1. Introduction
\( {CO_{2}} \) emissions to the atmosphere from daily activities could be attributed to the greenhouse gases which caused severe environmental problems such as global warming, air pollution and sea acidic levels to the society. Most activities have CO2 participated or used up; therefore, this gas is over-released to the environment and causes problems [1]. Global warming is one of the most common issues and is caused by the excessive greenhouse gas emissions to the air. In figure 1 it demonstrates the several pathways of carbon dioxide utilization, such as chemicals raw material, combustion, transportation, storage etc. \( {CO_{2}} \) plays a vital role in almost every side for humans from household necessary appliance usage to agriculture and industry. In the past fifty years, the average temperature has been raised in a range of 1.1 \( ℃ \) which is due to the emissions of \( {CO_{2}} \) and other greenhouse gases [2]. Meanwhile, carbon dioxide is the most emitted gas in all greenhouse gases and based on statistical data, the global atmospheric \( {CO_{2}} \) concentration has increased sharply from 356.56 ppm in 1979 to 419.51 ppm in 2023 with a relative change is 25% [2, 3]. These numbers confirmed that carbon dioxide is accumulated now which is the primary reason the global warming by the temperature rising continuously in fifty years. Thus, finding a technical way to regulate the negative sides caused by carbon dioxide is urgently needed.
It is suggested that using carbon dioxide to produce valuable chemical fuels is a win-win situation which may not only reduce the carbon footprint in general but also generate clean source energy to use as recycled. The \( {CO_{2}} \) conversion could be facilitated under hydrogenation catalysis to produce methanol as a fuel commercially. The catalysis conversion pathways include conventional thermocatalytic, photocatalysis and electrochemical conversion. Currently, the hydrogeneration approach has been implemented on a big scale but for other aspects like photo and electrochemical, it has not been upgraded to quantity production yet. In the following text, a brief description of different \( {CO_{2}} \) conversion pathways will be explained accompanied by catalysts selected based on the availability during conversion.
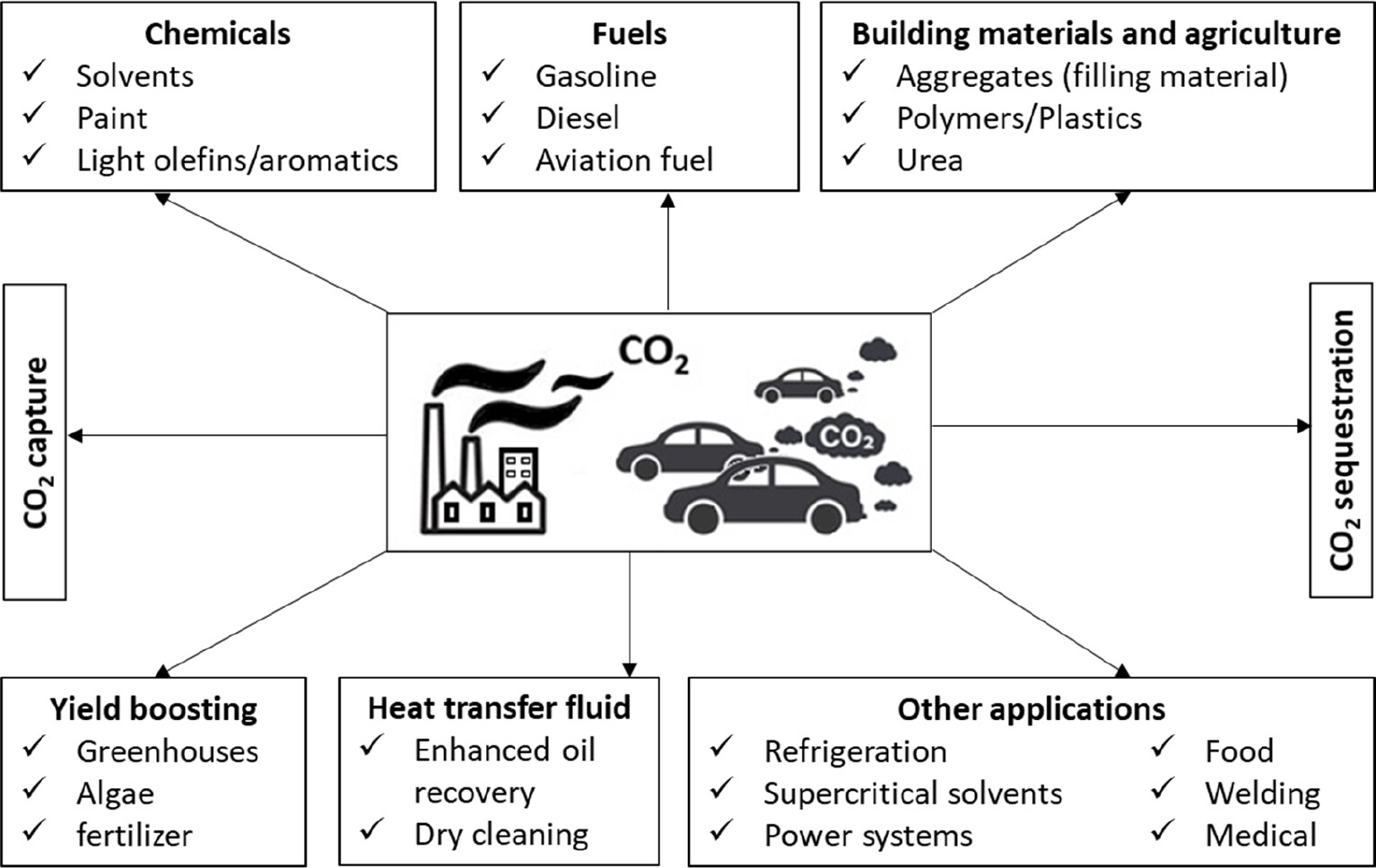
