1. Introduction
The development of LIBs dates back to the 1970s when the American chemist M. Stanley Whittingham proposed the concept who later was awarded with Nobel prize with John B. Goodenough and Akira Yoshino. However, commercialization did not occur until the 1990s when portable electronics became the target market. In 1991, Sony introduced the first commercially successful LIBs [1]. Since the early 2000s, LIB technology has made significant advancements, with a focus on improving safety, energy density, and cycle life. Various cathode materials, such as LiFePO4 and LiMn2O4, have been developed to enhance battery performance. These advancements have resulted in the widespread adoption of LIBs in cellphones, laptops, electric vehicles (EVs), and energy storage systems. Despite their successes, LIBs do have several drawbacks. One limitation is their limited energy density, meaning they can store a limited amount of energy per unit of weight or volume. This limitation affects the overall energy capacity of devices powered by these batteries [2]. Additionally, LIBs have relatively low charging and discharging rates, which means they may not be able to handle high-demand applications efficiently. Safety concerns are another drawback of LIBs. Overheating, overcharging, or physical damage can lead to thermal runaway, causing the battery to catch fire or explode [3]. While significant efforts have been made to improve the safety of LIBs, incidents still occur occasionally. Fortunately, nanotechnology offers promising solutions to mitigate these problems. Nanotechnology involves manipulating materials and systems at the nanoscale level, typically ranging from 1 to 100 nanometers. The following sections will introduce the nanotechnology being employed to improve the performance and safety of LIBs.
2. Application of nanotechnology in cathode materials
2.1. LiCoO2 cathode
LiCoO2 (LCO), illustrated in Figure 1, is considered the preferred and optimal cathode material for several reasons. These include its high energy density, elevated operation voltage, ease of synthesis, effective capacity retention at moderate rates, and stability in ambient atmosphere [4].
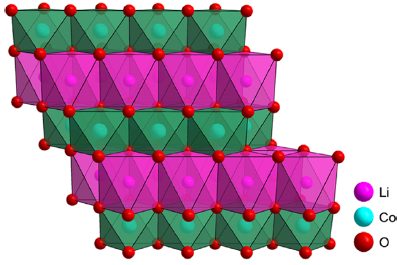
Figure 1. Crystal structure of LiCoO2 [5].
However, researchers found that capacity fading may occur to LCO or even nanosized LCO. This decrease in capacity primarily stems from the elevated Co dissolution in the electrolyte, which is closely linked to a significant anisotropic alteration in the crystal structure [6]. Luckily, with the addition of nano powders the diffusion path length of Li ions can be shortened which mitigates the capacity fading phenomenon [7]. Furthermore, applying a 2 nm Al2O3 coating layer directly onto the cathode proves to enhance its capacity retention stability significantly by preventing surface breakdown. Experimental data supports this claim: an LCO cathode coated with Al2O3 was subjected to charge/discharge cycling between 3.3 and 4.5 V (vs. Li/Li+). Initially, the cycling was performed at a current rate of 16 mA/g (0.1 C) for the first three charge-discharge cycles and later increased to 500 mA/g (2.8 C) for subsequent cycles. Remarkably, the cathode maintained 100% capacity after 200 charge-discharge cycles, compared to the fourth discharge capacity. In contrast, the untreated LCO cathode lost almost all of its capacity after the same number of cycles [7, 8].
2.2. LiMn2O4 cathode
A different category of cathode materials, which has demonstrated its effectiveness in commercial vehicles, consists of lithium manganese oxide spinel as shown in Figure 2. As a result, LiMn2O4 (LMO) provides multiple Li ion transportation pathways, which results in a more efficient Li ion intercalation.
