1. Introduction
Domestic wastewater treatments play an important role in the human society. Basically, water treatments can be separated into three levels of water processing. The first level is primary treatment, which is the process of solid removal; the second level is secondary treatment, which is the decomposition of bacteria; the third level is tertiary treatment, which is extra filtration of water. Therefore, Different levels of treatment get rid of certain matter inside the source water, from macro to micro matters. Through these levels of processing, the water would become safe for municipal use.
To be specific, the primary treatment includes coagulation and sedimentation, the physical separation. With the method of primary sedimentation tank, which is gravity-fed, removes the suspended solids or organic matter such as grits and mud clot as they settle down and be filtrated by clarifier. Primary treatment could remove about 50% to 70% of suspended solids, and 25% to 40% of biological oxygen demand (BOD) [1]. The secondary treatment includes suspended-growth systems, the biological decomposition [2]. For example, the aerobic granular sludge (AGS) technology, which serves for dissolved matter removal, gets rid of chemical oxygen demand (COD), nitrogen (N), and phosphorus (P) inside the water through aerobic, anoxic anaerobic layers processes in a single granule with microorganisms [3]. Tertiary treatment is the process of the final purification of the water, which is also known as advanced treatment, includes further biological nutrient removal after secondary treatment.
To generalize the property of tertiary treatment, this treatment is the final disinfection process that is important to the ultimate water quality of water transmitted to domestic users, which is the main focus of this thesis: the authors made an effort in researching, summarizing, and comparing the advantages and disadvantages between various tertiary treatment techniques, therefore presenting the necessity of tertiary treatment in overall domestic wastewater treatment.
2. Three ways used in tertiary treatment
2.1. Membrane filtration
Membrance filtration has the pervasive and common method of pressure-driven. As the water solution is permeating through different membranes with different sizes of pores, thus achieving the physical filtration of the undesired matter. Here are some of the pressure-driven filtrations: micro filtration (MF), ultra filtration (UF), nano filtration (NF), and reverse osmosis (RO). Besides, there are also electrodialysis, gas separation, and pervaporation, which are not the most commonly used method that won’t be discussed in this passage.
Firstly, Pressures between 100 and 400 kPa are generally set for micro filtration modules to function at, which has a pore size on a scale of 1 μm [4]. These pressures enable the removal of bacteria, protozoa, and other debris like sand, clay, and cracks. Secondly, smaller pore sizes allow ultra filtration (UF) to reject bigger dissolved molecules. Thirdly, nano filtration (NF) group of membranes includes those that can reject tiny dissolved molecules and divalent ions. These membranes include pores that are 1 nm in size. Last but not least, reverse osmosis (RO) lacks holes and separates materials based on how quickly various solutes diffuse through the membrane’s polymer. Even monoval
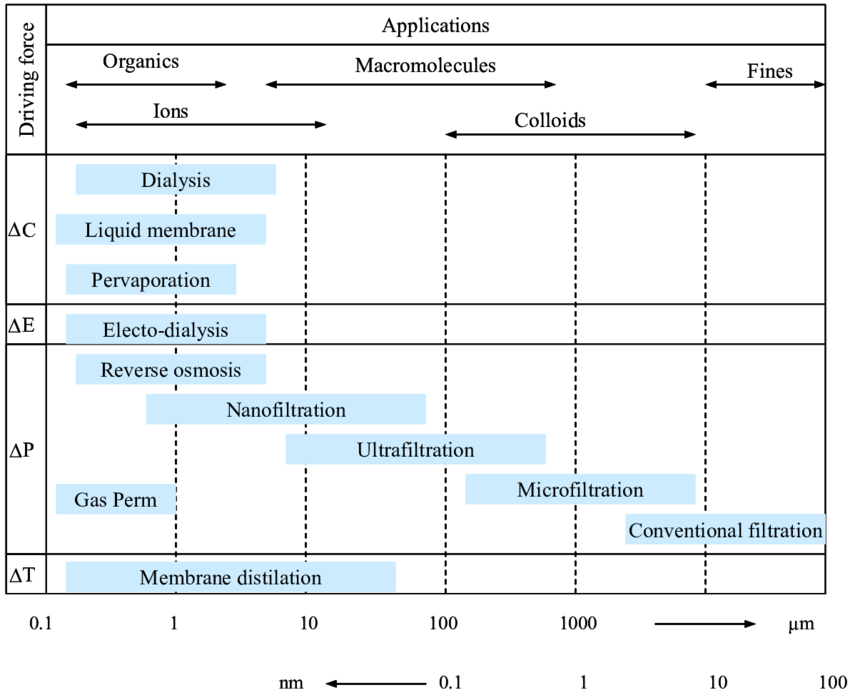
Figure 1. Range of membrane filtration [5].
