but the cycle and rate of new energy are poor. The lattice of the titanium-based materials is stable, and titanium-based materials have the characteristics of high safety, high life, and high rate, but the theoretical capacity is low. Lithium metal materials have high-capacity characteristics but are not safe enough. The application of anode material is still in the theoretical stage.
Due to those defects, the non-graphite carbon and non-carbon-based anode materials are difficult to commercialize on a large scale. Since graphite modification is more feasible, graphite and its main modification methods will be introduced later.
2. Graphite modification
Graphite is one of the most commonly used anode materials in lithium ion batteries.
Natural graphite has great electrical conductivity, high crystallinity, and good layered structure. Natural graphite is well-suited for the process of lithium ion intercalation and deintercalation, allowing for the creation of a lithium-graphite interlayer compound. The graphite has excellent charge and discharge performance, the charge and discharge capacity can achieve more than 300 mAh/g, and the efficiency of charging and discharging exceeds 90%. The irreversible capacity of natural graphite is lower than 50 mAh/g.
Though graphite anode has such great properties, graphite has the disadvantages of poor compatibility with solvents and low charge and discharge capacity at high rates. During the first charge and discharge process, the solvated lithium ions will be embedded into the graphite interlayer, and the solvent molecules will be reduced and decomposed into new substances, resulting in the collapse of the layered structure. With the extension of storage time, the solid electrolyte interface (SEI) film will continue to grow at the defect position and consume a large amount of active lithium, causing the internal resistance of the battery to increase and the storage capacity to decline, affecting the overall performance of the battery. Figure 1 shows the formation mechanism of SEI film on the graphite anode surface. During the charging process, lithium ions are extracted from the active materials of the positive electrode. They then move into the electrolyte by passing through the separator and subsequently re-enter the anode materials. Finally, these lithium ions reintegrate into the layered spaces within the anode, completing a full deintercalation and intercalation cycle. At this point, electrons flow back from the positive electrode through the external circuit and enter the carbon material of the anode. Within the electrolyte, a redox reaction takes place involving electrons, solvents, and lithium ions. Solvent molecules accept electrons and combine with lithium ions to produce the SEI, as well as generate gases such as H2, CO, and C2H4.
As the SEI layer gradually thickens, it forms a passivation layer, eventually becoming impenetrable to electrons. This inhibits the ongoing redox reaction.
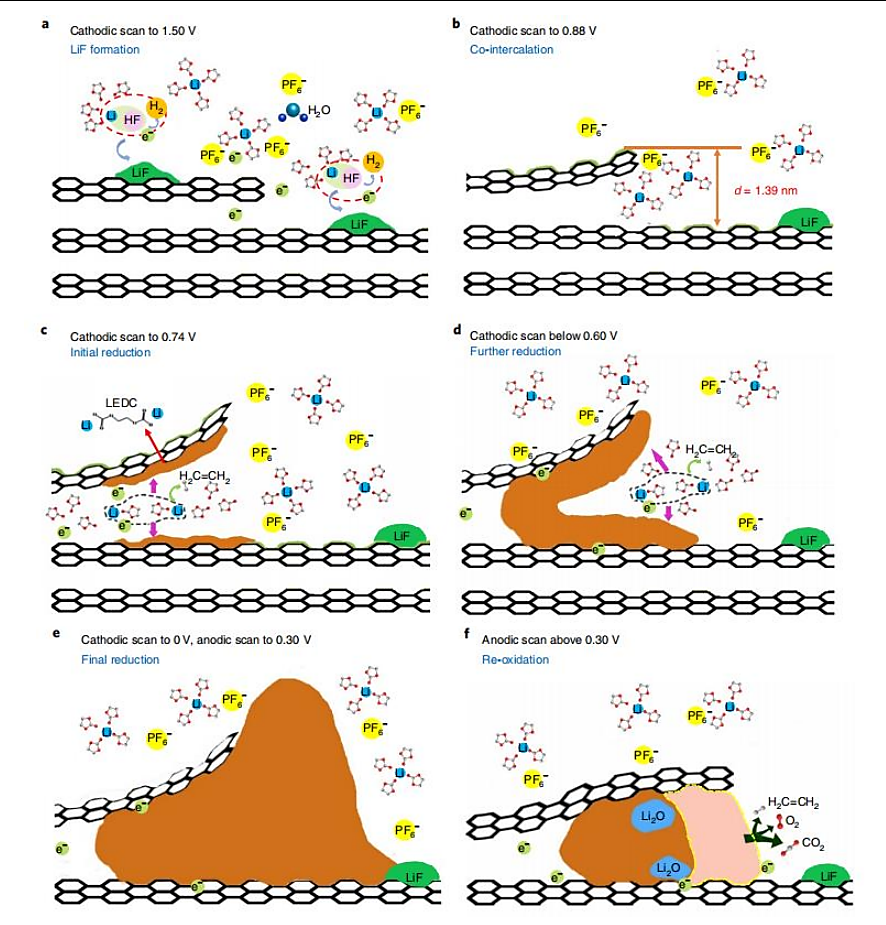
Figure 1. The formation mechanism of SEI film on graphite anode surface [3].
