1. Introduction
Crystallography is a multidisciplinary technique that has played a pivotal role in the advancement of numerous scientific fields. By analyzing the diffraction patterns of X-rays, electrons, or neutrons interacting with crystalline materials, crystallography provides valuable insights into the arrangement of atoms or molecules within a crystal lattice. This ability stems from a series of significant historical developments:
In 1912, Max von Laue and his team observed the diffraction of X-rays through a crystal for the first time, which showed that the atoms in the crystal had a regular internal structure. Subsequently, the Braggs provided a method to calculate the spacing of atoms within crystals through Bragg’s Law, further advancing the accuracy and feasibility of crystal structure analysis.
X-ray crystallography has had a revolutionary impact in the fields of chemistry and biology in particular. For example, the determination of the double helix structure of DNA in 1953 was achieved using X-ray diffraction technology, and this discovery revealed the physical carrier structure of genetic information, which was a major breakthrough in the history of biology.
With the development of technology, electron and neutron diffraction has also been applied to crystallographic studies. Electron diffraction because the wavelength of electrons is shorter than that of X-rays, it can provide higher resolution structural information, especially for small-size samples or films. Neutron diffraction can provide unique information about the position and magnetic structure of atoms, especially for light atoms that are not sensitive to X-rays.
What these diffraction techniques have in common is that they all take advantage of diffraction phenomena as waves interact with periodic arrangements of atoms in a crystal. Diffraction patterns provide an indirect picture of the internal structure of a crystal, and scientists analyze these diffraction patterns to determine the precise positions of atoms and molecules. This approach allows scientists to understand the properties of materials, how biological macromolecules work, and the properties and reaction mechanisms of various chemicals.
This review paper aims to provide a comprehensive overview of the myriad applications of crystallography across various disciplines. From biological macromolecules, material science, earth and planetary science, and so on. This paper can provide corresponding references for crystallography-related researchers.
2. Basic principles of crystallography
Basic Principles of Crystallography Understanding the fundamental principles of crystallography is essential to grasp its applications fully. We discuss Bragg’s law, which is one of the most significant discoveries in the study of crystallography.
Bragg conceptualized the crystal as a collection of parallel planes. As pulses travel through the crystal, a fraction of their energy is reflected off these planes. Such reflections accumulate to form a peak interference pattern, with the separation between the pulses being 2dcosθ, where θ is the angle of incidence relative to the normals of these planes, and d represents the distance between these consecutive planes. Essentially, crystals act akin to a grating, bending specific wavelengths of light. Each incident pulse is thus broken down into a series of subsidiary pulses, which correspond to wavelengths λ, λ/2, λ/3, λ/4, etc., where λ equals 2dcosθ. This concept laid the groundwork for what is known as Bragg’s formula, which was later refined to nλ = 2dsinθ in subsequent publications [1], with θ now representing the angle between the incoming light and the plane. Bragg demonstrated this theory using a simple spectrometer at the Cavendish Laboratory, directing a fine X-ray beam at a piece of mica set at an 80° angle and supported by a thin aluminum sheet. The result was a photographic plate that, after exposure and development, clearly showed the spots of both reflected and incident light [2].
3. X-ray crystallography in chemistry
X-ray Crystallography in Chemistry has revolutionized the field of chemistry by enabling the determination of the three-dimensional structures of small organic molecules, inorganic compounds, and coordination complexes. We explore how X-ray crystallography has been instrumental in elucidating molecular structures.
X-ray crystallography allows scientists to directly observe the three-dimensional arrangement of molecules and atoms. From the diffraction data, the position of the atoms in the molecule can be precisely determined, and a three-dimensional model of the molecule can be constructed (see Figure 1)..
