1. Introduction
These guidelines, written in the style of a submission, show the best layout for your paper using Microsoft Word. If you don’t wish to use the Word template provided, please use the following page setup measurements. An industry that manufactured acetaldehyde and vinyl compounds and used mercury (II) oxide as a catalyst discharged methylmercury (MeHg) into the Minamata Bay, which contaminates aquatic plants and animals. When the rate of intake of methylmercury by aquatic animals is higher than the rate of excretion, bioaccumulation occurs. Minamata disease is the first incident caused by a large amount of consumption of contaminated aquatic foods by inhabitants, like fishermen and their families, and was determined between 1953 and 1956. From 1953 to 1961, 88 cases of Minamata had been recorded. Among the patients of Minamata disease, males and females made up 56 percent and 32 percent of the population, respectively. The rest of the 25 percent are children who are under the age of ten. As a result, 35 people died because of mercury poisoning, which was composed of 21% of males and 14% of females [1].
Under exposure to methylmercury, people’s central nervous system, kidneys, and livers are influenced [2]. The amount of methylmercury was high in concentration at the beginning of the contamination, but the concentration of inorganic mercury, I-Hg, will gradually increase with time. This was discovered in patients who endure chronic cases of Minamata disease. Inorganic mercury may also alter the body’s function and characteristics.
1.1. The history of Minamata Disease
Organic mercury, such as dimethylmercury, is the material of the synthesis of Trimethyl gallium. In history, although dimethylmercury deaths are rare, only four with Karen. In 1865, two laboratory assistants died after synthesizing dimethylmercury. Also, Minamata disease, caused by the ingestion of fish and shellfish, which is contaminated. This disease is the same as methylmercury poisoning and annoys many people with neurological disorders such as ataxia, speech disturbance, and constriction of visual fields [3]. Secondly, Karen Wetterhahn is one of the pioneers who researches toxic metal exposure. During the experiment, when she transferred, due to the lack of some nature of the dimethylmercury, she only wore a pair of rubber gloves. The dimethylmercury can reach the skin through the gloves. The exposure to a few drops of a toxic metal—dimethylmercury—in a laboratory accident in August 1996 led to the insidious onset of neurological symptoms and signs of poisoning over 3 months later [4].
Based on an extensive review of the Absorption and Excretion of Mercury in Man, Leonard J. Goldwater and Arthur C. Ladd(1962) argue that the reason why, toxicologically, there are differences between the behavior of organic and inorganic mercury compounds [5]. It has been repeatedly noted that degrees of exposure and levels of urine excretion seem to correlate very well in the case of inorganic mercury. The situation for organic mercury compounds is not totally apparent in this regard. The majority of commentators on the matter concur that there is very little connection between urine mercury excretion levels and the emergence of clinical symptoms of poisoning.
1.2. The properties of organic mercury
An important step in this paper is deciding on organic mercury’s type and respective characteristics. Organic mercury compounds are a class of organic compounds that contain mercury and play a significant role in organic chemistry. There is a wide variety of organic mercury compounds, including methylmercury, methylmercury, and phenylmercury, among others. These compounds differ in their structures, primarily in the organic groups they are attached to. Furthermore, the different types of organic mercury compounds exhibit varying levels of toxicity and pose different risks to human health. For example, the term “methylmercury” serves as a shorthand for the hypothetical “methylmercury cation,” also known as a methylmercury (II) cation. This functional group is composed of a methyl group bonded to an atom of mercury, whose chemical formula is CH3Hg+. The Methylmercury compound has an overall charge of +1, and Hg in the +2 oxidation state. Methylmercury exists as a substituent in many complexes of the type MeHgL (L = Lewis base) and MeHgX(X = anion)[6]. As a positively charged ion, it readily reacts with anions such as chloride, hydroxide, and nitrate. It has a special affinity for anions that include sulfur, especially thiols. Thiols are generated when the amino acid cysteine and the peptide glutathione form strong complexes with methylmercury. The equation below is to generate thiols when the amino acid cysteine and the peptide glutathione form strong complexes wit methylmercury[7].