Figure 1. Classification of CO2 utilisation pathways in life [1].
2. Conventional Heterogeneous Catalysis
In methanol production, carbon dioxide could be consumed and thus mitigate its impact on the environment. Three approaches are introduced in the following aim to transfer the undesired greenhouse gas into a worthy fuel product, by implementing strategies which are hydrogenation with catalysts, photocatalysis and electrochemical conversion.
\( {CO_{2}} \) is a thermodynamically stable molecule with a high degree of oxidation and little reactivity. It is necessary to get over a thermodynamic barrier to activate \( {CO_{2}} \) . Hence, a metal catalyst is beneficial to convert \( {CO_{2}} \) and copper-based catalyst is mostly used as the metal catalyst. Hydrogenation using metal catalysts is one of the traditional and major approaches in industry to produce methanol by reacting carbon dioxide. Heterogeneous hydrogenation of \( {CO_{2}} \) is already well-established on the industrial scale under certain harsh conditions [4]. The processes involve methane reforming in addition to adding hydrogen, \( CO \) and \( C{O_{2}} \) into reactions with certain catalysts in order to produce methanol as a clean fuel. The key components and routes under hydrogenation are shown from equations 1 to 5 [5]:
\( C{H_{4}}+{H_{2}}O ↔ CO+{3H_{2}} \) (1)
\( C{H_{4}}+{2H_{2}}O ↔ C{O_{2}}+{4H_{2}} \) (2)
\( C{O_{2}}+{3H_{2}} ↔ C{H_{3}}OH+{3H_{2}}O \) (3)
\( CO+{H_{2}}O ↔ C{O_{2}}+{H_{2}} \) (4)
\( {CO_{2}}+{2H_{2}} ↔ C{H_{3}}OH \) (5)
In this methanol synthesis pathway, firstly in equation (1) & (2) methane reacts with water to generate both \( CO \) and \( {CO_{2}} \) . Equation (3) & (4) indicate that after hydrogen takes participation with the \( {CO_{2}} \) , \( {CO_{2}} \) is adsorbed onto the interface of the catalysts and then breaking the highest C-O energy bond (750 kJ/mol) [6]. Next, hydrogen molecules may participate with \( {CO_{2}} \) to form intermediates. Finally, as shown in equation (5), the intermediates turn out to be methanol eventually as the desired product and water produced as the by-product [7]. The reaction conditions could be strict since the optimal operating temperature is around 230 \( ℃ \) to 270 \( ℃ \) [8] and the ideal pressure range is between 5 Mpa to 10 Mpa [8] to achieve the best yield results.
