1. Introduction
In recent decades, as the level of industrialisation has gradually risen, so has the standard of living. However, this progress has come at a cost. The overreliance on human demand for fossil fuels, substantial emissions of industrial gas waste and vehicle exhaust, has led to an escalation in carbon dioxide in the environment in the past few decades. As predicted, global energy consumption will increase by 0.5 times of the current level by 2040. At that time, fossil fuel resources will not be enough to supply the growing energy demand [1]. This has contributed to a range of environmental issues, including environmental warming, glacier melting, and sea level rising [2]. Consequently, the demand for new energy sources and pollution control is becoming increasingly urgent. Thus, methods of reducing CO2 emissions and reusing it have become global research focus. Currently, the techniques for reducing CO2 emissions are mainly categorised: CO2 capture and storage [3] and CO2 chemical conversion and consumption [4]. However, capturing and storing CO2 is not a long-term applicable solution; merely relocating the gas spatially. This method has also brought up practical challenges, such as possible leaks and storage costs [3]. Different from it, the CO2 chemical conversion and consumption can reduce the CO2 content and solve the environment crisis. At the same time, it can also convert CO2 into valuable fuels or other organic substances, which can lead to the fuel recovery and solve the energy crisis. Therefore, this method has become one of the research hotspots worldwide.
Given the high stability of CO2’s structure, breaking the C=O bonds requires substantial energy [5]. By the inspiration from natural photosynthesis, researchers have attempted to use clean, economical, and safe solar energy for CO2 photoreduction. A significant breakthrough occurred in 1972 when Honda and Fujishima used a TiO2 electrode to reduce CO2 to CH3OH and CHO by photocatalysis [6], demonstrating the feasibility of using photocatalysis to convert CO2. Additionally, by varying the number of electrons and protons involved in the redox reaction process, it is practicable to obtain different valuable molecule chemical materials or fuel products, such as CO [7], CH4 [8], HCOOH [9], CH3OH [10] and, etc. As a result, this approach achieves a closed carbon cycle, offering promising avenues for further research and development [11].
Metal-organic frameworks (MOFs) generally refer to the periodic multidimensional skeleton structure formed by the metal ions and bridged organic ligands. They are porous solid materials included by organic-inorganic hybridisation. The earliest appearance of MOFs can be traced back to the research of Robson and Hoskins in 1989 [12]; they prepared the 3D network coordination compounds and inferred that they may have larger pores and holes than zeolite molecular sieve from which began the research boom of MOFs. However, the early synthesised MOFs’ pore structure and skeleton is not very stable and easier to collapse. It was not until 1995 that Yaghi et al. reported a coordination compound with a stable pore structure prepared by the reaction of trimesic acid (BTC) and Cobalt (Co), and called it MOF [13], which made it possessed practical. With the rapid development of modern X-ray crystallisation, the original problems in crystal structure testing and structure analysis have been well solved, and the rapid growth of new crystal data has extensively promoted the research progress of MOFs.
Compared with traditional photocatalytic materials, MOFs have the following advantages: (1) The special pore structure of MOFs is conducive to CO2 adsorption and enhances their catalytic performance. (2) The structure of MOFs can be functionalised and modified; (3) MOFs can be combined with other functional materials to form composite materials, maintaining the structural properties of MOFs while also possessing the excellent properties of additional materials, etc. [14, 15]. Therefore, they are used in catalysis, gas separation, storage and conversion, dye decomposition, etc. Among them, catalysis is one of the fastest-growing application fields of MOFs with the longest research time [16]. Recently, with more research, MOFs have shown the following advantages in photocatalysis and electrocatalysis. On the one hand, MOFs’ high specific surface area and unique porous structure can make active sites evenly dispersed. Because its internal environment can be regulated by synthetic means, its light absorption range can also be adjusted, making the reaction easier. On the other hand, MOFs can remain stable in solution, even in moderately acidic or alkaline solutions [17]. In the reaction system, they can be simply separated so that they can be reused. Therefore, they have high economic and environmental benefits.
In the process of photocatalysis, as the photon energy is higher than or equal to the band gap of MOFs, firstly, MOFs can generate electrons with reducing ability and holes with oxidising ability after receiving photoexcitation. Secondly, the electron-hole pair moves to the surface of the catalyst [18]. During their movement, part of the electron-hole pairs will recombine, and the other part will reach the active site to participate in the photocatalytic reaction. Thus the CO2 has been adsorbed and reduced. The more CO2 is adsorbed, the more number of active sites of the photocatalyst can be contacted, which is also beneficial for the reduction reaction. The unsaturated coordination site of metal ions in MOFs can be used as the active site of catalytic reactions [19]. The organic ligand and large surface area are beneficial for loading catalytic active molecules and metal active components [20]. In the adsorption and separation of CO2, MOFs have good CO2 adsorption capacity and unique semiconductor behaviour [21]. It has a good application prospect in CO2 photoreduction.
However, MOFs’ visible light-capturing ability is weak, and the electron-hole recombination is relatively severe. The existing research directions for improve the efficiency of photoreduction are mainly divided into metal node modification and functional modification of organic ligands, embedded semiconductors, precious metal particles and other materials with light absorption ability, and prepared MOFs-derived materials. This paper is a review of the MOFs for photocatalytic CO2 reduction. It discusses recent developments in photoactive MOFs and their application in CO2 photoreduction reactions. This paper is composed of (1) functional modification of pure MOFs, (2) MOF-composite materials and (3) MOF-derived materials as photocatalysts. In addition to these, it also makes the prospects for future development.
2. Function modification of pure MOFs
MOFs have semiconductor-like properties, so that pure MOFs can be used as photocatalytic materials. The presence of organic ligands can stabilise and support the frame structure and can excite electrons under specific wavelengths of sunlight, then move electrons to the activated metal clusters to participate in the photocatalytic reactions [22,23], giving MOFs semiconductor properties. Reasonable modification of the frameworks of MOFs can effectively reduce the energy difference between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO), thereby improving the light capture ability of MOFs and enhancing the catalytic performance of MOFs for photocatalyst CO2 reduction. Common methods include introducing organic ligands with photosensitive functional groups, electron-rich conjugated systems, or metallising organic ligands of MOFs.
2.1. Amino-modified organic ligands
The unique structures of MOFs have led to widespread interest in their utilisation for photoreduction of CO2 under visible light exposure. The amino group presently is the most extensively applied among the active functional groups. Its potent electron-donating capacity serves a dual purpose. On the one hand, acting as a weak base, it facilitates the adsorption of acidic gases like CO2, thereby augmenting CO2 adsorption capability. On the other hand, the incorporation of auxochrome groups causes a displacement of the absorption wavelength of MOFs towards the visible spectrum. This shift intensifies the absorption of light and consequently substantially elevates the catalytic proficiency of MOFs in CO2 photoreduction. In organic ligand modifications, NH2-MIL-125(Ti) and other amino-functionalised MOFs have shown promise in improving the photocatalytic ability for CO2 reduction (MIL= Materials of Institute Lavoisier).
NH2-MIL-125(Ti) is synthesised by introducing amino-functionalised organic ligands (such as H2ATA) within the framework of MIL-125(Ti), considering its structure [24]. The process typically entails mixing a Ti precursor, such as titanium tetrachloride (TiCl4), with a linker molecule containing amino groups, such as 2-aminoterephthalic acid (ATA). The mixture is then heated under appropriate conditions, allowing the Ti nodes and ATA linker to assemble and form the porous framework structure of NH2-MIL-125(Ti). The resulting MOFs possess a substantial surface area and exhibits remarkable thermal stability, making it suitable for many prospective applications, such as gas separation and catalysis. The presence of amino groups does not compromise the structural stability of the parent MOF, leads to improvements in optical absorption and CO2 adsorption, but affects the charge transfer within the metal cluster, making the NH2-MIL-125(Ti) display a wide absorption range. The broadened absorption range allows the MOF to harness more visible light energy for photocatalytic processes. Then, the introduction of amino-functionalised ligands enhances the interaction between NH2-MIL-125(Ti) and CO2 molecules. The amino groups provide weak basic sites that can effectively interact with the acidic CO2, leading to higher CO2 adsorption capacity [25]. NH2-MIL-125(Ti) demonstrates a significantly greater CO2 uptake ability than unmodified MIL-125(Ti).
In addition to this, it exhibits enhanced photocatalytic ability for CO2 reduction. When exposed to visible light, NH2-MIL-125(Ti) can catalytic the reduction from absorbed CO2 to HCOO- with the presence of sacrificial agent like triethanolamine (TEOA) and triethylamine (TEA). The excited electrons from the amino-functionalised ligands transfer to Ti(IV), reducing it to Ti(III), which plays an indispensable role in promoting CO2 reduction (Figure 1). This is the first time that photoactive Ti-MOFs have been used for the CO2 photoreduction. TEOA, as an electron donor, is essential for photocatalytic process, as it provides the necessary electrons for holes, simultaneously suppressing the recombination of electron-hole pairs in reducing Ti(IV) as well as subsequent CO2 conversion [26].

Figure 1. Under visible light irradiation, the speculated mechanism of NH2-MIL-125(Ti) CO2 photoreduction [24].
NH2-UiO-66(Zr) refers to a variant of the Metal-organic Framework UiO-66(Zr) that has been functionalised with amino groups [24] (UiO=University of Oslo). ATA is used as a ligand during the synthesis process to achieve the functionalisation. ATA contains amino (NH2) groups that can form coordination bonds with the zirconium ions present in UiO-66(Zr). These amino groups serve as anchor points for the attachment of the ligand to the metal nodes, leading to the NH2-UiO-66(Zr). This modification has been shown to improve the MOF’s photocatalytic activity and its light absorption capabilities.
A prominent benefit of NH2-UiO-66(Zr) is its improved adsorption capacity for CO2. This makes it particularly interesting for applications in CO2 capture and sequestration. Moreover, this MOF demonstrates enhanced photocatalytic activity, which can convert the adsorbed CO2 into formic acid, a useful industrial chemical. An interesting modification of NH2-UiO-66(Zr) involves partially replacing ATA with 2,5-diaminoterephthalic acid (DTA) to create a mixed-ligand MOF [27]. This results in further enhancements in photocatalytic performance and spectral absorption range. The photocatalytic reaction mechanism of it is in Figure 2.
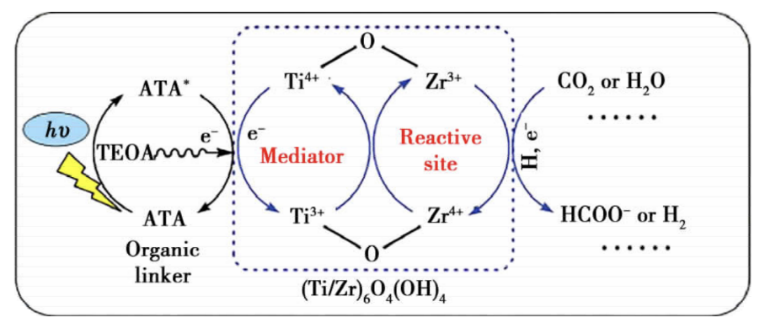
Figure 2. Photocatalytic reaction mechanism of NH2-UiO-66(Zr/Ti) [27].
Furthermore, post-synthetic modification (PSM) of UiO-66(Zr) with a catechol-functionalised organic ligand (catbdc) and incorporation of Cr(III) and Ga(III) metal ions resulted in M(III)-monocatecholato functionalised MOFs with improved visible light absorption ability and electron transfer properties [27]. The modified MOFs showed higher photocatalytic activity for CO2 reduction, with UiO-66-CrCAT demonstrating a high light responsiveness and formic acid yield. NH2-UiO-66(Zr) also demonstrates higher catalytic ability than UiO-66(Zr) in various reactions, such as cross-aldol condensation and jasminaldehyde synthesis [28]. This multi-catalytic activity is attributed to these MOFs’ NH2 groups and oxygen atoms.