\( {H_{3}}C-{Hg^{+}}+RSH→{H_{3}}C-Hg-SR+{H^{+}} \)
1.3. The harm of organic mercury to the human body
In addition, we will have further discussion on the focus of how organic mercury gradually influences the human body and harms it. Organic mercury compounds are highly toxic and can easily enter the human body, causing severe health implications. This is due to their unique ability to react with biological molecules within the body, disrupting normal physiological functions. Organic mercury compounds primarily enter the body through ingestion, inhalation, and dermal contact, and can circulate throughout the body via the bloodstream. The adverse effects of organic mercury primarily manifest in the nervous, respiratory, and immune systems. They can lead to neurological damage, cognitive impairments, and in severe cases, paralysis and even death. Furthermore, organic mercury can induce respiratory disorders, abnormal lung function, immune system dysregulation, and an increased risk of developing cancer. Current treatment approaches for organic mercury poisoning primarily focus on detoxification methods. These include the use of compounds that can bind to organic mercury, forming insoluble substances that can be eliminated from the body. Additionally, prevention of organic mercury poisoning is crucial, which involves minimizing exposure to organic mercury and implementing robust protective measures. Understanding the synthesis principles of organic mercury compounds and their detrimental effects on human health is of paramount importance. Further research in these areas is essential to effectively safeguard human health and mitigate the hazards posed by organic mercury compounds.
2. The chemical properties and effects of methylmercury on the human body
2.1. The formation of methylmercury
Methylmercury (MeHg) is the simplest organomercury compound. It derives from inorganic mercury with the activity of microbial communities living in aquatic situations, such as rivers, lakes, oceans, soils, wetlands, and sediments [8]. For example, anaerobic bacteria mainly contribute to the formation of methylmercury. The equations below are two possible reactions of anaerobic bacteria to make inorganic mercury methylation [9]. The natural production of methylmercury also includes eruptions of volcanoes, volatilizations of the ocean, weathering of rocks containing mercury, and forest fires [10].
\( {2R-CH_{3}}+{Hg^{2+}}→{H_{3}}C-Hg-{CH_{3}}→{H_{3}}C-{Hg^{+}} \)
\( {R-CH_{3}}+{Hg^{2+}}→{H_{3}}C-{Hg^{+}}\overset{R-{CH_{3}}}{→}{H_{3}}C-Hg-{CH_{3}} \)
Besides, a large amount of methylmercury is produced artificially, such as burning of inorganic mercury, and burning of fossil fuels [11]. The amount of MeHg is mainly decided by methylation and demethylation, as shown in Figure 1. MeHg forms through methylation. The removal of MeHg is done by demethylation by replacing CH3 with hydrogen.
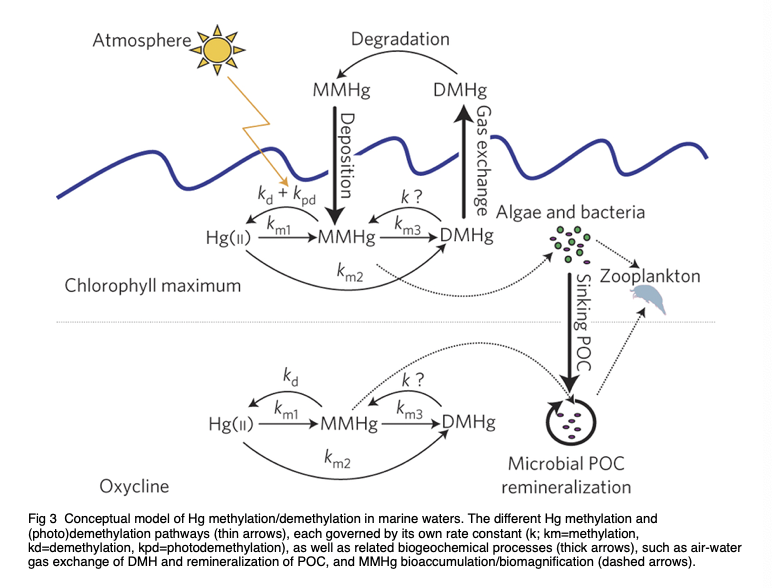
Figure 1. Conceptual model of Hg methylation/ demethylation in marine waters. The various Hg methylation and (photo)demethylation pathways (thin arrows), each governed by their respective rate constants (k; km = methylation, kd = demethylation, kpd = photo demethylation), along with associated biogeochemical process (thick arrows), such as air-water gas exchange of DMHg and remineralization of POC, and MMHg bioaccumulation/biomagnification (dashed arrows) [12].