Many scholars have done a lot of research on graphite modification in order to reduce these defects and obtain graphite anodes with better performance. The measures of graphite modification can be divided into three categories including spheroidization treatment, surface coating, and doping modification.
2.1. Spheroidization treatment
In order to modify the shaping of natural graphite and improve the natural graphite powder’s electrochemical performance, researchers have carried out spheroidization treatment on graphite. Spheroidization treatment can control the size of graphite particles, reduce the specific surface area, increase the tap density, and reduce the side reaction between graphite and electrolyte.
Spheroidization principle of natural flake graphite is that the natural flake graphite particles collide, shear and rub in the spheroidizing machine, and the large flake particles undergo plastic deformation to become spherical or quasi-spherical particles, while the weak particles are adsorbed on the main core. After friction and de-angularization, spherical graphite is obtained. Spheroidized natural flake graphite exhibits a reduced specific surface area, increased tap density, elevated initial Coulombic efficiency, greater reversible specific capacity, and enhanced cycle stability.
At present, Yang et al. spheroidized the flake graphite with a carbon content of more than 98%, which was obtained by impact mechanical pulverizer and natural microcrystalline graphite with purity of 99% [4]. The results show that under the same speed and time, the aspect ratio of flake graphite is reduced by 32.75% and 48.94%, respectively, compared with that before spheroidization. The tap density increased by more than 85%, and the spheroidizing rate is high; The aspect ratio of natural microcrystalline graphite decreased by 26.88% and 50.20% compared with that before shaping. The tap density increased by 8.13%.
Teng et al. used an LNP-18A shaping experimental machine to spheroidize natural graphite with a carbon content of 94.83%, and analyzed the influence of rotation speed of spheroidizing wheel on spheroidizing effect, the influence of air volume change on spheroidization, the influence of rotation speed of classification wheel on spheroidization and the influence of different spheroidization time on spheroidization effect of graphite [5]. The results show that the increase of spheroidizing wheel speed and spheroidizing time increases the tap density obviously. The tap density of spherical graphite increased from 0.643 g/m3 to 0.913 g/m3 after 180 min of shaping under the condition of 6000 r/min of spheroidizing wheel speed, 3900 r/min of classification wheel speed and 4.54 m3/min of air volume, and the yield was 67%. Wang et al. conducted a spheroidization experiment on flake graphite with a fixed carbon content of about 95%, produced high-purity spherical graphite with a mixed acid purification process, and analyzed the spheroidization efficiency, purification efficiency, and initial electrochemical performance [6]. The results show that this flake graphite is suitable for spheroidizing production. After purification, the carbon content of spherical graphite reached 99.96%. After the half-cell test, the initial Coulombic efficiency reached 88.53%, and the initial discharge capacity was 346.20 mAh/g.
2.2. Surface coating
Surface coating usually adopts a core-shell structure design to solve the problem of uneven graphite surface and large specific surface area, and improve cycle stability. Surface coating can effectively a). resist the interaction between graphite and electrolyte. b). constrain and alleviate the volume expansion of the active center of electrode material and inhibit the agglomeration phenomenon of nanoparticles. c). prevent the electrolyte from penetrating into the active center and maintain the stability of the electrode material interface.
Commonly used substances coated on the surface include amorphous carbon, metal and metallic oxide, and polymer. Coating amorphous carbon on the surface of spherical graphite is one of the most effective methods to improve the electrochemical performance of graphite anode. Metals and metal oxides have the potential to enhance the lithium-ion diffusion coefficient of the material and mitigate side reactions between the graphite anode and the electrolyte. Polymers have great ionic conductivity and thus can provide a great conductive network for graphite. Besides, polymers can also reduce the direct contact area of the graphite material and electrolyte and enhance the stability of the material.
Pitch, one of the most commonly used carbon sources when modifying graphite, has attracted much attention from researchers.