Recently, as the abundant iron resources on Earth, Li et al. also studied the photocatalytic reduction from CO2 to HCOO- by three iron-based MOFs: NH2-MIL-53(Fe), NH2-MIL-101(Fe), and MIL-88B(Fe) [29]. They proposed that the dual excitation pathway present in the photocatalytic process is the main reason for the enhanced catalytic performance. Namely, electrons can be directly excited through Fe-O clusters. From O2- to Fe3+, the ligand can absorb light, generate electron transfer to Fe3+ reduce to Fe2+, and undergo a CO2 reduction reaction. The combined action of two excitation pathways significantly boosted the photocatalytic activity compared to the parent MOFs. Similarly, TEOA serves as an electron sacrificial agent to supplement the consumed electrons, enabling the catalytic reaction to continue. Additionally, Incorporating Ti into NH2-UiO-66(Zr) to create NH2-UiO-66(Zr/Ti) further enhances its photocatalytic ability. The addition of Ti facilitates metal-metal charge transfer and promotes charge transfer from the ligand to the Zr-O centre, thereby improving the photocatalytic reduction of CO2.
In conclusion, the functionalisation of MOFs with amino groups, incorporation of Ti or other substituents in bimetallic or modified MOFs, and the introduction of electron-withdrawing groups enhance their photocatalytic and catalytic activities. This expands their potential applications in CO2 reduction and other reactions, positioning them as promising tools for addressing environmental challenges such as greenhouse gas emissions.
2.2. Porphyrin-modified organic ligand
Porphyrins are a class of macromolecular heterocyclic compounds with a nearly planar 18 π electron-rich structure. In natural photosynthesis, antenna light collection is accomplished through porphyrin-like pigments [30]. Porphyrin organic ligands are rigid, so porphyrin-based metal-organic frameworks generally have good stability. In addition, porphyrin is an important photosensitive compound due to its highly conjugated aromatic electron system, which can broaden the light absorption of MOFs to the entire visible light region, with high light absorption efficiency and intense interaction with CO2 [31]. Therefore, it is widely used in CO2 photocatalytic reduction reactions.
Liu et al. [32] reported on the first rhodium porphyrin metal-organic skeleton (Rh-PMOF-1). Firstly, they prepared the Rhodium porphyrin complex Rh(TCPPCO2Me)Cl (TCPP = tetrakis(4-carboxyphenyl)porphyrin) with ZrCl4), by hydrolyzing it, they obtained Rh(TCPP)Cl. Then, they put all the raw materials together in a glass vial, including Rh(TCPP)Cl, ZrCl4, benzoic acid, etc. After 48 hours at 120°C, they obtained the red crystals——Rh-PMOF-1. Their study revealed that the material had a greater adsorption ability for CO2 at 298K compared to MOFs without porphyrin modification, which is conducive for enhancing the photocatalytic activity of MOFs. In addition, they also reported that the selective catalytic reduction rate of HCOO- in this reaction is as high as 99%, which has good application prospects.
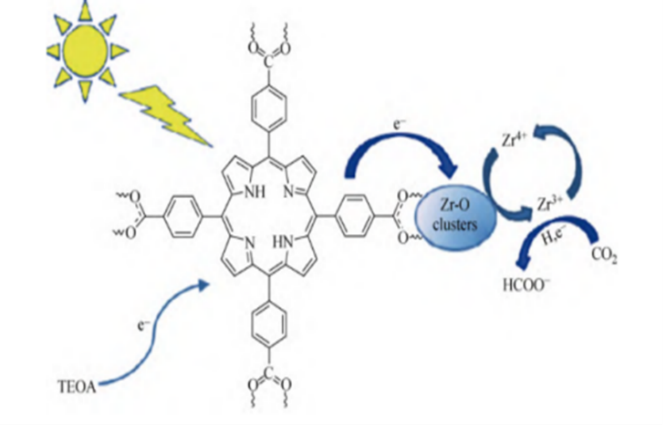
Figure 3. Photocatalytic mechanism of the double catalytic centres in Rh-PMOF-1 [33].
Based on experimental and theoretical results, it is inferred that there are dual catalytic centres in this reaction. As the “antenna”, the metal porphyrin ligand first captures photons to form an excited state, generates photogenerated electrons and transfers them to the metal Zr6O8 cluster and the metal Rh porphyrin ring. Subsequently, a CO2 reduction reaction is carried out (Figure 3). During the reaction, the sacrificial agent TEOA offers electrons to consume the holes for the reaction smoothly. In addition, the team also studied the application of bimetallic PMOF in this direction and reported a Cu Al/PMOF [33]. Liu et al. measured the adsorption capacity for CO2 and proved it was much higher than that of Al/PMOF, resulting in a nearly seven times increase in the amount of methanol precipitated from the reaction. The results indicate that incorporating Cu ions is helpful in improving the adsorption capacity of photocatalysts for CO2. It is speculated that CO2 can adsorb at the copper site, causing linear CO2 molecules to bend, thereby reducing the reaction potential energy and enhancing the catalytic reaction efficiency.
Feng [34] synthesised a highly stable zirconium-based porphyrin PCN-222 (Porous Coordination Network), also known as MOF-545, using Fe-TCPP as the ligand and highly stable ZrCl4 clusters as the nodes. Xu et al. [35], through a series of experiments, found that this material exhibits the visible light absorption ability of porphyrin ligands, coupled with its CO2 adsorption capacity and high specific surface area, which can prominently enhance the photocatalytic efficiency of CO2 reduction to HCOO-. Their research speculates that the mechanism of this process is that the porphyrin ligand H2TCPP serves as the light absorption centre, and the photogenerated electrons generated after absorption transition to the Zr-O cluster, which will reduce Zr(IV) to Zr(III). At the same time, Zr(III) acts as a reducing agent, reducing CO2 to HCOO- (Figure 4). TEOA is also an electron sacrificial agent that participates in the reaction. In addition, the ultrafast transient absorption spectrum indicates that in PCN-222, the appearance of ultra-long lifetime electron trap states significantly suppressed the recombination of electrons and holes during the transfer to metal clusters, thereby improving the efficiency of CO2 photoreduction.
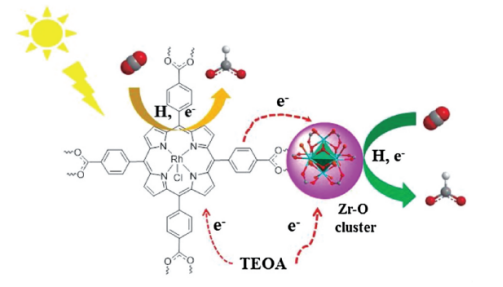
Figure 4. Photocatalytic mechanism of PCN-222 photoreduction CO2 [34].
2.3. Metallising organic ligands
In recent years, transition metals have shown good catalytic activity for CO2 photoreduction in homogeneous systems such as iron, copper, cobalt, manganese, etc. In addition, precious metal complexes such as rubidium and iridium have also been widely used as photosensitisers or homogeneous photocatalysts due to their good photoresponse ability [36-41]. The structure of MOFs has unique adjustability, which can be adjusted by fixing metal complexes into the MOFs framework, combining their advantages, thereby adjusting the function and structure of MOFs. After introducing metal complexes, the photocatalytic ability of MOFs can be enhanced, and the rapid deactivation of active centres can also be inhibited [42].
Elcheikh et al. [43] synthesised a novel Zr-based MOF, AUBM-4 (AUBM=American University of Beirut Materials), using bis(4’(4-carboxylphenyl) pyridinyl) Ru(II) complex as ligand. The preparation process of AUBM-4 material is summarised in Figure 5. This complex exhibits excellent absorbance of the metal complex Ru(II), and the time-resolved photoluminescence (TRPL) spectrum results show that the recombination of ALBM-4 electron-hole pairs is effectively suppressed, resulting in a longer fluorescence lifetime. The density functional theory calculation (DFT) results indicate that the catalytic centre of the CO2 photoreduction reaction is most likely located on the complex ligand. After Ru absorbs light, it generates electron-hole pairs, and electrons move to the complex ligand to form free radical anions, thereby reducing the adsorbed CO2 to HCOO-. The sacrificial agent TEOA will reduce the holes (Figure 6).
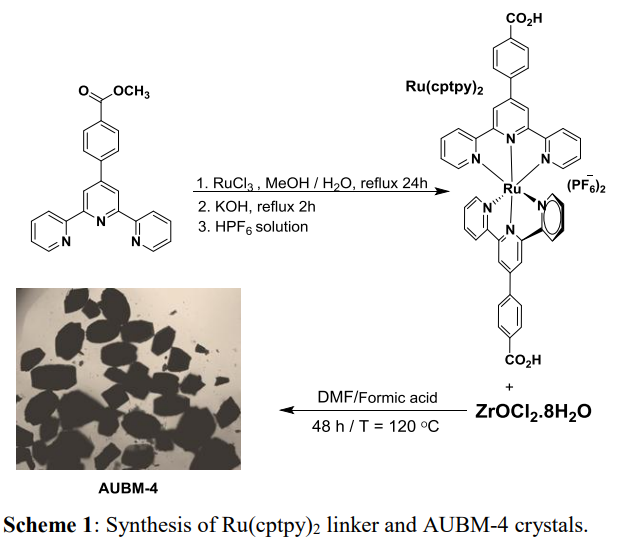
Figure 5. Process of synthesizing AUBM-4 through Ru(cptpy)2 [43].
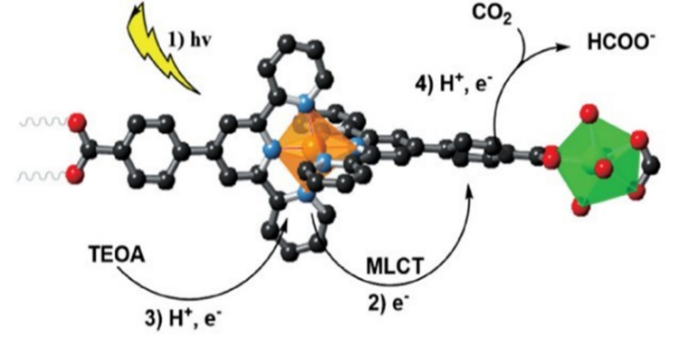
Figure 6. The mechanism of AUBM-4 catalyzed CO2 photoreduction [43].
Although Ru and Ir complexes have quite ideal application prospects in photosensitisers (PSs), developing catalysts for non-noble metal complexes is another hot field of current research due to their limited reserves, expensive, and high toxicity. Feng et al. [44] reported that the multifunctional MOFs comprising Cu-PSs and Re-based CO2 reduction catalyst (mPT-Cu/Re) were prepared. They used the Earth’s most abundant Cu complex as PS-ligands and the Re complex as the active centre in the reaction. It shows an excellent catalytic performance for the reduction reaction from CO2 to CO, with a turnover value (TON) of 95 times that of a homogeneous system under the same conditions. Cohen et al. [45] introduced Cr(III) and Ga(III) complexes into the UiO-66’s framework through a post-synthesis strategy. This operation enhanced the modification of the metal complex and metallised the organic ligand, promoting the electron transfer process within the MOF and enhancing the photocatalytic ability.
Despite the above advantages, due to the poor stability and difficult separation of metal complexes in the catalytic process, they often cause environmental pollution and cannot achieve the recycling and reuse of photocatalysts, resulting in poor atomic economy. Therefore, this is also one of the problems that researchers need to work hard to solve and improve in the future.