There are both some advantages and disadvantages of membrane filtration. For advantages, firstly, membrane filtration is largely available from commercial manufacturers, and it has a number of applications: Diverse real-world uses include clarifying or sterile filtration (MF), polymer separation (UF), the removal of multivalent ions and nonionic solutes (ED), the desalination and generation of pure water (RO), and the extraction of salts from polymer solutions [6]. Secondly, it efficiently eliminates particles, suspended solids, and microorganisms by MF, UF, NF, and RO process. Volatile and nonvolatile organics are removed by NF and RO as well, thus the final effluent has high-quality of clearness. For Disadvantages, firstly, membrane filtration is costly for small industries to put into use, the design of membrane would be different according to environment, and energy consumed by constant pressure input is high. Finally, the membrane itself may face the problem of clogging as high concentration of water solution would generate bio-fouling onto the membrane [7].
2.2. Chlorination
2.2.1. Chlorination is extensively employed in tertiary treatment as one of the most favored disinfection methods. The rationale behind its widespread use lies not only in the ready availability of chlorine in gaseous, liquid, and solid forms but also in its ease of application owing to its high solubility in water. Among the three chlorine states, gaseous chlorine stands out as the most cost-effective approach in larger public water treatment facilities, offering economical disinfection. Additionally, when compared to chlorine solutions, gaseous chlorine demands less storage space.
2.2.2. A series of reactions occurs during the chlorination process with chlorides. Hydrolysis takes place when chlorine reacts with water, resulting in the formation of hypochlorous acid and hydrochloric acid.
\( {Cl_{2}} + {H_{2}}O → HOCl + HCl \)
Sodium hypochlorite reacts with water as follows:
\( NaOCl + {H_{2}}O → NaOH + NaCl \)
Chlorine reacts with water, producing hypochlorous acid (HOCl), which dissociates to form the hypochlorite ion [8].
\( HOCl → {OCl^{-}} + {H^{+}} \)
2.2.3. There are both advantages and disadvantages to using chlorination in tertiary treatment. Regarding the advantages, as previously mentioned, chlorine is readily available in all its forms and exhibits high solubility in water. Chlorine proves to be the most effective disinfectant for deactivating waterborne pathogens. Moreover, it’s essential to recognize that waterborne diseases were prevalent until the early 20th century. While earlier treatment methods eliminated many contaminants from drinking water, chlorine was the only solution that effectively reduced pathogens in the water supply. Since the widespread adoption of chlorination in the United States, waterborne illnesses such as typhoid, dysentery, and cholera have largely vanished [9]. As for the disadvantages, chlorine has a distinct odor. Furthermore, it can lead to skin irritation, as it opens up pores and strips the skin of its natural oils. This eventually results in dryness, irritation, and itchiness, with individuals with sensitive skin being particularly affected. This is due to excessive chlorine exposure, which can cause skin rashes, redness, and inflammation, potentially developing into blisters with continued chemical exposure [10].
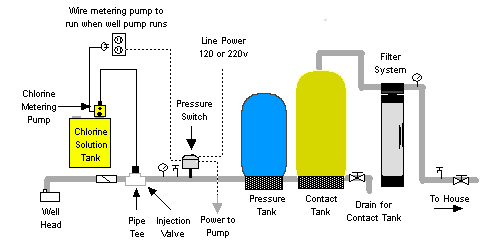
Figure 2. CHLORINATORS | Water Treatment | Waste Water Treatment | Water Treatment Process & Plant Design [1].
2.3. Photo-Fenton
Photo fenton is widely used among the advanced oxidation processes because of its low operational costs. In the study of removing sulfamethazine, the researchers found that this kind of antibiotics is totally degradated, but they also found that the TOC (total organic carbon) reduction reached to 56% [11]; on another study of photo-fenton directed by Trovo, when they use the photo-fenton process, the reduction of amoxicillin is perfect, and the TOC removal reached 81% [12].
It was quite effective of the photo-fenton (using sunlights) that they can easily remove the ARG (aquifer remediation goal) and ARB (anoxic recirculation basin)’s waste. As the research proposed by Miralles-Cuevas, when the combination of NF (nanofiltration) and solar photo-fenton is used, the various kinds of pH, like carbamazepine, flumequine, and so on, can be almost totally removed from the urban wastewater [13]. Moreover, when the process of solar photo-fenton is employed with SBR (sequencing batch reactor), this process can be used to address the concern of the antibiotic wastewater performance, and finally succeed in letting about 89% of the soluble COD decreasing [14].