2.2. Methods and technologies for mercury detection
To understand the concentration of mercury remaining in Minamata Bay and the intake of mercury by animals or humans living nearby, we can use different methods and technologies to assist us in identifying the existence of mercury in the tissue of samples or the concentration of mercury in sediments of Minamata Bay, comprehending the effect of methylmercury in patients who endure Minamata disease better.
1. Mercury in the sample’s tissue can be determined by using ammonium sulfide (NH4)2S and hydrogen sulfate through the observation of brownish back granules [13]. Coprecipitation can be attempted for the removal of residual Hg (II). In the experiment, the coprecipitating agents are bismuth (III), lanthanum (III), and iron (III). The precipitation of 100 ppb Hg(II) or MeHg can be made in 1000 ppm La (III), Bi (III), or Fe (III) with or without the complexing agents, which are L-cysteine and thiourea. Metal hydroxides are precipitated with triethylmaine (TEA). Then, the separation of coprecipitations is made with ammonium sulfide [14].
2. Using diphenyl thiocarbazide, dithizone, and rhodizonate, we can detect the mercury in the sample’s tissue by observing colored precipitation in the metal chelating mechanism. Also, Mercury in the sample’s tissue can be detected by the precipitation reaction by using stannous chloride [13].
3. Methylmercury can be detected by gas chromatography. It is possible that the mercury compound in shellfish was methyl(methylthio)mercury, which is the derivative of methylthio. The methylthio group is removed first when it reacts with alkali because of the higher bond energy of Hg-S than the Hg-NH or Hg-OH bond. The formation of the Hg-S bond is not water-soluble as the Hg-OH and Hg-NH bond do. Also, when the distillation of benzene is under room temperature with reduced pressure, the extraction with ammonium or sodium hydroxide is carried out smoothly without the prevention caused by methanethiol and hydrogen sulfide. The extract is acidified with hydrochloric acid (HCl) to obtain the organomercury compound with benzene, and the benzene solution will be used to determine the methylmercury by gas chromatography after drying with anhydrous sodium sulfate [15].
4. Golgi silver impregnation can help us to identify mercury in human tissues. However, the pre-experiment includes the preparation of thick tissue blocks. The crystallization of granules containing metal silver occurs in the individual cells’ membrane produces black precipitates, which helps us to better observe the cells’ details and dendrites under the golden background [16].
5. Autoradiography is a sensitive and simple technology. It can used to record the spatial distribution of a radiolabeled compound sample. In the study that was conducted by Lippman, Findle, and Gillette, 1951, 203Hg was utilized [17]. The sample of autoradiography is usually a film or section with a low mass of tissues. Thus, a high concentration of radioisotope and exposure time are required for the experiment [18].
6. High precision of identification of stable Hg isotope ratios helps us trace the concentration of mercury in Minamata Bay. The isotopic composition of a pool serves as a signature of the mercury. The history of the mercury’s biogeochemical transformation can be reflected by the signature. Based on the signature, we can trace the amount of mercury through mass-dependent fractionation (MDF) or mass-independent fractionation (MIF). Mass-dependent fractionation signature of Hg can be used to determine the source and activities of Hg in the aquatic system. Mass-independent fractionation can provide information about the source and transformation of Hg in the environmental compartment. In addition, it could provide the condition of MeHg ingestion in the food web [19].
2.3. The health effects caused by methylmercury
However, a mass of MeHg is harmful, since it is toxic due to its volatility and ability to pass through the biological membrane, such as the blood-brain barrier (BBB) and placenta barrier [20]. When we judge the toxicity of a substance, we consider its form, concentration, duration, and route of exposure. For methylmercury, it mainly attacks our central nervous system, which consists of our brain and spinal cord. According to the study by Masumi Marumoto, there is a loss of granules in the cerebellum of subjects who are contaminated by methylmercury [2]. Granules shape the maturation of purkinje cell structure, which is responsible for the inhibition of gamma-aminobutyric acid (GABA) in the human body. If there is excessive GABA, people will present manic symptoms. MeHg also influences people’s cerebrum, which contains the hippocampus, basal ganglia, and olfactory bulb that are related to explicit memory, implicit memory, and sense of smell, respectively.