3. MOF-based composite materials
The pure MOFs photocatalyst still needs to be improved, such as its poor response to visible light and easy-to-generate photogenerated electron-hole recombination. The improvement of photocatalytic CO2 reduction efficiency depends on separating photogenerated charges and inhibiting charge recombination [46]. Therefore, to improve catalytic performance, MOFs can be combined with other materials to quickly migrate photogenerated electrons within the composite material. The material produced by this method has the dual characteristics of the parent MOFs and the binding material. It can cause more efficient charge separation while improving the selectivity of photocatalysts. The commonly used method is to design MOF-based composite materials with high catalytic activity by combining MOFs with semiconductors, precious metals, etc [47].
3.1. Semiconductor-MOF composites
TiO2, as a classic photocatalyst, has been extensively and deeply explored since its excellent CO2 photoreduction activity was discovered in 1972 [6]. However, it still has some disadvantages, such as high electron-hole pair recombination rate and low CO2 adsorption capacity. To improve the photocatalytic effect of TiO2, researchers have proposed combining MOFs with TiO2 for CO2 photoreduction.
Wang et al. [48] modified ZIF-67 (Zeolitic Imidazolate Framework) with a multifunctional amorphous TiO2(a-TiO2) layer using a hydrothermal and low-temperature calcination method. The resulting composite, ZIF-67@a-TiO2, exhibited improved conversion efficiency and selectivity for CO2 photoreduction under visible light. The a-TiO2 layer facilitated light scattering, suppressed charge recombination, introduced mesoporous properties, and inhibited photocorrosion, enhancing performance and stability. TiO2/HKUST-1 (Hong Kong University of Science and Technology-1) catalysts were prepared by functionalising TiO2 nanocrystals with phosphonic acid (PHA) using a slow diffusion method. The resulting TiO2/HKUST-1 nanocomposite demonstrated significantly higher photocatalytic efficiency for CO2 reduction to CH4 under sunlight than the individual components. Figure 7 [49] presents a schematic illustration of the mechanism involved in the reduction of CO2. When the reaction system is exposed to visible light, the photosensitiser absorbs the light, leading to the generation of photogenerated electron-hole pairs and an excited state ([Ru(bpy)3]2+*). The presence of the ZIF-67@a-TiO2 layer enhances the light capture capacity of the photosensitiser through multiple scattering effects. The photoinduced electrons are rapidly injected into the a-TiO2 layer, where surface defects temporarily trap them. Subsequently, these electrons are transported to ZIF-67 and react with CO2. The a-TiO2 layer also acts as a blocking layer, impeding the reverse flow of electrons as well as reducing surface recombination, thus facilitating the acceleration of CO2 reduction. Furthermore, the robust structure of the a-TiO2 shell enhances the stability of the reduction process during cycling.
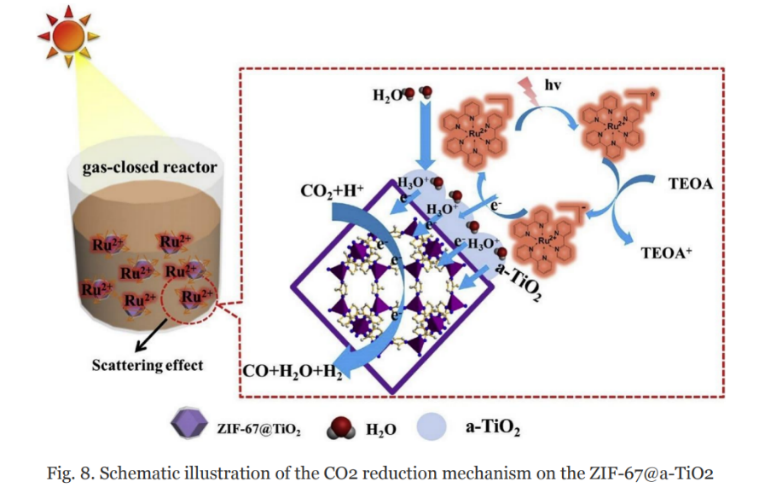
Figure 7. Schematic illustration of the CO2 photoreduction mechanism by ZIF-67@a-TiO2 [49].
Except for TiO2, BiVO4 is another highly regarded photocatalyst according to its exceptional photocatalytic activity. When subjected to light within the visible spectrum, BiVO4 can generate electron-hole pairs, which are crucial in various photochemical reactions. BiVO4’s optimal band-edge positions and appropriate bandgap contribute to its efficiency as a photocatalyst. To understand the impact of Cu on the photoactivity of the BiVO4-Bi2O3 semiconductor, researchers investigated the CO2 photoreduction catalyst by CuO [50]. They analysed the concentrations of the resulting products after a 2-hour photoreaction, setting the experimental conditions based on previous studies, including temperature, supporting electrolyte, pH, and reaction time. The findings, depicted in Figure 8, revealed variations in the concentrations of methanol and acetone, the products of CO2 reduction, depending on the amount of Cu found within the BiVO4-Bi2O3 photocatalyst. Interestingly, the types of molecules produced remained consistent across all cases. However, higher levels of copper doping (>1.0%) did not improve the photoactivity for CO2 reduction. Instead, the concentrations of methanol and acetone decreased below the levels achieved with the unmodified BiVO4-Bi2O3 semiconductor. This indicates that excessive copper doping may introduce recombination centres, leading to a decline in photocatalytic performance.
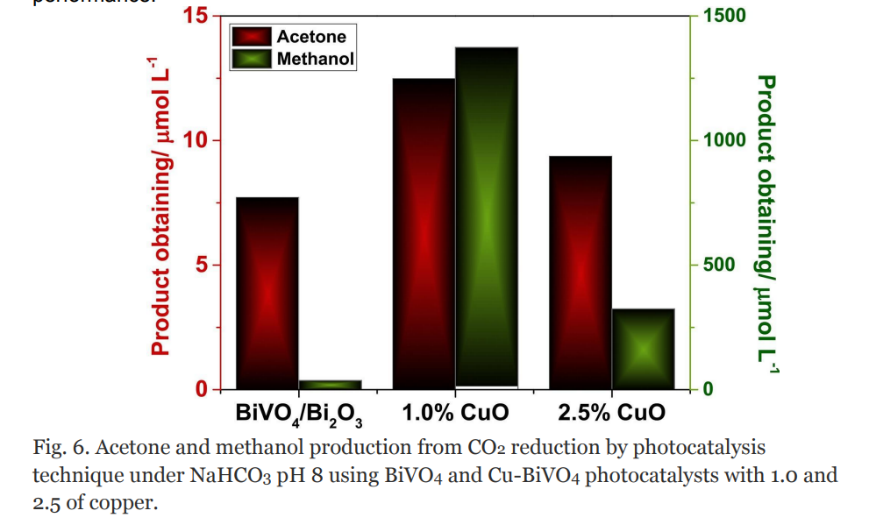
Figure 8. The chart of Acetone and methanol production from CO2 photoreduction by using BiVO4 and Cu-BiVO4 photocatalysts [50].
3.2. Rare earth-MOF composites
Rare Earth Metal-Organic Frameworks (RE-MOFs) share several characteristics with other MOF families. Nevertheless, they also unveil distinct structures and attributes attributed to the distinctive characteristics of RE elements, such as their high coordination numbers and unique optical properties [51]. Endowed with favourable Lewis acid characteristics, RE compounds hold promise as catalysts. Mirroring other MOF families, RE-MOFs leverage consume CO2 as a reactant, ingeniously adsorbing and subsequently converting it into valuable refined chemicals [52].
As per the IUPAC classification, the RE elements primarily encompass the 15 lanthanides positioned within the periodic table (Figure 9) [53]. The RE elements’ concentration within their primary minerals seldom exceeds 10–15%, while their crustal abundance fluctuates from 0.5 to 60 parts per million. Notably, nearly all RE elements maintain a stable trivalent oxidation state (Ln3+), although certain RE elements also endure alternative oxidation states (e.g., Eu2+, Ce4+, Sm2+, Pr4+, Yb2+, and Tb4+) [54]. The preponderance of lanthanides existing in the trivalent oxidation state safeguards their heightened photostability, facilitating well-defined 4f to 4f transitions characterised by precise luminescence spectra spanning from ultraviolet-visible regions to near-infrared (NIR) regions [55].
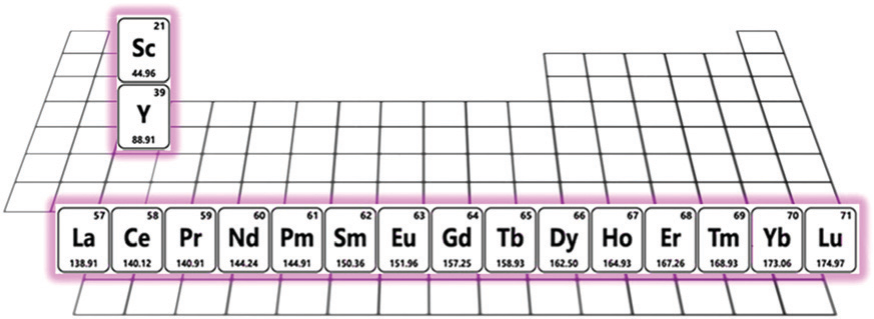
Figure 9. A periodic table which is introduced the rare-earth (RE) elements [53].
Due to constraints on f-f transitions between different configurations, the absorption effect of Ln3+ is weak [56]. However, it can be suppressed by integrating Ln3+ ions into the crystalline framework of MOFs. Within the coordination framework, the ligand acts as an antenna, absorbing photons to obtain energy [57]. Subsequent radiative transitions of absorbed photon energy (T1) from the antennas to the emitting states of RE nodes, facilitated by ligand-metal charge transfer (LMCT) mechanisms, culminate in the emergence of well-defined, sharp, and narrow luminescent emissions characteristic of RE-MOFs [55, 58].
There are seven distinct synthesis methods for RE-MOFs, each characterised by unique features and requirements. These methods encompass both traditional and novel approaches, with one of them offering the ability to generate a wide array of RE-MOF sizes. One prominent technique is the solvo(hydro)thermal synthesis method [51], which employs electrical heating to precipitate RE-MOFs directly under autogenous pressure at varying temperatures (100-300℃). This approach requires hours to days for synthesis. Alternatively, microwave irradiation can be employed to grow RE-MOFs under autogenous pressure at lower temperatures (<180℃), resulting in synthesis times ranging from minutes to hours. This method offers the advantage of straightforward reaction conditions for controlling shape, size, or crystallinity, and it accelerates reactant reactivity, especially within a microwave oven. However, products may contain contaminants due to unreacted ionic elements, and energy consumption can be substantial.
Another method, the sonochemical synthesis, utilises ultrasonic waves to generate RE-MOFs of varying sizes through a simple or modulator-based technique. This method assembles RE-MOFs within minutes to hours using atmospheric-pressure, low-temperature (25-100℃) ultrasonic vapour-phase scattering. The resulting RE-MOFs exhibit high crystallinity in diverse forms like nanoparticles and nanotubes. Nonetheless, the quantitative yield is modest, and the increase in ultrasound frequency may compromise the thermodynamic stability of RE-MOFs [59, 60].