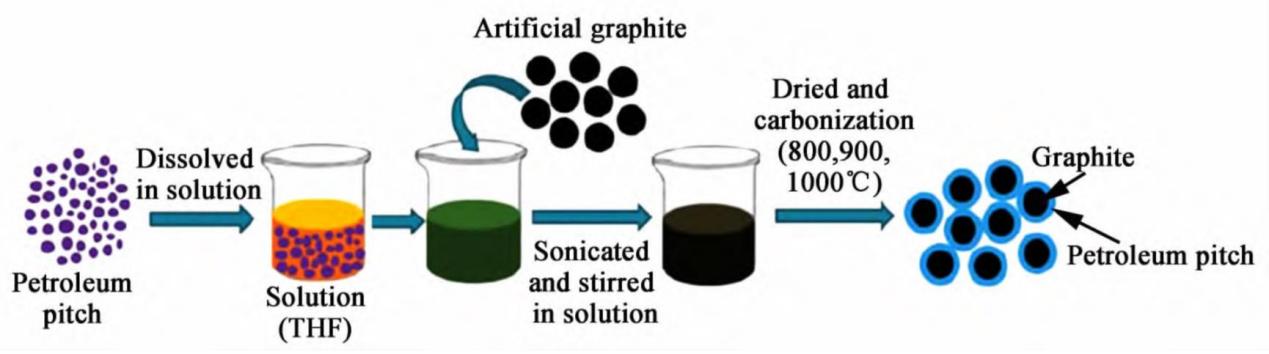
Figure 2. Preparation process of artificial graphite coated with petroleum pitch [7].
Figure 2 shows the procedure for producing artificial graphite covered with a petroleum-based pitch coating. Petroleum pitch was dissolved in solution, and then the artificial graphite was sonicated and stirred in the solutions, treated by liquid phase coating. After drying and carbonization in the specific temperature, the process was completed.
Zhang et al. coated and carbonized the high-purity spherical graphite with pitch as the carbon source [8]. The initial discharge capacity of the big ball product was 366.6 mA·h/g and that of the small ball product was 364.3 mA·h/g. The initial coulomb efficiency was 93.40% and 92.32%, respectively. After 30 cycles of charge and discharge, the capacity retention rate was 99.40 % and 99.15%. Coated carbonized products have high discharge capacity and good cycle stability.
Liu et al. used pitch with a softening point of 280 °C to coat graphite with a carbon content greater than 99.95% in the liquid phase and solid phase under negative pressure or normal pressure, respectively [9]. The results show that liquid phase coating can uniformly coat a layer of amorphous carbon on the surface of natural graphite, so that the surface is smooth, the surface area is reduced, and the defects of the marginal structure are effectively reduced. The electrochemical test results that coating under negative pressure and normal pressure can both improve the wettability of natural graphite and electrolyte, reduce the interfacial mass transfer resistance, and improve the electrode cycle stability and high current charge-discharge properties.
Youn Jae Woong et al. used a dry speed mixer to investigate the electrochemical performance of pitch-coated natural graphite [10]. The experiment is conducted by modifying parameters such as the mixer’s rotational speed, duration, graphite composition, and the softening point of the pitch. When subjecting the pitch-coated graphite to testing conditions with a mixer speed set at 9000 RPM, a graphite content of 10wt%, a 2-hour mixing duration, and a pitch softening point of 150 °C, the highest observed capacity at a discharge rate of 0.1 C is 324.5 mA·h/g. Impressively, even after 50 cycles, the capacity retention remains high at 98.9%.
2.3. Doping modification
Exotic atoms can change carbon material’s microstructure and electrochemical property, then promote the deintercalation and intercalation of lithium ions.
Doping modification is classified according to different elements, usually divided into non-metallic element doping and metal element doping. The non-metallic elements used for doping mainly are N, P, B, Si, S, etc., while the metallic elements mainly are Fe, Co, Ni, Zn, Cu, Ag, etc. The doping of non-metallic elements can change the electron distribution around graphite crystallites, improve the binding ability between lithium ions and graphite crystallites, strengthen the insertion and extraction behavior of lithium ions, improve the conductivity of electrode materials, and realize the comprehensive improvement of battery performance.
The addition of silicon and tin elements can improve the specific capacity of graphite anode materials. Boron doping can reduce the repulsive force between embedded and pre-embedded lithium ions and improve the rate performance.