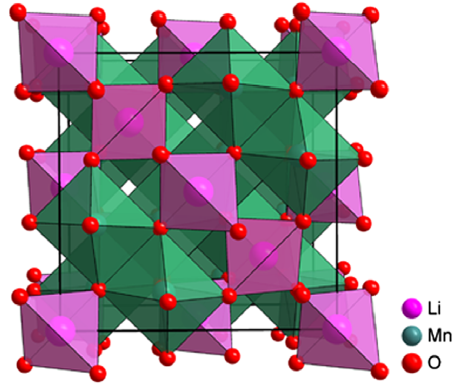
Figure 2. Crystal structure of LiMn2O4 [5].
An inherent challenge associated with LMO cathodes is the structural instability they exhibit. This instability often manifests as Jahn-Teller distortion, particularly when the cathode operates at a low state of charge, leading to the formation of Mn3+ ions and subsequently affecting the overall cycle life. To address this concern, a technique was utilized that entails the nanoscale doping of the cathode surface with aluminum. This approach resulted in an impressive retention rate of 70% of the initial charge after 1100, 1800, 2700, and 4600 cycles for materials subjected to calcination temperatures of 400 ◦C, 500 ◦C, 600 ◦C, and 700 ◦C, respectively, while undergoing charge/discharge cycling at a high current rate of 1000 mAg−1 across the potential range of 0.2–1.3 V (SCE) [9]. Another method that might also be adopted is surface modification by nano oxide coatings such as ZnO, Al2O3, and NiO [10]. Recent research has shifted its attention towards alternative oxides like porous iron oxide (PION). The introduction of a nanolayer of PION onto the surface of the LMO cathode has proven effective in mitigating surface degradation. This prevention of undesirable Li2MnO3 formation and growth is notable. Additionally, the PION layer brings about a significant reduction in charge transfer resistance between the electrode and electrolyte phases. This is supported by the following data: for a pristine LMO cathode, the charge transfer resistance measures 8.62 Ω, whereas for the coated cathode, it is reduced to just −0.507 Ω [11].
2.3. LiFePO4 cathode
LiFePO4 (LFP), characterized by its olivine structure as depicted in Figure 3, represents another highly acclaimed and widely utilized battery technology. Its notable attributes encompass exceptional power density, extended operational lifespan, and a commendable level of safety [12].
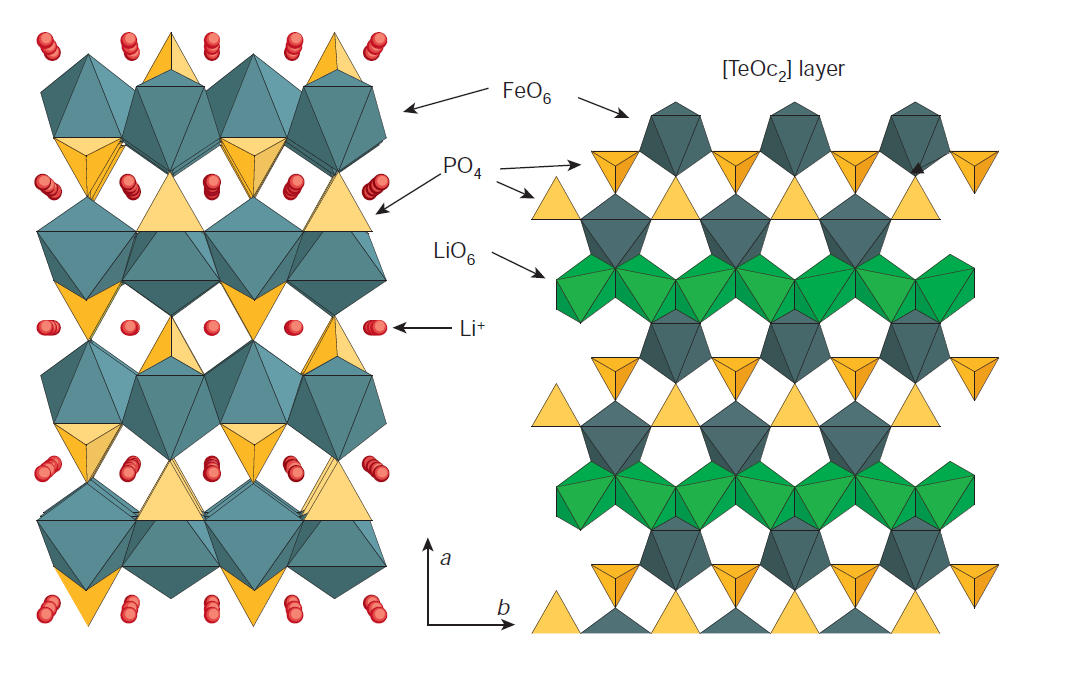
Figure 3. Crystal structure of LiFePO4 [13].