Extensive exploration into lanthanide chemistry has spotlighted the exceptional reactivity of the Eu(II) ion in reduction processes [61, 62]. A notable study by Kong et al. in 2018 [63] featured the Eu-Ru(phen)3-MOF (phen = 1,10-phenanthroline). This remarkable structure entailed Ru(phen)3-derived tricarboxylic acid linkers interconnecting binuclear nodes of Eu(III)-clusters. This distinctive configuration operated as a photocatalyst, deftly harnessing and channelling solar energy into chemical energy. Figure 10 shows the catalytic mechanism of photoreduction from CO2 to HCOOH. The photoexcitation of metal ligands initiated the injection of electrons into nodes, engendering dinuclear {Eu(II)}2 active sites that selectively executed CO2 conversion through an impressive two-electron process, clocking a remarkable reduction rate. A standout feature is the self-assembled nature of the Eu-Ru(phen)3 MOF, underscoring the prowess of selective CO2 photoreduction within the Ln-MOF realm. Noteworthy is its superior photocatalytic activity when pitted against NH2-MIL-125(Ti) [64], NH2-UiO-66(Zr) [65], PCN-222 [66], and certain light-responsive semiconductors [67, 68]. This augmented efficacy owes its credit to the robust photosensitising and light-harvesting attributes of the Ru(phen)3 group, particularly evident in the visible light spectrum.
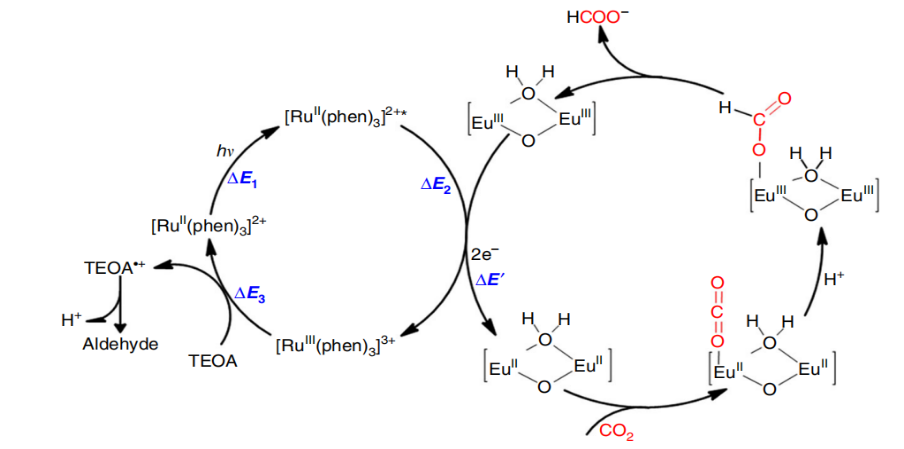
Figure 10. The catalytic mechanism of photoreduction from CO2 to HCOOH [63].
The synthesis of this exceptional RE-MOF involves encapsulating Eu(NO3)3·6H2O, H3L, 2-FBA, and DMF within a 20 mL Teflon-lined autoclave. This amalgamation is subjected to a 105°C temperature over a span of 70 hours in a preheated oven, and once cooled to room temperature, red block-shaped crystals emerge, yielding around 44%.
A pioneering study by He et al. in 2016 [69] brought forth the inaugural application of Ln-MOFs in photocatalytic CO2 reduction. Gb-TCA (Trichloroacetic acid) was identified as a potent photosensitiser for light-driven H2 evolution and CO2 reduction. The well-suited microporous structure of Gb-TCA, optimised for selective CO2 capture and conversion, exhibited superior attributes due to its finely tuned pore characteristics and high charge carrier mobility. Furthermore, the negative excited state potential of Gd-TCA proved pivotal in facilitating the reduction of Ni(Cyclam)Cl2, a well-regarded catalyst in electrochemical CO2 reduction [70, 71, 72, 73]. Figure 11 shows the process of Gd-TCA photocatalytic reduction of CO2.
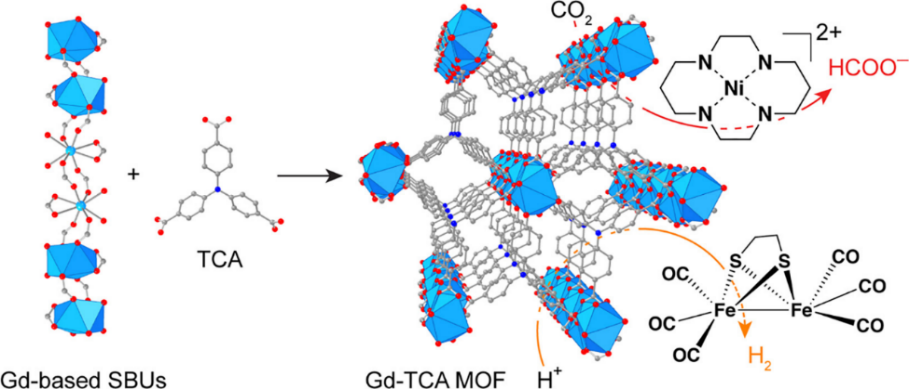
Figure 11. Catalytic process in the photocatalytic Gd-TCA system. Atom colors: Gb, blue polyhedra; O, red; C, gray; N, blue. H atoms are omitted for clarity [69].
The meticulous preparation of this extraordinary RE-MOF through a solvothermal reaction encompasses the dissolution of a blend of 4,4′,4″-tricarboxytriphenylamine (H3TCA) and Gd(NO3)3·6H2O within a DMF-ethanol mixture. This amalgamation is then exposed to a temperature of 100 °C for a span of 3 days in an oven. The outcome comprises yellow block-shaped crystals, subsequently acquired via filtration and vacuum drying, yielding an impressive approximately 60%. The process of reducing carbon dioxide entails saturating the solution with CO2, followed by pH adjustment to 11.0 within a CH3CN/H2O mixture solvent at 25 °C. Subsequently, the solution containing a Gd-TCA suspension, a redox catalyst, trace quantities of Ni(cyclam)Cl2, and Et3N undergoes 12 hours of irradiation. In the presence of Ni(cyclam)Cl2, Gb-TCA catalyses the light-driven photocatalytic reduction of CO2, yielding HCOO- [69].
4. MOF-derived materials
Through appropriate treatment, MOFs can be transformed into nanomaterials of related porous carbon, nitrogen-doped carbon, metal oxides, or other metal compounds. Compared with MOF materials, MOF-derived nanomaterials have higher catalytic activity due to their good organisational structure, dispersed metal centres, high porosity, and larger specific surface area, making them ideal carriers for multiphase catalytic materials [74].
Recently, due to its various structures and excellent adjustability, hollow architectures have been developed as effective photosensors for many applications in photoreactions, such as CO2 photoreduction [75]. The hollow reduces the length of electron transitions to catalytic sites, accelerates the separation of electron-hole pairs and provides a large surface area and more catalytic active sites. By reason of their special structure, suitable bond energy, and adjustable optical properties, metal sulfides exhibit certain catalytic activity in light reactions [76]. Therefore, Wang et al. prepared a multidimensional In2S3-CdIn2S4 nanotube with a heterogeneous structure [77]. They used MOF (MIL-68) as the reaction precursor, first prepared In2S3 nanosheets through a liquid phase substitution process, and then prepared In2S3-CdIn2S4 through a cation exchange reaction (Figure 12 & Figure 13).
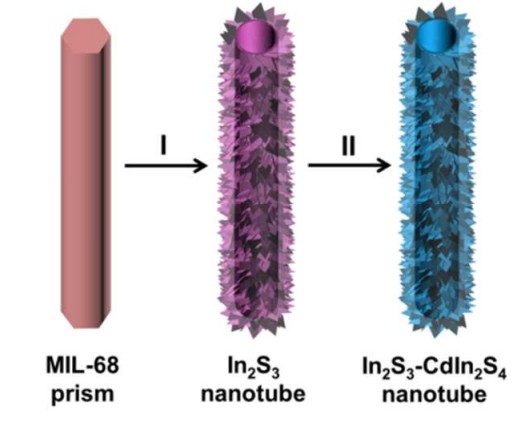
Figure 12. Schematic diagram of In2S3-CdIn2S4 nanotube preparation process. (I) liquid phase sulfidation, (II) cation exchange reaction [77].

Figure 13. Comparison chart of catalytic production of CO through different catalysts [77].
Due to its unique heterostructure, it not only improves the separation and migration of photogenerated electron-hole pairs but inhibits their recombination. It promotes CO2 adsorption ability as well as provides rich active sites for surface photocatalysis reactions. According to their experimental results, the pure In2S3 sample has a CO2 reduction rate of only 68% μmol h-1g-1; when detecting In2S3-CdIn2S4, the performance of CO2 reduction reaction significantly improved, reaching a maximum of 825 μmol h-1g-1, 12 times higher than In2S3 material. Therefore, the optimised In2S3-CdIn2S4 nanotubes exhibit significant CO2 reduction performance under visible light exposure, with a high CO generation rate and excellent stability.
In another work, they used In-MIL-58 as a precursor for photocatalytic CO2 conversion to CO, proposing a sandwich-shaped ZnIn2S4-In2O3 multidimensional tubular heterostructure with excellent performance and high stability [78]. This material is obtained by assembling ZnIn2S4 on the surfaces of In2O3 microtubules. This design has similar catalytic properties to In2S3-CdIn2S4 nanotubes and can also efficiently catalyse the photoreduction reaction from CO2 to CO. According to experimental measurements, pure In2O3 has almost no catalytic activity for CO2 photoreduction, and the reduction rate of CO2 by a single ZnIn2S4 material is approximately 900-1300 μmol h-1g-1. However, with the emergence of the multidimensional catalyst ZnIn2S4-In2O3, this material exhibits the highest reduction rate for CO2 reduction, approximately 3000 μmol h-1g-1 exhibits catalytic activity for up to eight hours.
In addition to metal sulfides, carbon nitride is also a nano-semiconductor material that can utilise visible light to catalyse reactions [79]. This material has the characteristics of a short bandgap, stable structure, and simple preparation. However, because of the good recombination of the electron-hole pair, its catalytic activity could be better, which also limits its application in photocatalysis. Meng et al. first used a facile thermal polymerisation method to obtain the bulk g-C3N4. Then, they prepared ZIF-67 by Co(NO3)2·6H2O and 2-methylimidazole as the raw material. Eventually, they mixed g-C3N4 with MOFs through stirring and ageing, and g-C3N4 was encapsulated in ZIF-67 composite photocatalyst, obtaining g-C3N4/ZIF-67 material (Figure 14 & figure 15) [80]. A heterojunction was successfully constructed between the two of them.
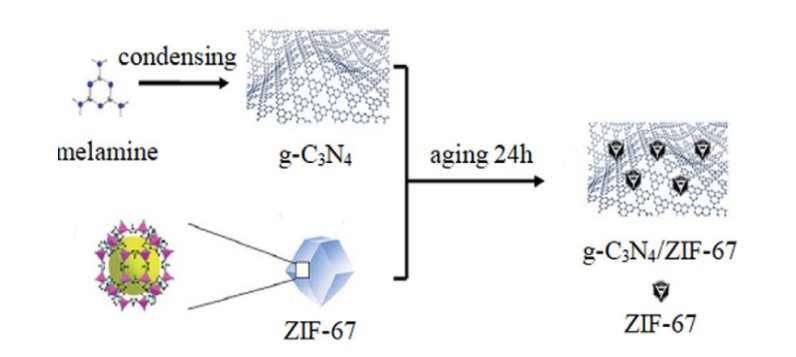
Figure 14. Schematic diagram of the synthetic process of the g-C3N4/ZIF-67 [78].
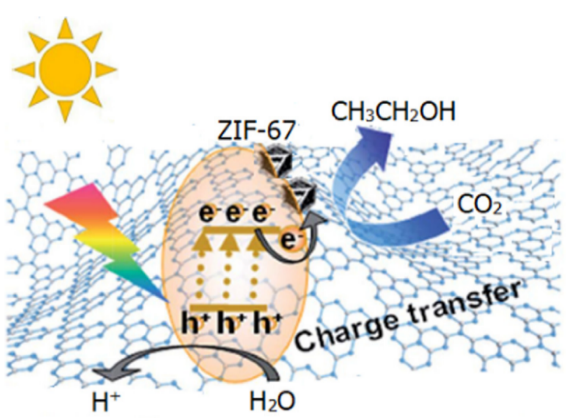
Figure 15. The mechanism of the photocatalytic process [78].