Scientifically, it has been proven that these meal-based catalysts may do a crucial job on methanol synthesis by improving the stability and activating the overall chemical conversion. Figure 2 is the schematic that illustrates the primary working mechanism of Cu-based catalysts participating with other oxidized additives under hydrogenation reaction to formulate methanol successfully [9]. The interface between the copper-based catalysts and those embedded on an oxidized material surface potentially being the region formate intermediates. After hydrogen comes, the reaction takes place, and the reactor should be used to fully accommodate the entire process to be done. The general order of this thermal catalysis process would be \( {CO_{2}} \) activation, hydrogenation, format and methanol generation [10]. The conversion result indicates that the selectivity for Cu- \( Zr{O_{2}} \) at three experimental temperatures 433 K, 453 K and 473 K are 0.6-100%, 1.2-85.5% and 2.2%-83.0% which are relatively higher than the Cu-ZnO/ \( Zr{O_{2}} \) catalyst [11]. Therefore, the oxide \( Zr{O_{2}} \) phase enables a good performance of \( {CO_{2}} \) absorption for the intermediate completion than Cu-ZnO interaction with a bad selectivity yield.
Overall, conventional catalytic hydrogenation seems to be a well-implemented choice to synthesize methanol. However, the stability of this process is limited, and selectivity is also a constraint. Thus, the following two approaches would be better replacements under certain situations.
Figure 2. Reaction mechanism for CO2 hydrogenation to methanol catalyzed by \( Cu-ZnO \) / \( Zr{O_{2}} \) [11]
3. Photocatalysis Conversion
Although the conventional approaches may be effective and practical in transferring carbon dioxide to methanol, they still have several drawbacks such as unsustainability, environmental issues in terms of pollution and over-weight energy consumption [12]. Regarding the quest for sustainable solutions to mitigate the effects of climate change and focusing on the green chemistry aspect, a promising shift to advanced methods of converting \( {CO_{2}} \) is underway.
The emergence of novel and innovative approaches not only offers a path to reducing greenhouse gas emissions but also creates the potential to revolutionise the way to produce methanol sustainably on an industrial scale, instead of the traditional way of hydrogenation. The new frontier conversions developed as photocatalytic catalytic conversion and electrochemical methodology.
Photocatalysis is a groundbreaking technique that transfers carbon dioxide into methanol by providing an alternative way of \( {CO_{2}} \) utilisation apart from hydrogenation. The primary principle of photocatalysis is capturing solar energy and applying different photocatalysts which may convert \( {CO_{2}} \) to methanol [13]. At its core, photocatalysis utilizes various materials to speed up in terms of photocatalysts implemented for the conversion from solar to chemical [14]. A typical photocatalyst is Titanium Oxide ( \( Ti{O_{2}} \) ) particles which are used in photocatalysis from \( {CO_{2}} \) reduction to methanol synthesis which yields 2330 \( μmol{g^{-1}}{h^{-1}} \) methanol [15]. It is commonly used due to its property with high stability and activation to convert, good operating performance and low cost [16]. Even though \( Ti{O_{2}} \) provides the basic potential to achieve photoreduction to produce methanol, the efficiency is still limited [17]. Experimental results shown that pure \( Ti{O_{2}} \) has a lower efficiency compared with incorporating Ag with \( Ti{O_{2}} \) , which the 1.5 wt% \( Ag/Ti{O_{2}} \) catalyst is the most effective. The amount of \( C{H_{3}}OH \) produced using pure \( Ti{O_{2}} \) and 1.5 wt% \( Ag/Ti{O_{2}} \) after 6 hrs photocatalysis of is 14.9 \( μmol/g \) and 25.2 \( μmol/g \) respectively, which proves the 1.5wt% \( Ag/Ti{O_{2}} \) has a better performance on photoreduction of carbon dioxide [18]. Hence, doping is a technique that enhances efficiency rather than a pure metal photocatalyst.