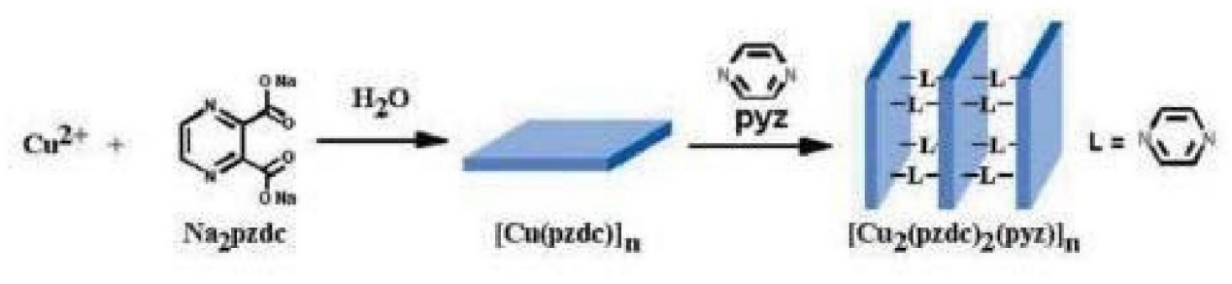
Figure 1. CPL-1’s structure chart
To be more specific, X-ray crystallography stands as a critical component and methodology within contemporary chemical research. A prominent area of investigation involves porous materials, recognized for their roles in gas adsorption and separation, storage, and catalytic activities, exemplified by substances like natural zeolite. To address diverse applications, there is a need to custom-synthesize porous materials such as molecular sieves and polymers, tailored to specific functionalities. X-ray crystallography is employed to ascertain the structural integrity of these synthetics, ensuring the porous architecture aligns with its intended use and monitoring structural variations during its application. Notably, Masami Takata’s research delved into the pore architecture of polydicarboxypyrazine copper salt (Cu-PZDC) pyrazine (L), known as CPL-1, and its proficiency in adsorbing O2 and H2 [3]. The minute granules of CPL-1 necessitate the resolution of its structure through powder diffraction techniques. The documented structure, CPL-1, reveals a layered arrangement connected via L with channels constituting a cavity framed by Cu-PZDC and L, measuring 4 A × 6 A. The O2 absorption process is noted during cooling from 300 K to 90 K, with significant diffraction spectrum alterations observed between 150 K and 130 K, indicating the onset of oxygen absorption and consequent structural modifications (see Figure 2). The structural elucidation was achieved using the Maximum Entropy (MEM) charge density alongside the Rietveld refinement approach, revealing O2’s presence in the channels as molecular entities, with adjacent O2 molecules forming dimers in nearby channels, maintaining a separation of 3.24 A and adjacent dimers being 4.69 A apart, without interacting with channel wall atoms [4].
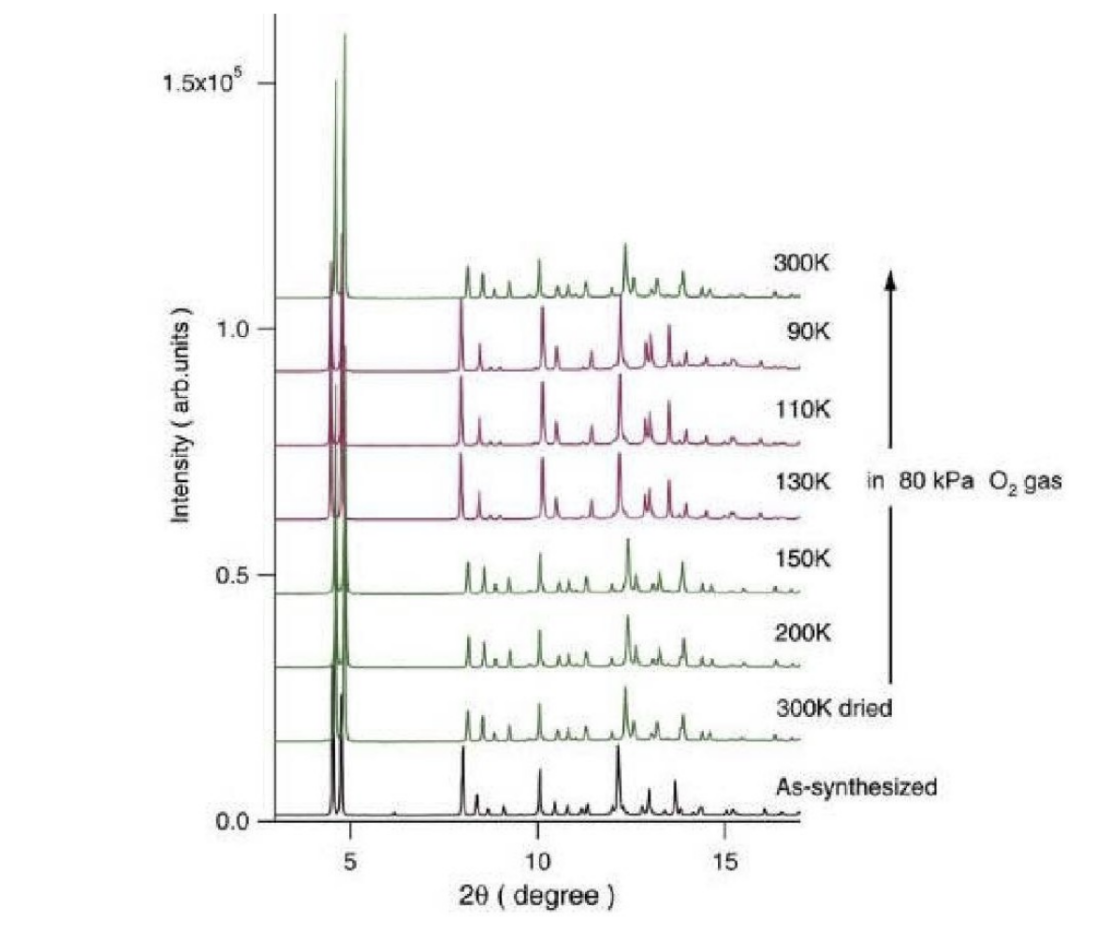
Figure 2. Diffraction spectrum of oxygen absorption process of CPL-1
4. Crystallography in materials science