This design shortens the distance of charge transfer, accelerating electron transfer, enhancing the separation rate of electron-hole pairs, and greatly improving its catalytic efficiency. Therefore, the recovery rate and stability increase with the increase of concentration. The above research demonstrates the special advantages of MOF derivatives in photocatalytic CO2 reduction.
5. Conclusion
MOFs are a new type of porous material with a unique structure that has broad application prospects. In recent years, due to the advantages such as high specific surface area, excellent structural adjustability, and high CO2 adsorption capacity, the use of inexhaustible solar energy for CO2 photoreduction has become one of the current research hotspots.
In this article, we discuss the improved MOF-based materials in CO2 photoreduction from the above three aspects. Firstly, they can improve catalytic efficiency through synthetic modifying organic ligands. Common methods include introducing organic ligands with photosensitive functional groups and electron-rich systems and applying metal complexes as ligands. This way can broaden the light absorption of MOFs to the entire visible light region and has shown a significantly higher CO2 absorption ability than unmodified materials. Secondly, MOFs-based hybrid materials that consist of other semiconductors or transition metals would offer better photocatalytic ability. Their respective advantages can also be well preserved and reflected. Finally, MOFs can be converted into other related materials which have photocatalytic properties as raw materials. The derived materials have higher specific surface area and pore size, resulting in higher catalytic activity than MOFs. However, the preparation process is complex and cannot be widely adopted.
However, there are still many things that could be improved in the photoreduction of CO2 in MOFs: (1) The utilisation rate of visible light in MOFs is still feeble, resulting in a lower reduction rate of CO2 in photocatalytic reduction. There is still a certain distance from its application in industry and the environment; (2) Most MOFs still have poor stability and are susceptible to structural collapse after several catalytic cycles, resulting in a rapid decrease in activity; (3) In most of the reported examples of MOFs participating in the photoreduction of CO2, organic sacrificial agents (such as TEOA, TEA) and organic solvents are still needed in the reaction system, which is undoubtedly an uneconomical and environmentally friendly measure. From these, future research on MOFs in CO2 photocatalysis should focus on addressing the above issues by functionalising MOFs and combining them with functional materials to improve their catalytic selectivity and stability. In short, MOF materials have their unique advantages in photocatalytic applications and will definitely be used in more vigorous developments in photocatalysis in the future.
References
[1]. Li, J. R., Ma, Y., McCarthy, M. C., Sculley, J., Yu, J., Jeong, H. K., ... & Zhou, H. C. (2011). Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coordination Chemistry Reviews, 255(15-16), 1791-1823.
[2]. Ola, O., & Maroto-Valer, M. M. (2015). Review of material design and reactor engineering on TiO2 photocatalysis for CO2 reduction. Journal of Photochemistry and Photobiology C: Photochemistry Reviews, 24, 16-42.
[3]. Sadeghi, N., Sharifnia, S., & Arabi, M. S. (2016). A porphyrin-based metal organic framework for high rate photoreduction of CO2 to CH4 in gas phase. Journal of CO2 Utilization, 16, 450-457.
[4]. Yu, S., Wilson, A. J., Kumari, G., Zhang, X., & Jain, P. K. (2017). Opportunities and challenges of solar-energy-driven carbon dioxide to fuel conversion with plasmonic catalysts. ACS Energy Letters, 2(9), 2058-2070.
[5]. Li, Y., Wang, W. N., Zhan, Z., Woo, M. H., Wu, C. Y., & Biswas, P. (2010). Photocatalytic reduction of CO2 with H2O on mesoporous silica supported Cu/TiO2 catalysts. Applied Catalysis B: Environmental, 100(1-2), 386-392.
[6]. Fujishima, A., & Honda, K. (1972). Electrochemical photolysis of water at a semiconductor electrode. nature, 238(5358), 37-38.
[7]. Zhu, C. Y., Zhang, Y. Q., Liao, R. Z., Xia, W., Hu, J. C., Wu, J., ... & Wang, F. (2018). Photocatalytic reduction of CO2 to CO and formate by a novel Co(II) catalyst containing a cis-oxygen atom: photocatalysis and DFT calculations. Dalton Transactions, 47(37), 13142-13150.
[8]. Tahir, M., & Amin, N. S. (2015). Indium-doped TiO2 nanoparticles for photocatalytic CO2 reduction with H2O vapors to CH4. Applied Catalysis B: Environmental, 162, 98-109.
[9]. Luo, D., Chen, C., Zhang, N., Hong, S., Wu, H., & Liu, Z. (2009). Characterization and DFT research of Nd/TiO2: Photocatalyst for synthesis of methanol from CO2 and H2O. Zeitschrift für Physikalische Chemie, 223(12), 1465-1476.
[10]. Fu, Y., Sun, D., Chen, Y., Huang, R., Ding, Z., Fu, X., & Li, Z. (2012). An amine‐functionalized titanium metal–organic framework photocatalyst with visible‐light‐induced activity for CO2 reduction. Angewandte Chemie International Edition, 51(14), 3364-3367.
[11]. Akhter, P., Hussain, M., Saracco, G., & Russo, N. (2015). Novel nanostructured-TiO2 materials for the photocatalytic reduction of CO2 greenhouse gas to hydrocarbons and syngas. Fuel, 149, 55-65.
[12]. Hoskins, B. F., & Robson, R. (1989). Infinite polymeric frameworks consisting of three dimensionally linked rod-like segments. Journal of the American Chemical Society, 111(15), 5962-5964.
[13]. Yaghi, O. M., Li, G., & Li, H. (1995). Selective binding and removal of guests in a microporous metal–organic framework. Nature, 378(6558), 703-706.
[14]. Kang, Y. S., Lu, Y., Chen, K., Zhao, Y., Wang, P., & Sun, W. Y. (2019). Metal–organic frameworks with catalytic centers: From synthesis to catalytic application. Coordination chemistry reviews, 378, 262-280.
[15]. Liu, S., Zhang, C., Sun, Y., Chen, Q., He, L., Zhang, K., ... & Chen, L. F. (2020). Design of metal-organic framework-based photocatalysts for hydrogen generation. Coordination Chemistry Reviews, 413, 213266.
[16]. Liu, X., Tang, B., Long, J., Zhang, W., Liu, X., & Mirza, Z. (2018). The development of MOFs-based nanomaterials in heterogeneous organocatalysis. Science bulletin, 63(8), 502-524.
[17]. Zhao, S. N., Wang, G., Poelman, D., & Van Der Voort, P. (2018). Metal organic frameworks based materials for heterogeneous photocatalysis. Molecules, 23(11), 2947.
[18]. Tu, W., Zhou, Y., Liu, Q., Tian, Z., Gao, J., Chen, X., ... & Zou, Z. (2012). Robust hollow spheres consisting of alternating titania nanosheets and graphene nanosheets with high photocatalytic activity for CO2 conversion into renewable fuels. Advanced Functional Materials, 22(6), 1215-1221.
[19]. Jiang, D., Mallat, T., Krumeich, F., & Baiker, A. (2008). Copper-based metal-organic framework for the facile ring-opening of epoxides. Journal of Catalysis, 257(2), 390-395.
[20]. Wang, C. C., Zhang, Y. Q., Li, J., & Wang, P. (2015). Photocatalytic CO2 reduction in metal–organic frameworks: a mini review. Journal of Molecular Structure, 1083, 127-136.
[21]. Alvaro, M., Carbonell, E., Ferrer, B., Llabrés i Xamena, F. X., & Garcia, H. (2007). Semiconductor behavior of a metal‐organic framework (MOF). Chemistry–A European Journal, 13(18), 5106-5112.
[22]. Horiuchi, Y., Toyao, T., Saito, M., Mochizuki, K., Iwata, M., Higashimura, H., ... & Matsuoka, M. (2012). Visible-light-promoted photocatalytic hydrogen production by using an amino-functionalized Ti (IV) metal–organic framework. The Journal of Physical Chemistry C, 116(39), 20848-20853.
[23]. Fu, Y., Sun, D., Chen, Y., Huang, R., Ding, Z., Fu, X., & Li, Z. (2012). An amine‐functionalized titanium metal–organic framework photocatalyst with visible‐light‐induced activity for CO2 reduction. Angewandte Chemie International Edition, 51(14), 3364-3367.
[24]. Arstad, B., Fjellvåg, H., Kongshaug, K. O., Swang, O., & Blom, R. (2008). Amine functionalised metal organic frameworks (MOFs) as adsorbents for carbon dioxide. Adsorption, 14, 755-762.
[25]. Alkhatib, I. I., Garlisi, C., Pagliaro, M., Al-Ali, K., & Palmisano, G. (2020). Metal-organic frameworks for photocatalytic CO2 reduction under visible radiation: A review of strategies and applications. Catalysis Today, 340, 209-224.
[26]. Zhu, M., Han, M., Du, Y., Yang, P., & Wang, X. (2010). The synthesis, light-harvesting, and photocatalysis of naphthylporphyrin-functionalized platinum nanocomposites. Dyes and Pigments, 86(1), 81-86.
[27]. Shen, Y., Pan, T., Wang, L., Ren, Z., Zhang, W., & Huo, F. (2021). Programmable logic in metal–organic frameworks for catalysis. Advanced Materials, 33(46), 2007442.
[28]. Liu, J., Chen, L., Cui, H., Zhang, J., Zhang, L., & Su, C. Y. (2014). Applications of metal–organic frameworks in heterogeneous supramolecular catalysis. Chemical Society Reviews, 43(16), 6011-6061.
[29]. Wang, D., Huang, R., Liu, W., Sun, D., & Li, Z. (2014). Fe-based MOFs for photocatalytic CO2 reduction: role of coordination unsaturated sites and dual excitation pathways. Acs Catalysis, 4(12), 4254-4260.
[30]. Chughtai, A. H., Ahmad, N., Younus, H. A., Laypkov, A., & Verpoort, F. (2015). Metal–organic frameworks: versatile heterogeneous catalysts for efficient catalytic organic transformations. Chemical Society Reviews, 44(19), 6804-6849.
[31]. Gao, W. Y., Chrzanowski, M., & Ma, S. (2014). Metal–metalloporphyrin frameworks: a resurging class of functional materials. Chemical Society Reviews, 43(16), 5841-5866.
[32]. Liu, J., Fan, Y. Z., Li, X., Wei, Z., Xu, Y. W., Zhang, L., & Su, C. Y. (2018). A porous rhodium (III)-porphyrin metal-organic framework as an efficient and selective photocatalyst for CO2 reduction. Applied Catalysis B: Environmental, 231, 173-181.
[33]. Liu, Y., Yang, Y., Sun, Q., Wang, Z., Huang, B., Dai, Y., ... & Zhang, X. (2013). Chemical adsorption enhanced CO2 capture and photoreduction over a copper porphyrin based metal organic framework. ACS applied materials & interfaces, 5(15), 7654-7658.
[34]. Feng, D., Gu, Z. Y., Li, J. R., Jiang, H. L., Wei, Z., & Zhou, H. C. (2012). Zirconium‐metalloporphyrin PCN‐222: mesoporous metal–organic frameworks with ultrahigh stability as biomimetic catalysts. Angewandte Chemie, 124(41), 10453-10456.
[35]. Xu, H. Q., Hu, J., Wang, D., Li, Z., Zhang, Q., Luo, Y., ... & Jiang, H. L. (2015). Visible-light photoreduction of CO2 in a metal–organic framework: boosting electron–hole separation via electron trap states. Journal of the American Chemical Society, 137(42), 13440-13443.