In both acute and subacute cases, the concentration of inorganic mercury I-Hg gradually increases in chronic cases [2]. Inorganic mercury is toxic, one main object to be attacked is the kidney. However, there is no significant change in the organ throughout the study, and there is no lesion observed. One possible explanation could be the protection from selenium (Se).
In addition, Hg ion is found in microglial cells of the calcarine cortex and glial cells of the cerebellum. The function of microglial is to mediate immune responses in the central nervous system, acting as macrophages by cleaning up dead neurons. For the glial cells, they are in the nervous system and support, nourish, and protect neurons. They may also play a role in learning and thinking.
2.4. Treatments of methylmercury exposure
In the early stages, the following treatments are recommended:
1. Exclusion of methylmercury exposure route and detection: Identify the route through which methylmercury is being exposed and eliminate it.
2. Promotion of methylmercury excretion:
a) Administration of chelating agents: Chemical compounds that can trap methylmercury and facilitate its excretion through urine.
b) Administration of high-affinity SH-compounds: Oral intake of thiol resin to prevent re-absorption of methylmercury from the intestines.
c) Hemodialysis with SH-compound L-cysteine.
d) Blood exchange.
3. Administration of antioxidants:
It has been observed that methylmercury induces cellular damage by increasing reactive oxygen species in tissue cells. Antioxidants such as vitamin E are being studied for their ability to eliminate reactive oxygen species and aid in excretion treatment.
4. Symptomatic treatment:
Since Minamata disease currently has no definitive cure, symptomatic therapies are being used to manage the symptoms.
In the chronic stages, the following treatments are recommended:
1. Rehabilitation:
Physical therapy and occupational therapy are used to improve or maintain physical functions. Fetal Minamata disease patients with progressive impairment in ambulation and dysphagia undergo gait training using techniques such as belt electrode skeletal muscle electrical stimulation (B-SES), HAL (hybrid assistive limb) Single Joint, and unpowered walking assist clutch. Swallowing exercises are also conducted using a combined low-frequency therapy device.
B-SES is a novel neuromuscular electrical stimulation technique that can simultaneously contract lower limb muscle groups. Unlike conventional pad-type electrodes, B-SES can prevent muscle atrophy and reduce skeletal muscle hypoxia, making it an appropriate strategy for preventing muscular contracture. However, its impact on muscle flexibility is yet to be validated.
HAL is an exoskeleton designed for gait training, featuring a hybrid system that enables both voluntary and autonomous modes of action. It is equipped with a control algorithm and accompanying devices, enabling independent control of each knee and hip joint.
2. Treatment
State-of-the-art therapeutic treatment tailored to address specific symptoms, such as muscle cramps, involuntary movement, and abnormal muscle tonus, has been implemented alongside drug therapies to alleviate these symptoms.
Various treatment options have been explored to alleviate the symptoms and improve the quality of life for affected individuals. Chelation therapy involving the administration of chelating agents such as dimercaptosuccinic acid (DMSA) has shown promise in removing mercury from the body. Additionally, supportive therapies like physical rehabilitation and speech therapy have been effective in managing the motor and communication difficulties associated with the disease.[21]
3. Result
The narrative of Minamata disease, which has been controversial since the 1950s, has traditionally attributed the cause of the disease to inorganic mercury and methylmercury. However, new Density Functional Theory (DFT) calculations discovered that methylmercury does not have an energetically viable mechanism for forming acetaldehyde. Instead, these calculations suggest that the dumped form may be α-mercuri-acetaldehyde or a related chemical species, which are currently unexplored organic mercury compounds. Known for their stability and high melting points, these compounds may have been responsible for the devastating Minamata disease outbreak linked to the Chisso factory. Furthermore, this research suggests that previous instances of organic mercury poisoning, where methylmercury was assumed to be the cause, may have been misidentified. This highlights the need for further toxicological studies on alternative organic mercury species. Identifying chemical species accurately in toxicological research is crucial for understanding the mechanisms affecting both biological and environmental health.