Crystallography in Materials Science plays a vital role in materials science, allowing researchers to characterize the structure of various materials, including metals, ceramics, and semiconductors. We highlight the applications of crystallography in material design, optimization, and quality control.
Take ceramics for example, the glass-ceramic system typically consists of boron and silicon, forming the fundamental glass network. This structure is augmented with monovalent or divalent alkaline oxides, which aid in restructuring the glass network. Initially, the material is in a glass phase prior to sintering, transforming into a ceramic with a crystalline phase post-nucleation and crystallization during the sintering process. Understanding and controlling the nucleation and crystallization processes are crucial for achieving desired glass-ceramic properties [5]. Effective control over crystallization hinges on proper nucleation. Different thermal treatments yield various grain sizes. Inappropriate nucleation temperatures or brief nucleation periods can result in low crystal nucleus concentrations, potentially leading to coarser grains later. Conversely, insufficient duration during the crystal growth phase may fail to produce the necessary crystal phase percentage. Optimal nucleation temperature and time are essential to achieve a sufficient concentration of nucleation, fostering the growth of fine grains and the desired crystallization rate. Additionally, the temperature and duration of crystal growth are significant; too high temperatures can cause the nuclei to dissolve or distort the sample, while too low temperatures or short durations can lead to inadequate grain growth and crystallization rate [6].
This section of research on glass-ceramic systems provides an in-depth understanding and detailed description of their structure, composition, and transformation processes. In particular, the structure of the basic glass network composed of boron and silicon, and the importance of doped basic oxides in the reconstruction of the glass network are emphasized. This study thoroughly investigated the phase transition of the material before and after sintering, highlighting the critical role of nucleation and crystallization in obtaining ideal glass-ceramic properties.