[36]. Bonin, J., Chaussemier, M., Robert, M., & Routier, M. (2014). Homogeneous photocatalytic reduction of CO2 to CO using iron (0) porphyrin catalysts: mechanism and intrinsic limitations. ChemCatChem, 6(11), 3200-3207.
[37]. Guo, Z., Yu, F., Yang, Y., Leung, C. F., Ng, S. M., Ko, C. C., ... & Robert, M. (2017). Photocatalytic conversion of CO2 to CO by a copper (II) quaterpyridine complex. ChemSusChem, 10(20), 4009-4013.
[38]. Chan, S. L. F., Lam, T. L., Yang, C., Yan, S. C., & Cheng, N. M. (2015). A robust and efficient cobalt molecular catalyst for CO2 reduction. Chemical Communications, 51(37), 7799-7801.
[39]. Bourrez, M., Orio, M., Molton, F., Vezin, H., Duboc, C., Deronzier, A., & Chardon‐Noblat, S. (2014). Pulsed‐EPR Evidence of a Manganese (II) Hydroxycarbonyl Intermediate in the Electrocatalytic Reduction of Carbon Dioxide by a Manganese Bipyridyl Derivative. Angewandte Chemie International Edition, 53(1), 240-243.
[40]. Kobayashi, K., Kikuchi, T., Kitagawa, S., & Tanaka, K. (2014). Selective generation of formamides through photocatalytic CO2 reduction catalyzed by ruthenium carbonyl compounds. Angewandte Chemie International Edition, 53(44), 11813-11817.
[41]. Genoni, A., Chirdon, D. N., Boniolo, M., Sartorel, A., Bernhard, S., & Bonchio, M. (2017). Tuning iridium photocatalysts and light irradiation for enhanced CO2 reduction. ACS Catalysis, 7(1), 154-160.
[42]. Woo, S. J., Choi, S., Kim, S. Y., Kim, P. S., Jo, J. H., Kim, C. H., ... & Kang, S. O. (2019). Highly selective and durable photochemical CO2 reduction by molecular Mn (I) catalyst fixed on a particular dye-sensitized TiO2 platform. ACS Catalysis, 9(3), 2580-2593.
[43]. Elcheikh Mahmoud, M., Audi, H., Assoud, A., Ghaddar, T. H., & Hmadeh, M. (2019). Metal–organic framework photocatalyst incorporating bis (4′-(4-carboxyphenyl)-terpyridine) ruthenium (II) for visible-light-driven carbon dioxide reduction. Journal of the American Chemical Society, 141(17), 7115-7121.
[44]. Feng, X., Pi, Y., Song, Y., Brzezinski, C., Xu, Z., Li, Z., & Lin, W. (2020). Metal–organic frameworks significantly enhance photocatalytic hydrogen evolution and CO2 reduction with earth-abundant copper photosensitizers. Journal of the American Chemical Society, 142(2), 690-695.
[45]. Lee, Y., Kim, S., Kang, J. K., & Cohen, S. M. (2015). Photocatalytic CO2 reduction by a mixed metal (Zr/Ti), mixed ligand metal–organic framework under visible light irradiation. Chemical Communications, 51(26), 5735-5738.
[46]. He, J., Yan, Z., Wang, J., Xie, J., Jiang, L., Shi, Y., ... & Sun, Y. (2013). Significantly enhanced photocatalytic hydrogen evolution under visible light over CdS embedded on metal–organic frameworks. Chemical communications, 49(60), 6761-6763.
[47]. Guo, K., Hussain, I., Fu, Y., Zhang, F., & Zhu, W. (2023). Strategies for improving the photocatalytic performance of metal-organic frameworks for CO2 reduction: A review. Journal of Environmental Sciences, 125, 290-308.
[48]. ZHANG Zhongwei, GUO Ruitang, QIN Yang, GUO Deyu, PAN Weiguo. Application of Metal-Organic Framework in CO2 Photocatalytic Reduction. Materials Reports, 2021, 35(21): 21058-21070.
[49]. Wang, H., Wu, D., Yang, C., Lu, H., Gao, Z., Xu, F., & Jiang, K. (2019). Multi-functional amorphous TiO2 layer on ZIF-67 for enhanced CO2 photoreduction performances under visible light. Journal of CO2 Utilization, 34, 411-421.
[50]. Corradini, P. G., de Brito, J. F., Blaskievicz, S. F., Salvati, B. S., Zanoni, M. V. B., & Mascaro, L. H. (2023). Contribution of CuO on lamellar BiVO4/Bi2O3-based semiconductor for photoconversion of CO2. Journal of Photochemistry and Photobiology A: Chemistry, 114901.
[51]. Baral, B., Reddy, K. H., & Parida, K. M. (2019). Construction of M-BiVO4/T-BiVO4 isotype heterojunction for enhanced photocatalytic degradation of Norfloxacine and Oxygen evolution reaction. Journal of colloid and interface science, 554, 278-295.
[52]. Younis, S. A., Bhardwaj, N., Bhardwaj, S. K., Kim, K. H., & Deep, A. (2021). Rare earth metal–organic frameworks (RE-MOFs): Synthesis, properties, and biomedical applications. Coordination Chemistry Reviews, 429, 213620.
[53]. Huang, C. H. (2011). Rare earth coordination chemistry: fundamentals and applications. John Wiley & Sons.
[54]. Saraci, F., Quezada-Novoa, V., Donnarumma, P. R., & Howarth, A. J. (2020). Rare-earth metal–organic frameworks: from structure to applications. Chemical Society Reviews, 49(22), 7949-7977.
[55]. Bünzli, J. C. G., & Eliseeva, S. V. (2013). Photophysics of Lanthanoid Coordination Compounds. In Comprehensive Inorganic Chemistry II (Second Edition): From Elements to Applications (pp. 339-398).
[56]. Nguyen, T. N., Ebrahim, F. M., & Stylianou, K. C. (2018). Photoluminescent, upconversion luminescent and nonlinear optical metal-organic frameworks: From fundamental photophysics to potential applications. Coordination Chemistry Reviews, 377, 259-306.
[57]. Cui, Y., Chen, B., & Qian, G. (2014). Lanthanide metal-organic frameworks for luminescent sensing and light-emitting applications. Coordination Chemistry Reviews, 273, 76-86.
[58]. Zhao, S. N., Wang, G., Poelman, D., & Voort, P. V. D. (2018). Luminescent lanthanide MOFs: A unique platform for chemical sensing. Materials, 11(4), 572.
[59]. Hu, D., Song, Y., & Wang, L. (2015). Nanoscale luminescent lanthanide-based metal–organic frameworks: properties, synthesis, and applications. Journal of Nanoparticle Research, 17, 1-21.
[60]. Kukkar, D., Vellingiri, K., Kim, K. H., & Deep, A. (2018). Recent progress in biological and chemical sensing by luminescent metal-organic frameworks. Sensors and Actuators B: Chemical, 273, 1346-1370.
[61]. Vincent, K. A., Tilley, G. J., Quammie, N. C., Streeter, I., Burgess, B. K., Cheesman, M. R., & Armstrong, F. A. (2003). Instantaneous, stoichiometric generation of powerfully reducing states of protein active sites using Eu (II) and polyaminocarboxylate ligands. Chemical communications, (20), 2590-2591.
[62]. Lee, C. C., Hu, Y., & Ribbe, M. W. (2012). ATP-independent substrate reduction by nitrogenase P-cluster variant. Proceedings of the National Academy of Sciences, 109(18), 6922-6926.
[63]. Yan, Z. H., Du, M. H., Liu, J., Jin, S., Wang, C., Zhuang, G. L., ... & Zheng, L. S. (2018). Photo-generated dinuclear {Eu (II)}2 active sites for selective CO2 reduction in a photosensitizing metal-organic framework. Nature Communications, 9(1), 3353.
[64]. Fu, Y., Sun, D., Chen, Y., Huang, R., Ding, Z., Fu, X., & Li, Z. (2012). An amine‐functionalized titanium metal–organic framework photocatalyst with visible‐light‐induced activity for CO2 reduction. Angewandte Chemie International Edition, 51(14), 3364-3367.
[65]. Sun, D., Fu, Y., Liu, W., Ye, L., Wang, D., Yang, L., ... & Li, Z. (2013). Studies on photocatalytic CO2 reduction over NH2‐Uio‐66 (Zr) and its derivatives: towards a better understanding of photocatalysis on metal–organic frameworks. Chemistry–A European Journal, 19(42), 14279-14285.
[66]. Zhang, H., Wei, J., Dong, J., Liu, G., Shi, L., An, P., ... & Ye, J. (2016). Efficient visible‐light‐driven carbon dioxide reduction by a single‐atom implanted metal–organic framework. Angewandte Chemie, 128(46), 14522-14526.
[67]. Fan, J., Liu, E. Z., Tian, L., Hu, X. Y., He, Q., & Sun, T. (2011). Synergistic effect of N and Ni2+ on nanotitania in photocatalytic reduction of CO2. Journal of Environmental Engineering, 137(3), 171-176.
[68]. Zhang, Q., Li, Y., Ackerman, E. A., Gajdardziska-Josifovska, M., & Li, H. (2011). Visible light responsive iodine-doped TiO2 for photocatalytic reduction of CO2 to fuels. Applied catalysis A: general, 400(1-2), 195-202.
[69]. Wu, P., Guo, X., Cheng, L., He, C., Wang, J., & Duan, C. (2016). Photoactive metal–organic framework and its film for light-driven hydrogen production and carbon dioxide reduction. Inorganic Chemistry, 55(16), 8153-8159.
[70]. Schneider, J., Jia, H., Kobiro, K., Cabelli, D. E., Muckerman, J. T., & Fujita, E. (2012). Nickel (II) macrocycles: highly efficient electrocatalysts for the selective reduction of CO2 to CO. Energy & Environmental Science, 5(11), 9502-9510.
[71]. Schneider, J., Jia, H., Kobiro, K., Cabelli, D. E., Muckerman, J. T., & Fujita, E. (2012). Nickel (II) macrocycles: highly efficient electrocatalysts for the selective reduction of CO2 to CO. Energy & Environmental Science, 5(11), 9502-9510.
[72]. Herrero, C., Quaranta, A., El Ghachtouli, S., Vauzeilles, B., Leibl, W., & Aukauloo, A. (2014). Carbon dioxide reduction via light activation of a ruthenium–Ni (cyclam) complex. Physical Chemistry Chemical Physics, 16(24), 12067-12072.
[73]. Froehlich, J. D., & Kubiak, C. P. (2012). Homogeneous CO2 reduction by Ni (cyclam) at a glassy carbon electrode. Inorganic chemistry, 51(7), 3932-3934.
[74]. Khaletskaya, K., Pougin, A., Medishetty, R., Rosler, C., Wiktor, C., Strunk, J., & Fischer, R. A. (2015). Fabrication of gold/titania photocatalyst for CO2 reduction based on pyrolytic conversion of the metal–organic framework NH2-MIL-125 (Ti) loaded with gold nanoparticles. Chemistry of Materials, 27(21), 7248-7257.
[75]. Wang, S., Guan, B. Y., Lu, Y., & Lou, X. W. D. (2017). Formation of hierarchical In2S3–CdIn2S4 heterostructured nanotubes for efficient and stable visible light CO2 reduction. Journal of the American Chemical Society, 139(48), 17305-17308.
[76]. Jiao, X., Chen, Z., Li, X., Sun, Y., Gao, S., Yan, W., ... & Xie, Y. (2017). Defect-mediated electron–hole separation in one-unit-cell ZnIn2S4 layers for boosted solar-driven CO2 reduction. Journal of the American Chemical Society, 139(22), 7586-7594.