Indeed, several limitations could be cons caused by less effective production in photocatalysis conversion, it still possibly handles carbon dioxide under the natural condition. Apart from photocatalysis, electrochemical approach alternatively offers another pathway to convert methanol, in a flexible and handy manner.
4. Electrochemical Conversion
The electrochemical aspect of converting \( {CO_{2}} \) into methanol is an innovative and environmentally friendly solution that aims to address the current problem of GHG (Green House Gases). Electrochemical \( {CO_{2}} \) conversion has not been applied on a big scale in the industry but has proven successful in labs. This approach transfers \( {CO_{2}} \) using electricity as the source of energy [19] and allows hydrogenation to methanol. It follows as an electrochemical cell is employed, at the anode side of the cell water is oxidized for electrochemical reduction and at the cathode side, carbon dioxide is reduced as an intermediate product for further conversion. The electrochemical reaction routine for \( {CO_{2}} \) transferring to methanol is shown from equations (6) to (8) [20]:
Cathode: \( C{O_{2}}+6{H^{+}}+6{e^{-}} ⇌ C{H_{3}}OH+{H_{2}}O \) (6)
Anode: \( 3{H_{2}}O⇌1.5{O_{2}}+6{H^{+}}+6{e^{-}} \) (7)
Overall: \( C{O_{2}}+2{H_{2}}O⇌C{H_{3}}OH+1.5{O_{2}} \) (8)
Additionally, the intermediate product could be converted with methanol under an electrochemical catalytic hydrogenation process [21]. Solid metal electrocatalysts and other alloys are beneficial under ambient conditions since their high selectivity and energy efficiency when formulating methanol. It is reported that the faradaic efficiencies for methanol ( \( C{H_{3}}OH \) ) production between electrodes in acidic solution are 1.1% for bulk Cu, 4.9% for nanostructures and 16% for Cu-Au alloys [20, 22]. As a result, alloying copper with other metals could improve the performance of \( {CO_{2}} \) conversion to \( C{H_{3}}OH \) .
This process offers the advantage of utilizing renewable source electricity for the reduction of \( {CO_{2}} \) and subsequent methanol production. The electrocatalysts selected may enhance the overall efficiency and yield higher methanol products. In terms of the long run, this methodology could be deployed to a large scale in industry and other disciplines.
5. Conclusion
In conclusion, carbon dioxide ( \( {CO_{2}} \) ) conversion to methanol ( \( C{H_{3}}OH \) ) could be an environmentally viable approach which brings a sustainable prospect. Even though this conversion process still faces several challenges and limitations yet, its potential could be fully achieved and progressed to the next stage later. Three methodologies discussed each reflects own characteristics on carbon dioxide reduction, including conventional heterogenous catalysis, photocatalysis and electrochemical conversion. Catalysts are required in the conversion process to sort out the selectivity problem to produce the amount of methanol expected. Nevertheless, the main challenges in the different catalysis are the high operating conditions in temperature, pressure and the catalysts selected. Ideal catalysts with high efficiency could be classified as metal alloyed type, doped metal catalyst in electrochemical and photocatalysis conversion. For the electrochemical approach, it is recommended since its flexibility and a high selectivity in \( {CO_{2}} \) reduction. The material type of electrocatalysts should be compared and selected well to avoid the loss of faradaic efficiency. Similarly, photocatalysis is also innovative and attractive by using renewable sources of energy like solar power. In future’s perspective, the conversions needed to scale up in both photocatalysis and electrochemical in order to be implemented massively in industry. The efficiency and product yield results are still limited so far, as the incompleteness in technologies and lack of cost. Lastly, research efforts and attention should be put in this specific area to possibly increase the \( {CO_{2}} \) reduction efficiency in the upcoming years.