One potential improvement of LFP is its low electronic conductivity and its low-rate performance. For the low electronic conductivity aspect, it is found that this occurs largely due to the covalency of the crystal structure of LFP [14]. A promising way to mitigate this issue is by nano-coating a layer of fluorine-doped carbon (FC) on the surface of LFP [15]. This advantageous structure offers benefits such as effective electrical contact between grains, reducing the Li+ diffusion distance at grain interfaces, and promoting swift electron transfer during the charge-discharge process. Introducing an FC coating has a substantial impact on the specific capacity of the cathode. This yields an initial discharge capacity of 122.6 mA h g−1, representing 72.1% of the theoretical capacity. Even after 1000 cycles, the retention rate of the reversible capacity stays notably high at 86.6%. Furthermore, a study showed that adding holey graphene as a conductive additive can also improve both the electron conductivity and rate performance [16].
2.4. Ternary cathode
Ternary cathodes encompass various composites, such as lithium nickel manganese cobalt oxide (NCM), which is also the most widely used in the industry. These materials find extensive industrial applications due to their elevated voltage, impressive theoretical capacity, robust cycling characteristics, and exceptional thermal stability. However, in the case of NCM, its structural stability is relatively low, which is mainly caused by the side reaction between electrode and electrolyte, leading to concerns about safety and a reduction in specific capacity. Nevertheless, by employing techniques like surface nano-coating and doping, these shortcomings can be substantially mitigated. Al2O3 has proven to be an excellent choice for a coating material. When it is discharging, the primary capacity is 197.1 mA h g−1 at 0.2 C within the voltage range of 2.8–4.5 V. Moreover, it boasts an impressive capacity retention of 91.0%, outperforming the uncoated cathode material, which maintains a retention of 82.9% after 30 cycles at 1 C [17]. The application of Li3PO4 as a coating on the cathode enhances its thermal stability by inhibiting the phase transition of charged NCM at elevated temperatures. This coating leads to notable improvements in electrochemical properties. Specifically, the specific capacity increases from 184.8 mA·h/g (uncoated) to 192.7 mA·h/g (coated) at a voltage of 4.7 V. The coated cathode exhibits a retention capacity of 44.1% at 10 C and 79.7% after 100 cycles [18]. The incorporation of gradient doping using a phosphate polyanion has demonstrated exceptional efficacy in mitigating unwanted reactions of the cathode and electrolyte. This technique substantially augments the thermal stability of the cathode, rendering it chemically inert. Consequently, there is a notable enhancement in capacity retention, which reaches an impressive 92.9%, even when subjected to challenging conditions such as high cut-off voltage (4.5 V) and elevated temperature (55 °C). In stark contrast, the undoped NCM cathode only manages a capacity retention of 55.7%. [19].
3. Application of nanotechnology in anode materials
3.1. Graphite anode
Graphite has been the preferred anode material since the inception of LIB production. This preference primarily stems from its stability and excellent electron conductivity. However, graphite does possess certain constraints. For example, widely used organic electrolytes like diethyl carbonate, and propylene carbonate can react with lithiated graphite irreversibly. These side reactions encompass phenomena such as the exfoliation of graphene sheets and the reduction or decomposition of the electrolyte. [20]. Fortunately, the introduction of a solid-electrolyte interface (SEI), as displayed in Figure 4, provides a protective layer that safeguards the electrode and prevents the dissociation of electrolytes [20].