[77]. Wang, S., Guan, B. Y., Lu, Y., & Lou, X. W. D. (2017). Formation of hierarchical In2S3–CdIn2S4 heterostructured nanotubes for efficient and stable visible light CO2 reduction. Journal of the American Chemical Society, 139(48), 17305-17308.
[78]. Wang, S., Guan, B. Y., & Lou, X. W. D. (2018). Construction of ZnIn2S4–In2O3 hierarchical tubular heterostructures for efficient CO2 photoreduction. Journal of the American Chemical Society, 140(15), 5037-5040.
[79]. Groenewolt, M., & Antonietti, M. (2005). Synthesis of g‐C3N4 nanoparticles in mesoporous silica host matrices. Advanced materials, 17(14), 1789-1792.
[80]. Meng, Y., Zhang, L., Jiu, H., Zhang, Q., Zhang, H., Ren, W., ... & Li, D. (2019). Construction of g-C3N4/ZIF-67 photocatalyst with enhanced photocatalytic CO2 reduction activity. Materials Science in Semiconductor Processing, 95, 35-41.
Cite this article
Xiao,X.;Huang,S.;Chen,H. (2024). Metal-organic frameworks (MOFs) for photoreduction of CO2. Applied and Computational Engineering,84,9-26.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Li, J. R., Ma, Y., McCarthy, M. C., Sculley, J., Yu, J., Jeong, H. K., ... & Zhou, H. C. (2011). Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coordination Chemistry Reviews, 255(15-16), 1791-1823.
[2]. Ola, O., & Maroto-Valer, M. M. (2015). Review of material design and reactor engineering on TiO2 photocatalysis for CO2 reduction. Journal of Photochemistry and Photobiology C: Photochemistry Reviews, 24, 16-42.
[3]. Sadeghi, N., Sharifnia, S., & Arabi, M. S. (2016). A porphyrin-based metal organic framework for high rate photoreduction of CO2 to CH4 in gas phase. Journal of CO2 Utilization, 16, 450-457.
[4]. Yu, S., Wilson, A. J., Kumari, G., Zhang, X., & Jain, P. K. (2017). Opportunities and challenges of solar-energy-driven carbon dioxide to fuel conversion with plasmonic catalysts. ACS Energy Letters, 2(9), 2058-2070.
[5]. Li, Y., Wang, W. N., Zhan, Z., Woo, M. H., Wu, C. Y., & Biswas, P. (2010). Photocatalytic reduction of CO2 with H2O on mesoporous silica supported Cu/TiO2 catalysts. Applied Catalysis B: Environmental, 100(1-2), 386-392.
[6]. Fujishima, A., & Honda, K. (1972). Electrochemical photolysis of water at a semiconductor electrode. nature, 238(5358), 37-38.
[7]. Zhu, C. Y., Zhang, Y. Q., Liao, R. Z., Xia, W., Hu, J. C., Wu, J., ... & Wang, F. (2018). Photocatalytic reduction of CO2 to CO and formate by a novel Co(II) catalyst containing a cis-oxygen atom: photocatalysis and DFT calculations. Dalton Transactions, 47(37), 13142-13150.
[8]. Tahir, M., & Amin, N. S. (2015). Indium-doped TiO2 nanoparticles for photocatalytic CO2 reduction with H2O vapors to CH4. Applied Catalysis B: Environmental, 162, 98-109.
[9]. Luo, D., Chen, C., Zhang, N., Hong, S., Wu, H., & Liu, Z. (2009). Characterization and DFT research of Nd/TiO2: Photocatalyst for synthesis of methanol from CO2 and H2O. Zeitschrift für Physikalische Chemie, 223(12), 1465-1476.
[10]. Fu, Y., Sun, D., Chen, Y., Huang, R., Ding, Z., Fu, X., & Li, Z. (2012). An amine‐functionalized titanium metal–organic framework photocatalyst with visible‐light‐induced activity for CO2 reduction. Angewandte Chemie International Edition, 51(14), 3364-3367.
[11]. Akhter, P., Hussain, M., Saracco, G., & Russo, N. (2015). Novel nanostructured-TiO2 materials for the photocatalytic reduction of CO2 greenhouse gas to hydrocarbons and syngas. Fuel, 149, 55-65.
[12]. Hoskins, B. F., & Robson, R. (1989). Infinite polymeric frameworks consisting of three dimensionally linked rod-like segments. Journal of the American Chemical Society, 111(15), 5962-5964.
[13]. Yaghi, O. M., Li, G., & Li, H. (1995). Selective binding and removal of guests in a microporous metal–organic framework. Nature, 378(6558), 703-706.
[14]. Kang, Y. S., Lu, Y., Chen, K., Zhao, Y., Wang, P., & Sun, W. Y. (2019). Metal–organic frameworks with catalytic centers: From synthesis to catalytic application. Coordination chemistry reviews, 378, 262-280.
[15]. Liu, S., Zhang, C., Sun, Y., Chen, Q., He, L., Zhang, K., ... & Chen, L. F. (2020). Design of metal-organic framework-based photocatalysts for hydrogen generation. Coordination Chemistry Reviews, 413, 213266.
[16]. Liu, X., Tang, B., Long, J., Zhang, W., Liu, X., & Mirza, Z. (2018). The development of MOFs-based nanomaterials in heterogeneous organocatalysis. Science bulletin, 63(8), 502-524.
[17]. Zhao, S. N., Wang, G., Poelman, D., & Van Der Voort, P. (2018). Metal organic frameworks based materials for heterogeneous photocatalysis. Molecules, 23(11), 2947.
[18]. Tu, W., Zhou, Y., Liu, Q., Tian, Z., Gao, J., Chen, X., ... & Zou, Z. (2012). Robust hollow spheres consisting of alternating titania nanosheets and graphene nanosheets with high photocatalytic activity for CO2 conversion into renewable fuels. Advanced Functional Materials, 22(6), 1215-1221.
[19]. Jiang, D., Mallat, T., Krumeich, F., & Baiker, A. (2008). Copper-based metal-organic framework for the facile ring-opening of epoxides. Journal of Catalysis, 257(2), 390-395.
[20]. Wang, C. C., Zhang, Y. Q., Li, J., & Wang, P. (2015). Photocatalytic CO2 reduction in metal–organic frameworks: a mini review. Journal of Molecular Structure, 1083, 127-136.
[21]. Alvaro, M., Carbonell, E., Ferrer, B., Llabrés i Xamena, F. X., & Garcia, H. (2007). Semiconductor behavior of a metal‐organic framework (MOF). Chemistry–A European Journal, 13(18), 5106-5112.
[22]. Horiuchi, Y., Toyao, T., Saito, M., Mochizuki, K., Iwata, M., Higashimura, H., ... & Matsuoka, M. (2012). Visible-light-promoted photocatalytic hydrogen production by using an amino-functionalized Ti (IV) metal–organic framework. The Journal of Physical Chemistry C, 116(39), 20848-20853.
[23]. Fu, Y., Sun, D., Chen, Y., Huang, R., Ding, Z., Fu, X., & Li, Z. (2012). An amine‐functionalized titanium metal–organic framework photocatalyst with visible‐light‐induced activity for CO2 reduction. Angewandte Chemie International Edition, 51(14), 3364-3367.
[24]. Arstad, B., Fjellvåg, H., Kongshaug, K. O., Swang, O., & Blom, R. (2008). Amine functionalised metal organic frameworks (MOFs) as adsorbents for carbon dioxide. Adsorption, 14, 755-762.
[25]. Alkhatib, I. I., Garlisi, C., Pagliaro, M., Al-Ali, K., & Palmisano, G. (2020). Metal-organic frameworks for photocatalytic CO2 reduction under visible radiation: A review of strategies and applications. Catalysis Today, 340, 209-224.
[26]. Zhu, M., Han, M., Du, Y., Yang, P., & Wang, X. (2010). The synthesis, light-harvesting, and photocatalysis of naphthylporphyrin-functionalized platinum nanocomposites. Dyes and Pigments, 86(1), 81-86.
[27]. Shen, Y., Pan, T., Wang, L., Ren, Z., Zhang, W., & Huo, F. (2021). Programmable logic in metal–organic frameworks for catalysis. Advanced Materials, 33(46), 2007442.
[28]. Liu, J., Chen, L., Cui, H., Zhang, J., Zhang, L., & Su, C. Y. (2014). Applications of metal–organic frameworks in heterogeneous supramolecular catalysis. Chemical Society Reviews, 43(16), 6011-6061.
[29]. Wang, D., Huang, R., Liu, W., Sun, D., & Li, Z. (2014). Fe-based MOFs for photocatalytic CO2 reduction: role of coordination unsaturated sites and dual excitation pathways. Acs Catalysis, 4(12), 4254-4260.
[30]. Chughtai, A. H., Ahmad, N., Younus, H. A., Laypkov, A., & Verpoort, F. (2015). Metal–organic frameworks: versatile heterogeneous catalysts for efficient catalytic organic transformations. Chemical Society Reviews, 44(19), 6804-6849.
[31]. Gao, W. Y., Chrzanowski, M., & Ma, S. (2014). Metal–metalloporphyrin frameworks: a resurging class of functional materials. Chemical Society Reviews, 43(16), 5841-5866.
[32]. Liu, J., Fan, Y. Z., Li, X., Wei, Z., Xu, Y. W., Zhang, L., & Su, C. Y. (2018). A porous rhodium (III)-porphyrin metal-organic framework as an efficient and selective photocatalyst for CO2 reduction. Applied Catalysis B: Environmental, 231, 173-181.
[33]. Liu, Y., Yang, Y., Sun, Q., Wang, Z., Huang, B., Dai, Y., ... & Zhang, X. (2013). Chemical adsorption enhanced CO2 capture and photoreduction over a copper porphyrin based metal organic framework. ACS applied materials & interfaces, 5(15), 7654-7658.
[34]. Feng, D., Gu, Z. Y., Li, J. R., Jiang, H. L., Wei, Z., & Zhou, H. C. (2012). Zirconium‐metalloporphyrin PCN‐222: mesoporous metal–organic frameworks with ultrahigh stability as biomimetic catalysts. Angewandte Chemie, 124(41), 10453-10456.
[35]. Xu, H. Q., Hu, J., Wang, D., Li, Z., Zhang, Q., Luo, Y., ... & Jiang, H. L. (2015). Visible-light photoreduction of CO2 in a metal–organic framework: boosting electron–hole separation via electron trap states. Journal of the American Chemical Society, 137(42), 13440-13443.
[36]. Bonin, J., Chaussemier, M., Robert, M., & Routier, M. (2014). Homogeneous photocatalytic reduction of CO2 to CO using iron (0) porphyrin catalysts: mechanism and intrinsic limitations. ChemCatChem, 6(11), 3200-3207.
[37]. Guo, Z., Yu, F., Yang, Y., Leung, C. F., Ng, S. M., Ko, C. C., ... & Robert, M. (2017). Photocatalytic conversion of CO2 to CO by a copper (II) quaterpyridine complex. ChemSusChem, 10(20), 4009-4013.
[38]. Chan, S. L. F., Lam, T. L., Yang, C., Yan, S. C., & Cheng, N. M. (2015). A robust and efficient cobalt molecular catalyst for CO2 reduction. Chemical Communications, 51(37), 7799-7801.
[39]. Bourrez, M., Orio, M., Molton, F., Vezin, H., Duboc, C., Deronzier, A., & Chardon‐Noblat, S. (2014). Pulsed‐EPR Evidence of a Manganese (II) Hydroxycarbonyl Intermediate in the Electrocatalytic Reduction of Carbon Dioxide by a Manganese Bipyridyl Derivative. Angewandte Chemie International Edition, 53(1), 240-243.