References
[1]. Ganji, P., R. K. Chowdari and B. Likozar (2023). "Photocatalytic Reduction of Carbon Dioxide to Methanol: Carbonaceous Materials, Kinetics, Industrial Feasibility, and Future Directions." Energy & Fuels 37(11): 7577-7602.
[2]. Hannah Ritchie, Max Roser and Pablo Rosado (2020) - "CO₂ and Greenhouse Gas Emissions". Published online at ourworldindata.org. Retrieved from: 'https://ourworldindata.org/co2-and-greenhouse-gas-emissions'
[3]. Climate change indicators: Atmospheric concentrations of greenhouse gases. Available at: https://www.epa.gov/climate-indicators/climate-change-indicators-atmospheric-concentrations-greenhouse-gases
[4]. LI, Y.-N., MA, R., HE, L.-N. & DIAO, Z.-F. 2014. Homogeneous hydrogenation of carbon dioxide to methanol. Catalysis Science & Technology, 4, 1498-1512.
[5]. Azhari, N. J., Erika, D., Mardiana, S., Ilmi, T., Gunawan, M. L., Makertihartha, I. G. B. N. & Kadja, G. T. M. 2022. Methanol synthesis from CO2: A mechanistic overview. Results in Engineering, 16, 100711.
[6]. Sajeda, A. A.-S. & Syed Javaid, Z. 2018. Carbon Dioxide Conversion to Methanol: Opportunities and Fundamental Challenges. In: IYAD, K., JANAH, S. & HASSAN, S. (eds.) Carbon Dioxide Chemistry, Capture and Oil Recovery. Rijeka: intechopen.
[7]. Methanol synthesis from CO 2 hydrogenation - chemistry Europe. Available at: https://chemistry- europe.onlinelibrary.wiley.com/doi/epdf/10.1002/cctc.201900401
[8]. Ye, R.-P., Ding, J., Gong, W., Argyle, M. D., Zhong, Q., Wang, Y., Russell, C. K., Xu, Z., Russell, A. G., Li, Q., Fan, M. & Yao, Y.-G. 2019. CO2 hydrogenation to high-value products via heterogeneous catalysis. Nature Communications, 10, 5698.
[9]. Zhong, J., Yang, X., Wu, Z., Liang, B., Huang, Y. & Zhang, T. 2020. State of the art and perspectives in heterogeneous catalysis of CO2 hydrogenation to methanol. Chemical Society Reviews, 49, 1385-1413.
[10]. Borisut, P. & Nuchitprasittichai, A. 2019. Methanol Production via CO2 Hydrogenation: Sensitivity Analysis and Simulation—Based Optimization. Frontiers in Energy Research, 7.
[11]. Arena F et al. Solid-state interactions, adsorption sites and functionality of Cu-zno/zro2 catalysts in the CO2 hydrogenation to CH3OH. Applied Catalysis A: General. 2008;350(1):16-23
[12]. Aleksandr Ya, R. 1989. Modern problems in the synthesis of methanol. Russian Chemical Reviews, 58, 41
[13]. Uil-López, R., Mota, N., Llorente, J., Millán, E., Pawelec, B., Fierro, J. L. G. & Navarro, R. M. 2019. Methanol Synthesis from CO2: A Review of the Latest Developments in Heterogeneous Catalysis. Materials, 12, 3902.
[14]. Hoque, M. A. & Guzman, M. I. 2018. Photocatalytic Activity: Experimental Features to Report in Heterogeneous Photocatalysis. Materials, 11, 1990.
[15]. Olowoyo J.O., Kumar M., Singh B., Oninla V.O., Babalola J.O., Valdes H., Vorontsov A.V., Kumar U. Self-assembled reduced graphene oxide-tio2 nanocomposites: Synthesis, DFTB+ calculations, and enhanced photocatalytic reduction of CO2 to methanol. Carbon. 2019;147:385–397.
[16]. Adekoya, D., Tahir, M. & Amin, N. A. S. 2019. Recent trends in photocatalytic materials for reduction of carbon dioxide to methanol. Renewable and Sustainable Energy Reviews, 116, 109389.