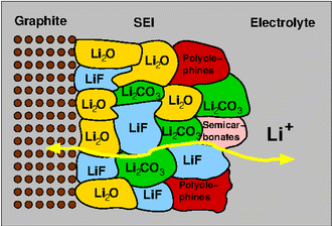
Figure 4. Demonstration of an SEI at the anodes of batteries [20].
An attractive option for anode material involves utilizing a composite of α-Fe2O3 nanoparticles with graphite. These α-Fe2O3 nanoparticles, ranging from 15 to 30 nm in size, were integrated within the layers of graphite, resulting in a layered porous nanostructure. This porous configuration, formed through the organization of graphite sheets, effectively mitigates the negative effects caused by the substantial volume fluctuations experienced by α-Fe2O3 particles during each cycle. Notably, the composite electrode demonstrates exceptional electrochemical performance, boasting an impressive reversible capacity of 1588 mAh g−1 after 100 cycles at 100 mA g−1 [21].
Another potential graphite composite is SiOx/Graphite composite. According to the study done by Zhang et al, the initial Coulombic efficiency (ICE) of SiOx/graphite composite anode was 88%. Following the application of a chemical prelithiation technique to the SiOx/graphite composite anodes, which constituted 95% of the active material mass loading on the electrode, the ICE experienced a notable boost. Specifically, the ICE surged from 88% to an impressive 98% within a mere 2-minute prelithiation period, thanks to the deployment of the aryl lithium reagent impregnation method [22].
4. Conclusion
In this paper, the cathodes discussed include LiCoO2, LiMn2O4, LiFePO4, and ternary NMC. While all these cathodes have their unique properties, structural dissolution can lead to reduced capacity for LiCoO2, LiMn2O4, and NMC. Fortunately, this issue can be addressed through techniques like nano-coating and doping. LiFePO4 exhibits lower electronic conductivity, which can be improved by employing techniques like FC coating or incorporating graphene conductivity additives. In terms of anode enhancement, the implementation of SEI proves effective in preventing graphene dissolution and minimizing side reactions with the electrolyte. Additionally, the exploration of various nanomaterial/graphene composites as an alternative to pure graphene shows promising potential for improving anode performance. While nanotechnology plays a crucial role in enhancing the performance of LIBs, it is imperative to acknowledge its drawbacks. Foremost among these is the potentially high production cost of nanomaterials, owing to the need for specialized equipment and processes. This could result in an overall increase in the manufacturing cost of LIBs. Additionally, the long-term stability and durability of nanomaterials in battery applications may still be under investigation. It is imperative to ensure that these materials can withstand the challenges of real-world use over extended periods.
References
[1]. Reddy M V, Mauger A and Julien C M, et al, 2000 Material 13 1884
[2]. Lain M J and Kendrick E, 2021 Journal of Power Sources 493 229690
[3]. Chen Y, Knag Y and Zhao Y, et al, 2021 Journal of Energy Chemistry 59 83-99
[4]. Myung S-T, Amine K and Sun Y-K, 2015 Journal of Power Sources 283 219-236
[5]. Zhang T, Li D, Tao Z and Chen J, 2013 Progress in Natural Science: Materials International 23 256-272
[6]. Reimers J N and Dahn J R, 1992 Journal of the Electrochemical Society 139 2091