4. Conclusion
This study begins by addressing the severe disease caused by mercury and subsequently focuses on providing fundamental information about organic mercury and its detrimental effects on human health. Additionally, this paper explores various methods used for detecting mercury. Through a thorough review of existing literature, our study consolidates past findings and emphasizes the need for further toxicological research to obtain a comprehensive understanding of the mechanism behind organic mercury exposure and its impacts on both humans and the environment. Previous experiments have shown that organic mercury primarily affects the nervous system, but the health effects are complex and multifaceted. To mitigate the adverse effects of organic mercury exposure on the general population, this paper also suggests several potential therapeutic approaches for individuals who have accidentally come into contact with mercury. However, it is important to note that due to limited access to primary data, the conclusions drawn in this study may be subject to some degree of uncertainty. It is imperative for future research to gather more first-hand evidence to ensure the reliability of the results.
Acknowledgement
Weiqiang Yan, Sinuo Li, and Zhenyu Li contributed equally to this work and should be considered co-first authors.
References
[1]. Takeuchi, T., et al. “A Pathological Study of Minamata Disease in Japan.” Acta Neuropathologica, vol. 2, no. 1, 1962, pp. 40–57, https://doi.org/10.1007/bf00685743.
[2]. Marumoto, M., et al. “Mercury and Selenium Localization in the Cerebrum, Cerebellum, Liver, and Kidney of a Minamata Disease Case.” ACTA HISTOCHEMICA et CYTOCHEMICA, vol. 53, no. 6, Dec. 2020, pp. 147–55, https://doi.org/10.1267/ahc.20-00009. Accessed 15 Feb. 2023.
[3]. Murata, K. and Sakamoto, M. (2013) ‘Minamata disease’, Encyclopedia of Environmental Health, pp. 401–407. https://doi:10.1016/b978-0-12-409548-9.02075-3.
[4]. Woolf, A.D. (2022) ‘Dimethylmercury Death—professor Wetterhahn, 1996’, History of Modern Clinical Toxicology, pp. 177–182. doi:10.1016/b978-0-12-822218-8.00054-5.
[5]. Goldwater, L. J., et al. “Absorption and Excretion of Mercury in Man. I. Relationship of Mercury in Blood and Urine.” Archives of Environmental Health: An International Journal, pp. 537–41, https://doi.org/10.1080/00039896.1962.10663327.
[6]. Canty, A. J.; Chaichit, N.; Gatehouse, B. M.; George, E. E.; Hayhurst, G. (1981). “Coordination chemistry of methylmercury(II). Synthesis, hydrogen-1 NMR, and crystallographic studies of cationic complexes of Me Hg(II) with ambidentate and polydentate ligands containing pyridyl and N-substituted imidazolyl donors and involving unusual coordination geometries”. Inorganic Chemistry. 20 (8): 2414–2422. https://doi.org/10.1021/ic50222a011
[7]. Nolan, E. M., Lippard, Stephen J. (2008). “Tools and Tactics for the Optical Detection of Mercuric Ion”. Chemical Reviews. 108 (9): 3443–3480. https://doi:10.1021/cr068000q. PMID 18652512
[8]. Ullrich, S. M., et al. “Mercury in the Aquatic Environment: A Review of Factors Affecting Methylation.” Critical Reviews in Environmental Science and Technology, vol. 31, no. 3, July 2001, pp. 241–93, https://doi.org/10.1080/20016491089226. Accessed 17 Aug. 2023.
[9]. Lin, H., Ascher, D.B., Myung, Y. et al. Mercury methylation by metabolically versatile and cosmopolitan marine bacteria. ISME J 15, 1810–1825 (2021). https://doi.org/10.1038/s41396-020-00889-4
[10]. “Mercury in the Environment.” Web.archive.org, 18 July 2015, web.archive.org/web/20150718192245/www.usgs.gov/themes/factsheet/146-00/. Accessed 17 Aug. 2023.
[11]. US EPA, OAR. “Technical Air Pollution Resources.” US EPA, 10 Aug. 2016, www.epa.gov/technical-air-pollution-resources. Accessed 17 Aug. 2023.
[12]. Lehnherr, I. & Louis, St & Hintelmann, H. & Kirk,J. L. (2011). Methylation of Inorganic Mercury in Polar Marine Waters. Nature Geoscience. 4. 298-302. https://doi:10.1038/ngeo1134.