[40]. Kobayashi, K., Kikuchi, T., Kitagawa, S., & Tanaka, K. (2014). Selective generation of formamides through photocatalytic CO2 reduction catalyzed by ruthenium carbonyl compounds. Angewandte Chemie International Edition, 53(44), 11813-11817.
[41]. Genoni, A., Chirdon, D. N., Boniolo, M., Sartorel, A., Bernhard, S., & Bonchio, M. (2017). Tuning iridium photocatalysts and light irradiation for enhanced CO2 reduction. ACS Catalysis, 7(1), 154-160.
[42]. Woo, S. J., Choi, S., Kim, S. Y., Kim, P. S., Jo, J. H., Kim, C. H., ... & Kang, S. O. (2019). Highly selective and durable photochemical CO2 reduction by molecular Mn (I) catalyst fixed on a particular dye-sensitized TiO2 platform. ACS Catalysis, 9(3), 2580-2593.
[43]. Elcheikh Mahmoud, M., Audi, H., Assoud, A., Ghaddar, T. H., & Hmadeh, M. (2019). Metal–organic framework photocatalyst incorporating bis (4′-(4-carboxyphenyl)-terpyridine) ruthenium (II) for visible-light-driven carbon dioxide reduction. Journal of the American Chemical Society, 141(17), 7115-7121.
[44]. Feng, X., Pi, Y., Song, Y., Brzezinski, C., Xu, Z., Li, Z., & Lin, W. (2020). Metal–organic frameworks significantly enhance photocatalytic hydrogen evolution and CO2 reduction with earth-abundant copper photosensitizers. Journal of the American Chemical Society, 142(2), 690-695.
[45]. Lee, Y., Kim, S., Kang, J. K., & Cohen, S. M. (2015). Photocatalytic CO2 reduction by a mixed metal (Zr/Ti), mixed ligand metal–organic framework under visible light irradiation. Chemical Communications, 51(26), 5735-5738.
[46]. He, J., Yan, Z., Wang, J., Xie, J., Jiang, L., Shi, Y., ... & Sun, Y. (2013). Significantly enhanced photocatalytic hydrogen evolution under visible light over CdS embedded on metal–organic frameworks. Chemical communications, 49(60), 6761-6763.
[47]. Guo, K., Hussain, I., Fu, Y., Zhang, F., & Zhu, W. (2023). Strategies for improving the photocatalytic performance of metal-organic frameworks for CO2 reduction: A review. Journal of Environmental Sciences, 125, 290-308.
[48]. ZHANG Zhongwei, GUO Ruitang, QIN Yang, GUO Deyu, PAN Weiguo. Application of Metal-Organic Framework in CO2 Photocatalytic Reduction. Materials Reports, 2021, 35(21): 21058-21070.
[49]. Wang, H., Wu, D., Yang, C., Lu, H., Gao, Z., Xu, F., & Jiang, K. (2019). Multi-functional amorphous TiO2 layer on ZIF-67 for enhanced CO2 photoreduction performances under visible light. Journal of CO2 Utilization, 34, 411-421.
[50]. Corradini, P. G., de Brito, J. F., Blaskievicz, S. F., Salvati, B. S., Zanoni, M. V. B., & Mascaro, L. H. (2023). Contribution of CuO on lamellar BiVO4/Bi2O3-based semiconductor for photoconversion of CO2. Journal of Photochemistry and Photobiology A: Chemistry, 114901.
[51]. Baral, B., Reddy, K. H., & Parida, K. M. (2019). Construction of M-BiVO4/T-BiVO4 isotype heterojunction for enhanced photocatalytic degradation of Norfloxacine and Oxygen evolution reaction. Journal of colloid and interface science, 554, 278-295.
[52]. Younis, S. A., Bhardwaj, N., Bhardwaj, S. K., Kim, K. H., & Deep, A. (2021). Rare earth metal–organic frameworks (RE-MOFs): Synthesis, properties, and biomedical applications. Coordination Chemistry Reviews, 429, 213620.
[53]. Huang, C. H. (2011). Rare earth coordination chemistry: fundamentals and applications. John Wiley & Sons.
[54]. Saraci, F., Quezada-Novoa, V., Donnarumma, P. R., & Howarth, A. J. (2020). Rare-earth metal–organic frameworks: from structure to applications. Chemical Society Reviews, 49(22), 7949-7977.
[55]. Bünzli, J. C. G., & Eliseeva, S. V. (2013). Photophysics of Lanthanoid Coordination Compounds. In Comprehensive Inorganic Chemistry II (Second Edition): From Elements to Applications (pp. 339-398).
[56]. Nguyen, T. N., Ebrahim, F. M., & Stylianou, K. C. (2018). Photoluminescent, upconversion luminescent and nonlinear optical metal-organic frameworks: From fundamental photophysics to potential applications. Coordination Chemistry Reviews, 377, 259-306.
[57]. Cui, Y., Chen, B., & Qian, G. (2014). Lanthanide metal-organic frameworks for luminescent sensing and light-emitting applications. Coordination Chemistry Reviews, 273, 76-86.
[58]. Zhao, S. N., Wang, G., Poelman, D., & Voort, P. V. D. (2018). Luminescent lanthanide MOFs: A unique platform for chemical sensing. Materials, 11(4), 572.
[59]. Hu, D., Song, Y., & Wang, L. (2015). Nanoscale luminescent lanthanide-based metal–organic frameworks: properties, synthesis, and applications. Journal of Nanoparticle Research, 17, 1-21.
[60]. Kukkar, D., Vellingiri, K., Kim, K. H., & Deep, A. (2018). Recent progress in biological and chemical sensing by luminescent metal-organic frameworks. Sensors and Actuators B: Chemical, 273, 1346-1370.
[61]. Vincent, K. A., Tilley, G. J., Quammie, N. C., Streeter, I., Burgess, B. K., Cheesman, M. R., & Armstrong, F. A. (2003). Instantaneous, stoichiometric generation of powerfully reducing states of protein active sites using Eu (II) and polyaminocarboxylate ligands. Chemical communications, (20), 2590-2591.
[62]. Lee, C. C., Hu, Y., & Ribbe, M. W. (2012). ATP-independent substrate reduction by nitrogenase P-cluster variant. Proceedings of the National Academy of Sciences, 109(18), 6922-6926.
[63]. Yan, Z. H., Du, M. H., Liu, J., Jin, S., Wang, C., Zhuang, G. L., ... & Zheng, L. S. (2018). Photo-generated dinuclear {Eu (II)}2 active sites for selective CO2 reduction in a photosensitizing metal-organic framework. Nature Communications, 9(1), 3353.
[64]. Fu, Y., Sun, D., Chen, Y., Huang, R., Ding, Z., Fu, X., & Li, Z. (2012). An amine‐functionalized titanium metal–organic framework photocatalyst with visible‐light‐induced activity for CO2 reduction. Angewandte Chemie International Edition, 51(14), 3364-3367.
[65]. Sun, D., Fu, Y., Liu, W., Ye, L., Wang, D., Yang, L., ... & Li, Z. (2013). Studies on photocatalytic CO2 reduction over NH2‐Uio‐66 (Zr) and its derivatives: towards a better understanding of photocatalysis on metal–organic frameworks. Chemistry–A European Journal, 19(42), 14279-14285.
[66]. Zhang, H., Wei, J., Dong, J., Liu, G., Shi, L., An, P., ... & Ye, J. (2016). Efficient visible‐light‐driven carbon dioxide reduction by a single‐atom implanted metal–organic framework. Angewandte Chemie, 128(46), 14522-14526.
[67]. Fan, J., Liu, E. Z., Tian, L., Hu, X. Y., He, Q., & Sun, T. (2011). Synergistic effect of N and Ni2+ on nanotitania in photocatalytic reduction of CO2. Journal of Environmental Engineering, 137(3), 171-176.
[68]. Zhang, Q., Li, Y., Ackerman, E. A., Gajdardziska-Josifovska, M., & Li, H. (2011). Visible light responsive iodine-doped TiO2 for photocatalytic reduction of CO2 to fuels. Applied catalysis A: general, 400(1-2), 195-202.
[69]. Wu, P., Guo, X., Cheng, L., He, C., Wang, J., & Duan, C. (2016). Photoactive metal–organic framework and its film for light-driven hydrogen production and carbon dioxide reduction. Inorganic Chemistry, 55(16), 8153-8159.
[70]. Schneider, J., Jia, H., Kobiro, K., Cabelli, D. E., Muckerman, J. T., & Fujita, E. (2012). Nickel (II) macrocycles: highly efficient electrocatalysts for the selective reduction of CO2 to CO. Energy & Environmental Science, 5(11), 9502-9510.
[71]. Schneider, J., Jia, H., Kobiro, K., Cabelli, D. E., Muckerman, J. T., & Fujita, E. (2012). Nickel (II) macrocycles: highly efficient electrocatalysts for the selective reduction of CO2 to CO. Energy & Environmental Science, 5(11), 9502-9510.
[72]. Herrero, C., Quaranta, A., El Ghachtouli, S., Vauzeilles, B., Leibl, W., & Aukauloo, A. (2014). Carbon dioxide reduction via light activation of a ruthenium–Ni (cyclam) complex. Physical Chemistry Chemical Physics, 16(24), 12067-12072.
[73]. Froehlich, J. D., & Kubiak, C. P. (2012). Homogeneous CO2 reduction by Ni (cyclam) at a glassy carbon electrode. Inorganic chemistry, 51(7), 3932-3934.
[74]. Khaletskaya, K., Pougin, A., Medishetty, R., Rosler, C., Wiktor, C., Strunk, J., & Fischer, R. A. (2015). Fabrication of gold/titania photocatalyst for CO2 reduction based on pyrolytic conversion of the metal–organic framework NH2-MIL-125 (Ti) loaded with gold nanoparticles. Chemistry of Materials, 27(21), 7248-7257.
[75]. Wang, S., Guan, B. Y., Lu, Y., & Lou, X. W. D. (2017). Formation of hierarchical In2S3–CdIn2S4 heterostructured nanotubes for efficient and stable visible light CO2 reduction. Journal of the American Chemical Society, 139(48), 17305-17308.
[76]. Jiao, X., Chen, Z., Li, X., Sun, Y., Gao, S., Yan, W., ... & Xie, Y. (2017). Defect-mediated electron–hole separation in one-unit-cell ZnIn2S4 layers for boosted solar-driven CO2 reduction. Journal of the American Chemical Society, 139(22), 7586-7594.
[77]. Wang, S., Guan, B. Y., Lu, Y., & Lou, X. W. D. (2017). Formation of hierarchical In2S3–CdIn2S4 heterostructured nanotubes for efficient and stable visible light CO2 reduction. Journal of the American Chemical Society, 139(48), 17305-17308.
[78]. Wang, S., Guan, B. Y., & Lou, X. W. D. (2018). Construction of ZnIn2S4–In2O3 hierarchical tubular heterostructures for efficient CO2 photoreduction. Journal of the American Chemical Society, 140(15), 5037-5040.
[79]. Groenewolt, M., & Antonietti, M. (2005). Synthesis of g‐C3N4 nanoparticles in mesoporous silica host matrices. Advanced materials, 17(14), 1789-1792.
[80]. Meng, Y., Zhang, L., Jiu, H., Zhang, Q., Zhang, H., Ren, W., ... & Li, D. (2019). Construction of g-C3N4/ZIF-67 photocatalyst with enhanced photocatalytic CO2 reduction activity. Materials Science in Semiconductor Processing, 95, 35-41.