[17]. Qin, G., Zhang, Y., Ke, X., Tong, X., Sun, Z., Liang, M. & Xue, S. 2013. Photocatalytic reduction of carbon dioxide to formic acid, formaldehyde, and methanol using dye-sensitized tio2 film. Applied Catalysis B: Environmental, 129, 599-605.
[18]. B. Yu, Y. Zhou, P. Li, W. Tu, P. Li, L. Tang, J. Ye, Z. Zou. Photocatalytic reduction of CO2 over Ag/tio2 nanocomposites prepared with a simple and rapid silver mirror method
[19]. Kuhl KP, Hatsukade T, Cave ER, Abram DN, Kibsgaard J, Jaramillo TF. Electrocatalytic conversion of carbon dioxide to methane and methanol on transition metal surfaces. Journal of American Chemical Society. 2014;136(40):14107-14113
[20]. Albo, J., Alvarez-Guerra, M., Castaño, P. & Irabien, A. 2015. Towards the electrochemical conversion of carbon dioxide into methanol. Green Chemistry, 17, 2304-2324.
[21]. Al-Rowaili, F. N., Jamal, A., Ba Shammakh, M. S. & Rana, A. 2018. A Review on Recent Advances for Electrochemical Reduction of Carbon Dioxide to Methanol Using Metal–Organic Framework (MOF) and Non-MOF Catalysts: Challenges and Future Prospects. ACS Sustainable Chemistry & Engineering, 6, 15895-15914.
[22]. Zhang, W., Qin, Q., Dai, L., Qin, R., Zhao, X., Chen, X., Ou, D., Chen, J., Chuong, T. T., Wu, B. & Zheng, N. 2018. Electrochemical Reduction of Carbon Dioxide to Methanol on Hierarchical Pd/sno2 Nanosheets with Abundant Pd–O–Sn Interfaces. Angewandte Chemie International Edition, 57, 9475-9479.
Cite this article
Chen,S. (2024). Preview of CO2 utilization in converting to methanol process. Applied and Computational Engineering,61,96-101.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Ganji, P., R. K. Chowdari and B. Likozar (2023). "Photocatalytic Reduction of Carbon Dioxide to Methanol: Carbonaceous Materials, Kinetics, Industrial Feasibility, and Future Directions." Energy & Fuels 37(11): 7577-7602.
[2]. Hannah Ritchie, Max Roser and Pablo Rosado (2020) - "CO₂ and Greenhouse Gas Emissions". Published online at ourworldindata.org. Retrieved from: 'https://ourworldindata.org/co2-and-greenhouse-gas-emissions'
[3]. Climate change indicators: Atmospheric concentrations of greenhouse gases. Available at: https://www.epa.gov/climate-indicators/climate-change-indicators-atmospheric-concentrations-greenhouse-gases
[4]. LI, Y.-N., MA, R., HE, L.-N. & DIAO, Z.-F. 2014. Homogeneous hydrogenation of carbon dioxide to methanol. Catalysis Science & Technology, 4, 1498-1512.
[5]. Azhari, N. J., Erika, D., Mardiana, S., Ilmi, T., Gunawan, M. L., Makertihartha, I. G. B. N. & Kadja, G. T. M. 2022. Methanol synthesis from CO2: A mechanistic overview. Results in Engineering, 16, 100711.
[6]. Sajeda, A. A.-S. & Syed Javaid, Z. 2018. Carbon Dioxide Conversion to Methanol: Opportunities and Fundamental Challenges. In: IYAD, K., JANAH, S. & HASSAN, S. (eds.) Carbon Dioxide Chemistry, Capture and Oil Recovery. Rijeka: intechopen.
[7]. Methanol synthesis from CO 2 hydrogenation - chemistry Europe. Available at: https://chemistry- europe.onlinelibrary.wiley.com/doi/epdf/10.1002/cctc.201900401
[8]. Ye, R.-P., Ding, J., Gong, W., Argyle, M. D., Zhong, Q., Wang, Y., Russell, C. K., Xu, Z., Russell, A. G., Li, Q., Fan, M. & Yao, Y.-G. 2019. CO2 hydrogenation to high-value products via heterogeneous catalysis. Nature Communications, 10, 5698.