[13]. Sakai, K., et al. “Histochemical Demonstration of Mercury in Humantissue Cells of Minamata Disease by Use of autoradiographic procedure.” ACTA HISTOCHEMICA et CYTOCHEMICA, vol. 8, no. 4, 1975, pp. 257–64, https://doi.org/10.1267/ahc.8.257. Accessed 11 Aug. 2023.
[14]. Arslan, Z. Determination of Methylmercury Levels in Mercury- Contaminated Soils from Oak Ridge TN by Cold Vapor Generation. 2018, nemc.us/docs/2018/presentations/pdf/Tuesday-Metals%20and%20Metals%20Speciation%20Analysis%20in%20Environmental%20Samples-15.3-Arslan.pdf. Accessed 17 Aug. 2023.
[15]. Westoo, G. “Determination of methylmercury compounds in foodstuffs.” Actu Chem (1966). Vol.20 pp.2131-2137
[16]. Watson, C. and George P. “Silver Impregnation - an Overview | ScienceDirect Topics.” Www.sciencedirect.com, 2010, www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/silver-impregnation. Accessed 11 Aug. 2023.
[17]. Lippman, R., et al. “Effect of Proteinuria on Localization of Radiomercury in Rat Kidney.” Sage Journals, May 1951, doi.org/10.3181/00379727-77-1868. Accessed 11 Aug. 2023.
[18]. Raje, M., and Gyan M. “Autoradiography - an Overview | ScienceDirect Topics.” Www.sciencedirect.com, 1998, www.sciencedirect.com/topics/agricultural-and-biological-sciences/autoradiography#:~:text=Autoradiography%20is%20a%20simple%20and. Accessed 11 Aug. 2023.
[19]. Balogh, S. J., et al. “Tracking the Fate of Mercury in the Fish and Bottom Sediments of Minamata Bay, Japan, Using Stable Mercury Isotopes.” Environmental Science & Technology, vol. 49, no. 9, Apr. 2015, pp. 5399–406, https://doi.org/10.1021/acs.est.5b00631.
[20]. Gupta, R, et al. “Methylmercury - an Overview | ScienceDirect Topics.” Www.sciencedirect.com, 9 Feb. 2018, www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/methylmercury#:~:text=Methylmercury%20is%20the%20most%20toxic. Accessed 5 Aug. 2023.
[21]. K. Eto, M. Marumoto, M. Takeya, “The Pathology of Methylmercury Poisoning (Minamata Disease)”, Neuropathology 30, 471 (2010)
Cite this article
Yan,W.;Li,S.;Li,Z. (2024). Properties of methylmercury and its physiological effect on the human body. Applied and Computational Engineering,84,27-34.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Takeuchi, T., et al. “A Pathological Study of Minamata Disease in Japan.” Acta Neuropathologica, vol. 2, no. 1, 1962, pp. 40–57, https://doi.org/10.1007/bf00685743.
[2]. Marumoto, M., et al. “Mercury and Selenium Localization in the Cerebrum, Cerebellum, Liver, and Kidney of a Minamata Disease Case.” ACTA HISTOCHEMICA et CYTOCHEMICA, vol. 53, no. 6, Dec. 2020, pp. 147–55, https://doi.org/10.1267/ahc.20-00009. Accessed 15 Feb. 2023.
[3]. Murata, K. and Sakamoto, M. (2013) ‘Minamata disease’, Encyclopedia of Environmental Health, pp. 401–407. https://doi:10.1016/b978-0-12-409548-9.02075-3.
[4]. Woolf, A.D. (2022) ‘Dimethylmercury Death—professor Wetterhahn, 1996’, History of Modern Clinical Toxicology, pp. 177–182. doi:10.1016/b978-0-12-822218-8.00054-5.
[5]. Goldwater, L. J., et al. “Absorption and Excretion of Mercury in Man. I. Relationship of Mercury in Blood and Urine.” Archives of Environmental Health: An International Journal, pp. 537–41, https://doi.org/10.1080/00039896.1962.10663327.