In addition, the study points to the importance of controlling crystallization, especially effective nucleation is essential to achieving the ideal grain size and crystallization rate. It is very important to analyze how different heat treatment processes affect the grain size, and to discuss the control of nucleation temperature, nucleation time, crystal growth temperature and time. The optimization of these factors is necessary to ensure high quality glass ceramic products.
Still, the study hints at possible challenges in practical applications, such as tightly controlling temperature and time conditions to avoid overgrowth or undergrowth of crystals. For future research, it may be necessary to explore more compositions and treatment methods to further optimize the properties of glass ceramics and expand their applications in industry and other fields. Overall, this study provides valuable insights into the field of glass ceramics and lays a solid foundation for future work.
5. Crystallography in earth and planetary sciences
Crystallography in Earth and Planetary Sciences has been instrumental in studying geological samples and meteorites to gain insights into the Earth’s geological history and the composition of extraterrestrial materials.
In envisioning the future of crystal chemistry, several transformative developments could emerge:
Advanced Predictive Modeling: The integration of artificial intelligence and machine learning could revolutionize crystallography, allowing for the rapid prediction of crystal structures and properties before they are synthesized. This could significantly accelerate the discovery of new materials and drugs.
Nanocrystal Engineering: Advances in nanotechnology might enable the precise manipulation and assembly of crystals at the atomic level, leading to the creation of novel nanocrystalline materials with unprecedented properties for use in electronics, photonics, and quantum computing.
3D Printing of Crystalline Structures: With the maturation of 3D printing technology, it might be possible to print complex crystalline structures directly. This could be particularly impactful in pharmaceuticals, where tailored crystal forms could lead to drugs with improved efficacy and solubility.
6. Conclusion
Crystallography has emerged as a cornerstone technique across various scientific disciplines. Its applications span from understanding the fundamental nature of matter to solving complex biological puzzles and designing novel materials. As technology continues to advance, crystallography remains at the forefront of scientific discovery. This paper lacks a combination of relevant specific cases of the application of crystallography in various aspects. Future research can also combine the development of technologies such as 3D printing to further study the development of crystallography.
References
[1]. BraggWH BraggWL. ThereflectionofX-raysbycrystals[J].ProcRoySoc.191388(A605): 428~438.
[2]. BraggWL.ThespecularreflectionofX-rays[J].Nature.191290(2250):410.
[3]. M Takata. Acta Cryst, 2008, A64:232-245
[4]. Maliton. A century of glory in X-ray crystallography [J]. Advances in Physics, 2014,34(02):47-117. DOI:10.13725/j.cnki.pip.2014.02.001
[5]. Raymond L Brown. IEEE MTT-S Digest., 1994. 1727-1730.
[6]. Wang Yuehui, Zhou Ji, Cui Xuemin, et al. Progress in materials science of low-temperature co-fired ceramics (LTCC) technology [J]. Journal of Inorganic Materials, 2006, (02): 267-276.
Cite this article
Gui,X. (2024). Crystallography applications: A comprehensive review. Applied and Computational Engineering,63,176-180.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. BraggWH BraggWL. ThereflectionofX-raysbycrystals[J].ProcRoySoc.191388(A605): 428~438.
[2]. BraggWL.ThespecularreflectionofX-rays[J].Nature.191290(2250):410.
[3]. M Takata. Acta Cryst, 2008, A64:232-245
[4]. Maliton. A century of glory in X-ray crystallography [J]. Advances in Physics, 2014,34(02):47-117. DOI:10.13725/j.cnki.pip.2014.02.001
[5]. Raymond L Brown. IEEE MTT-S Digest., 1994. 1727-1730.
[6]. Wang Yuehui, Zhou Ji, Cui Xuemin, et al. Progress in materials science of low-temperature co-fired ceramics (LTCC) technology [J]. Journal of Inorganic Materials, 2006, (02): 267-276.