[9]. Zhong, J., Yang, X., Wu, Z., Liang, B., Huang, Y. & Zhang, T. 2020. State of the art and perspectives in heterogeneous catalysis of CO2 hydrogenation to methanol. Chemical Society Reviews, 49, 1385-1413.
[10]. Borisut, P. & Nuchitprasittichai, A. 2019. Methanol Production via CO2 Hydrogenation: Sensitivity Analysis and Simulation—Based Optimization. Frontiers in Energy Research, 7.
[11]. Arena F et al. Solid-state interactions, adsorption sites and functionality of Cu-zno/zro2 catalysts in the CO2 hydrogenation to CH3OH. Applied Catalysis A: General. 2008;350(1):16-23
[12]. Aleksandr Ya, R. 1989. Modern problems in the synthesis of methanol. Russian Chemical Reviews, 58, 41
[13]. Uil-López, R., Mota, N., Llorente, J., Millán, E., Pawelec, B., Fierro, J. L. G. & Navarro, R. M. 2019. Methanol Synthesis from CO2: A Review of the Latest Developments in Heterogeneous Catalysis. Materials, 12, 3902.
[14]. Hoque, M. A. & Guzman, M. I. 2018. Photocatalytic Activity: Experimental Features to Report in Heterogeneous Photocatalysis. Materials, 11, 1990.
[15]. Olowoyo J.O., Kumar M., Singh B., Oninla V.O., Babalola J.O., Valdes H., Vorontsov A.V., Kumar U. Self-assembled reduced graphene oxide-tio2 nanocomposites: Synthesis, DFTB+ calculations, and enhanced photocatalytic reduction of CO2 to methanol. Carbon. 2019;147:385–397.
[16]. Adekoya, D., Tahir, M. & Amin, N. A. S. 2019. Recent trends in photocatalytic materials for reduction of carbon dioxide to methanol. Renewable and Sustainable Energy Reviews, 116, 109389.
[17]. Qin, G., Zhang, Y., Ke, X., Tong, X., Sun, Z., Liang, M. & Xue, S. 2013. Photocatalytic reduction of carbon dioxide to formic acid, formaldehyde, and methanol using dye-sensitized tio2 film. Applied Catalysis B: Environmental, 129, 599-605.
[18]. B. Yu, Y. Zhou, P. Li, W. Tu, P. Li, L. Tang, J. Ye, Z. Zou. Photocatalytic reduction of CO2 over Ag/tio2 nanocomposites prepared with a simple and rapid silver mirror method
[19]. Kuhl KP, Hatsukade T, Cave ER, Abram DN, Kibsgaard J, Jaramillo TF. Electrocatalytic conversion of carbon dioxide to methane and methanol on transition metal surfaces. Journal of American Chemical Society. 2014;136(40):14107-14113
[20]. Albo, J., Alvarez-Guerra, M., Castaño, P. & Irabien, A. 2015. Towards the electrochemical conversion of carbon dioxide into methanol. Green Chemistry, 17, 2304-2324.
[21]. Al-Rowaili, F. N., Jamal, A., Ba Shammakh, M. S. & Rana, A. 2018. A Review on Recent Advances for Electrochemical Reduction of Carbon Dioxide to Methanol Using Metal–Organic Framework (MOF) and Non-MOF Catalysts: Challenges and Future Prospects. ACS Sustainable Chemistry & Engineering, 6, 15895-15914.
[22]. Zhang, W., Qin, Q., Dai, L., Qin, R., Zhao, X., Chen, X., Ou, D., Chen, J., Chuong, T. T., Wu, B. & Zheng, N. 2018. Electrochemical Reduction of Carbon Dioxide to Methanol on Hierarchical Pd/sno2 Nanosheets with Abundant Pd–O–Sn Interfaces. Angewandte Chemie International Edition, 57, 9475-9479.