[6]. Canty, A. J.; Chaichit, N.; Gatehouse, B. M.; George, E. E.; Hayhurst, G. (1981). “Coordination chemistry of methylmercury(II). Synthesis, hydrogen-1 NMR, and crystallographic studies of cationic complexes of Me Hg(II) with ambidentate and polydentate ligands containing pyridyl and N-substituted imidazolyl donors and involving unusual coordination geometries”. Inorganic Chemistry. 20 (8): 2414–2422. https://doi.org/10.1021/ic50222a011
[7]. Nolan, E. M., Lippard, Stephen J. (2008). “Tools and Tactics for the Optical Detection of Mercuric Ion”. Chemical Reviews. 108 (9): 3443–3480. https://doi:10.1021/cr068000q. PMID 18652512
[8]. Ullrich, S. M., et al. “Mercury in the Aquatic Environment: A Review of Factors Affecting Methylation.” Critical Reviews in Environmental Science and Technology, vol. 31, no. 3, July 2001, pp. 241–93, https://doi.org/10.1080/20016491089226. Accessed 17 Aug. 2023.
[9]. Lin, H., Ascher, D.B., Myung, Y. et al. Mercury methylation by metabolically versatile and cosmopolitan marine bacteria. ISME J 15, 1810–1825 (2021). https://doi.org/10.1038/s41396-020-00889-4
[10]. “Mercury in the Environment.” Web.archive.org, 18 July 2015, web.archive.org/web/20150718192245/www.usgs.gov/themes/factsheet/146-00/. Accessed 17 Aug. 2023.
[11]. US EPA, OAR. “Technical Air Pollution Resources.” US EPA, 10 Aug. 2016, www.epa.gov/technical-air-pollution-resources. Accessed 17 Aug. 2023.
[12]. Lehnherr, I. & Louis, St & Hintelmann, H. & Kirk,J. L. (2011). Methylation of Inorganic Mercury in Polar Marine Waters. Nature Geoscience. 4. 298-302. https://doi:10.1038/ngeo1134.
[13]. Sakai, K., et al. “Histochemical Demonstration of Mercury in Humantissue Cells of Minamata Disease by Use of autoradiographic procedure.” ACTA HISTOCHEMICA et CYTOCHEMICA, vol. 8, no. 4, 1975, pp. 257–64, https://doi.org/10.1267/ahc.8.257. Accessed 11 Aug. 2023.
[14]. Arslan, Z. Determination of Methylmercury Levels in Mercury- Contaminated Soils from Oak Ridge TN by Cold Vapor Generation. 2018, nemc.us/docs/2018/presentations/pdf/Tuesday-Metals%20and%20Metals%20Speciation%20Analysis%20in%20Environmental%20Samples-15.3-Arslan.pdf. Accessed 17 Aug. 2023.
[15]. Westoo, G. “Determination of methylmercury compounds in foodstuffs.” Actu Chem (1966). Vol.20 pp.2131-2137
[16]. Watson, C. and George P. “Silver Impregnation - an Overview | ScienceDirect Topics.” Www.sciencedirect.com, 2010, www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/silver-impregnation. Accessed 11 Aug. 2023.
[17]. Lippman, R., et al. “Effect of Proteinuria on Localization of Radiomercury in Rat Kidney.” Sage Journals, May 1951, doi.org/10.3181/00379727-77-1868. Accessed 11 Aug. 2023.
[18]. Raje, M., and Gyan M. “Autoradiography - an Overview | ScienceDirect Topics.” Www.sciencedirect.com, 1998, www.sciencedirect.com/topics/agricultural-and-biological-sciences/autoradiography#:~:text=Autoradiography%20is%20a%20simple%20and. Accessed 11 Aug. 2023.
[19]. Balogh, S. J., et al. “Tracking the Fate of Mercury in the Fish and Bottom Sediments of Minamata Bay, Japan, Using Stable Mercury Isotopes.” Environmental Science & Technology, vol. 49, no. 9, Apr. 2015, pp. 5399–406, https://doi.org/10.1021/acs.est.5b00631.
[20]. Gupta, R, et al. “Methylmercury - an Overview | ScienceDirect Topics.” Www.sciencedirect.com, 9 Feb. 2018, www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/methylmercury#:~:text=Methylmercury%20is%20the%20most%20toxic. Accessed 5 Aug. 2023.
[21]. K. Eto, M. Marumoto, M. Takeya, “The Pathology of Methylmercury Poisoning (Minamata Disease)”, Neuropathology 30, 471 (2010)