[7]. Scott I D, Jung Y S and Cavanagh A S, et al, 2011 Nano Letters 11 414-418
[8]. Myung S, Izumi K and Komaba S, et al, 2007 The Journal of Physical Chemistry C 111 4061-4067
[9]. Yuan A, Tian L, Xu W and Wang Y, 2010 Journal of Power Sources 195 5032-5038
[10]. De Taeya L L, Teirlynck I and Vereecken P M, 2021 Advanced Functional Materials 31 2105180
[11]. Schuppert N D, Mukherjee S and Bates A, et al, 2020 Energy Storage 2 e143
[12]. Lu J, Chen Z and Ma Z, et al, 2016 Nature nanotechnology 11 1031-1038
[13]. Tarascon J M and Armand M 2001 Nature 414 359-367
[14]. Choe Y S, Han S B, Ahn J H and Kim C, 2021 Solid State Communications 328 114231
[15]. Wang X, Feng Z and Hou Z, et al, 2020 Chemical Engineering Journal 379 122371
[16]. Aurbach D, Markovsky B and Weissman I, et al, 1999 Electrochimica Acta 45 67-86
[17]. Chen Y, Zhang Y and Wang F, et al, 2014 Journal of Alloys and Compounds 611 135-141
[18]. Lee S W, Kim M S and Jeong J H, et al, 2017 Journal of Power Sources 360 206-214
[19]. Ran Q, Zhao H and Wang Q, et al, 2019 Electrochimica Acta 299 971-978
[20]. Jeong S K, Inaba M and Iriyama Y, et al, 2002 Electrochimica Acta 47 1975-1982
[21]. Ma L, Wang Z and Tian S, et al, 2020 Nanotechnology 31 435404
[22]. Zhang X, Hou X and Hou Y, et al, 2023 ACS Applied Energy Materials 6 7996-8005
Cite this article
Liu,J. (2024). The application of nanotechnology in the field of lithium-ion battery. Applied and Computational Engineering,61,24-28.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Reddy M V, Mauger A and Julien C M, et al, 2000 Material 13 1884
[2]. Lain M J and Kendrick E, 2021 Journal of Power Sources 493 229690
[3]. Chen Y, Knag Y and Zhao Y, et al, 2021 Journal of Energy Chemistry 59 83-99
[4]. Myung S-T, Amine K and Sun Y-K, 2015 Journal of Power Sources 283 219-236
[5]. Zhang T, Li D, Tao Z and Chen J, 2013 Progress in Natural Science: Materials International 23 256-272
[6]. Reimers J N and Dahn J R, 1992 Journal of the Electrochemical Society 139 2091
[7]. Scott I D, Jung Y S and Cavanagh A S, et al, 2011 Nano Letters 11 414-418
[8]. Myung S, Izumi K and Komaba S, et al, 2007 The Journal of Physical Chemistry C 111 4061-4067
[9]. Yuan A, Tian L, Xu W and Wang Y, 2010 Journal of Power Sources 195 5032-5038
[10]. De Taeya L L, Teirlynck I and Vereecken P M, 2021 Advanced Functional Materials 31 2105180
[11]. Schuppert N D, Mukherjee S and Bates A, et al, 2020 Energy Storage 2 e143
[12]. Lu J, Chen Z and Ma Z, et al, 2016 Nature nanotechnology 11 1031-1038
[13]. Tarascon J M and Armand M 2001 Nature 414 359-367
[14]. Choe Y S, Han S B, Ahn J H and Kim C, 2021 Solid State Communications 328 114231
[15]. Wang X, Feng Z and Hou Z, et al, 2020 Chemical Engineering Journal 379 122371
[16]. Aurbach D, Markovsky B and Weissman I, et al, 1999 Electrochimica Acta 45 67-86
[17]. Chen Y, Zhang Y and Wang F, et al, 2014 Journal of Alloys and Compounds 611 135-141
[18]. Lee S W, Kim M S and Jeong J H, et al, 2017 Journal of Power Sources 360 206-214
[19]. Ran Q, Zhao H and Wang Q, et al, 2019 Electrochimica Acta 299 971-978
[20]. Jeong S K, Inaba M and Iriyama Y, et al, 2002 Electrochimica Acta 47 1975-1982
[21]. Ma L, Wang Z and Tian S, et al, 2020 Nanotechnology 31 435404
[22]. Zhang X, Hou X and Hou Y, et al, 2023 ACS Applied Energy Materials 6 7996-8005