Wu et al. prepared K+-doped graphite electrode materials by mixing KCl with graphite and sintering at high temperatures [11]. When the sintering temperature was 850 °C, the gravimetric specific capacity was 437.6 mAh/g at 0.1 C discharge condition, and 269.7 mAh/g at 1 C discharge condition, which improved the capacity and rate performance of graphite. Chenguang Bao et al. obtained graphite material doped with boron with accelerated dynamics of Li diffusion, produced by using boron carbide powder as a graphitization catalyst at a lower graphitization temperature of 2500 ℃ [12]. Their findings revealed a significant boost in the lithium diffusion coefficient by approximately two orders of magnitude, attributed to the rapid formation of lithium carbonate and lithium fluoride-rich SEI layer at around 1.1 V, thanks to the boron doping process.
Doping modification can significantly improve the reversible capacity and energy density of graphite materials to a certain extent. However, in the process of element doping, the controllability, uniformity and active atom content of doped atoms still need to be solved. Besides, after doping, the material is more likely to expand in volume.
3. Conclusion
The market demand for lithium batteries continues increasing in the areas of mobile devices, electric vehicles and other industries, because lithium battery has the advantages of no memory effect, large specific energy, small self-discharge and mature technology. With the increasing demand, the energy density, cycle performance and manufacturing cost of lithium battery limit its further development. The modification of the materials involved in the internal structure of a lithium ion battery can effectively improve its performance.
In the realm of material modification, particularly focusing on graphite, which is the most commonly used anode material for lithium batteries, numerous scholars have dedicated their efforts, and their research has been discussed before. Processes like spheroidization treatment and surface coating have demonstrated their ability to enhance the electrochemical properties of the material interface. Nonetheless, these methods face challenges in significantly increasing the energy density of the material interface. While doping modification has the potential to improve energy density, it often falls short in achieving uniformity and stability. It is the future research direction of graphite modification to solve the defect of poor cycle stability of graphite anode by synergistic modification in various ways.
References
[1]. Lu Y, Yu L, Lou X, et al. 2018 Chem 4 972
[2]. Shi J, Liu Q, Zang H, et al. 2019 New chemical materials 47 42-46
[3]. Liu T, Lin L, Bi X, et al. 2019 Nat Nanotechnol 14 50-56
[4]. Yang Y, Chen X, Gai G, et al. 2004 Journal of Process Engineering z1 319-323
[5]. Teng D, Li P, Yuan N, et al. 2021 Chinese powder technology 27 70-76
[6]. Wang B. 2022 Mining and Metallurgy 31 82-88
[7]. Jiang C, Sun W, Wang L, et al. 2023 Battery industry 3 1-7
[8]. Zhang Y, Qiu Y, Zhang L, et al. 2023 Mining and metallurgy engineering 43 147-153
[9]. Liu Y, Li G, Guo X, et al. 2022 Carbon technology 41 46-50
[10]. Youn J W, Lee J D. 2021 Korean Chemical Engineering Research 59 410-416
[11]. Wu Y, Wang L, Li Y, et al. 2017 The Journal of Physical Chemistry C 121 13052-13058
[12]. Ba C, Liu Z, Yang Z, et al. 2023 Electrochimica Acta. 463 142821
Cite this article
Xia,X. (2024). Modification of graphite anode for lithium ion battery. Applied and Computational Engineering,60,159-164.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Lu Y, Yu L, Lou X, et al. 2018 Chem 4 972
[2]. Shi J, Liu Q, Zang H, et al. 2019 New chemical materials 47 42-46
[3]. Liu T, Lin L, Bi X, et al. 2019 Nat Nanotechnol 14 50-56
[4]. Yang Y, Chen X, Gai G, et al. 2004 Journal of Process Engineering z1 319-323
[5]. Teng D, Li P, Yuan N, et al. 2021 Chinese powder technology 27 70-76
[6]. Wang B. 2022 Mining and Metallurgy 31 82-88
[7]. Jiang C, Sun W, Wang L, et al. 2023 Battery industry 3 1-7
[8]. Zhang Y, Qiu Y, Zhang L, et al. 2023 Mining and metallurgy engineering 43 147-153
[9]. Liu Y, Li G, Guo X, et al. 2022 Carbon technology 41 46-50
[10]. Youn J W, Lee J D. 2021 Korean Chemical Engineering Research 59 410-416
[11]. Wu Y, Wang L, Li Y, et al. 2017 The Journal of Physical Chemistry C 121 13052-13058
[12]. Ba C, Liu Z, Yang Z, et al. 2023 Electrochimica Acta. 463 142821