Not Photo-fenton is more effective in removing the ARG and ARB, but solar photo-fenton is very kind to the environment impact. As the report directed by Rodriguez and co-workers, [15]. when they study the impact to the environment by proposing the LCA (life cycle assessment) into heterogeneous and homogeneous fenton process, they found that heterogeneous fenton emit lower GHG (green house gas) of 0.04 carbon dioxide (aquifer), and when Gallego- Schmid and his co-workers used this data to do further research, [16]. they found that in the acid pH condition, solar photo-fenton addressing the urban wastewater treatment can release about 554 carbon dioxide (aquifer)/1000m^3, which is lower than the operational system in the neutral pH condition.
In this way, considering all the research above, the specializer concludes that this process can be successfully used to make the environment continuous because of their improvement in degradation of recalcitrant pollutants.
2.4. Advantages and disadvantages Comparison
All three methods for water treatment have different advantages and disadvantages. Here is a comparison for those three methods.
First, For Membrane filtration, one of its major advantages is its ability to effectively remove various contaminants, including bacteria, viruses, suspended solids, and certain chemicals. This process ensures the production of clean and safe drinking water. Another advantage is the flexibility of the membrane filtration system, which can be easily scaled up or down according to the specific needs of the water treatment facility. In addition, membrane filtration provides a high level of automation, reducing the need for manual intervention and minimizing operational costs. However, there are some disadvantages to consider. Membrane filtration systems are relatively expensive to install and maintain, requiring regular cleaning and replacement of membranes. The pollution of organic matter or scale on the membrane will reduce the efficiency of the membrane and increase the operating cost. In addition, membrane filtration may not be effective in removing certain contaminants, such as dissolved salts or certain organic compounds. In such cases, additional treatment processes may be required, increasing the complexity and cost of the entire water treatment system.
Second, Chlorination is a commonly used method for water treatment. It is a powerful disinfectant that can kill a wide range of harmful microorganisms, including bacteria, viruses, and parasites. It helps to prevent the spread of waterborne diseases and can provide residual protection by remaining in the water distribution system, preventing the growth of microorganisms and ensuring the water remains safe during storage and distribution. Also, Chlorine is relatively inexpensive and widely available, making it a cost-effective option for large-scale water treatment which is still being commonly used.
However, Chlorine can react with organic matter in water to form disinfection byproducts, such as trihalomethanes (THMs) and haloacetic acids (HAAs). Some of these DBPs are known to be carcinogenic and can pose health risks. Then Chlorine can impart a noticeable taste and odor to the water, which some people find unpleasant. This can affect the overall acceptability of the treated water. Also, Environmental impact: Chlorine can have adverse effects on aquatic life and ecosystems when discharged into the environment. It can harm fish and other organisms in water bodies. And even though chlorine is effective against many microorganisms, it may not be as effective against certain pathogens which has a protective outer shell that can resist chlorine disinfection [17].
Furthermore, Photo-Fenton is also a good choice for domestic water treatment, it is a powerful oxidation process that can effectively degrade a wide range of organic pollutants in water. It can break down complex organic compounds into simpler and less harmful substances. This method can be applied to treat various types of water pollutants, including organic dyes, pesticides, pharmaceuticals, and industrial wastewater. It offers versatility in addressing different types of contaminants. The reaction rate of Photo-Fenton is relatively fast, allowing for efficient treatment of contaminated water within a shorter time frame compared to some other treatment methods. It utilizes natural sunlight or artificial UV light as the energy source, eliminating the need for additional chemicals in the treatment process. This can reduce the overall chemical usage and associated costs.
However, Photo-Fenton relies on the availability of light sources, whether natural sunlight or artificial ultraviolet light, to activate the process. This dependence may limit its applicability in areas where sunlight exposure is limited or artificial UV rays are not readily available. The efficiency of photofenton is affected by water quality parameters such as pH, temperature, and the presence of certain ions. In order for the process to work effectively, optimum conditions need to be maintained. The oxidation process in photofenton produces sludge, which requires proper treatment and disposal. Sludge management adds complexity and cost to the overall treatment process. If artificial ultraviolet light is used as a light source, it consumes a lot of energy, resulting in higher operating costs compared to natural sunlight.
When considering the use of photo-fenton for water treatment, it is important to evaluate these advantages and disadvantages in the context of specific water quality and treatment requirements. Proper optimization and monitoring are essential to ensure the effectiveness and efficiency of the process.
3. Conclusion
In our daily life, water is becoming more and more necessary for our daily life, and as the technological progress has greatly boosted, the tertiary treatment for the daily wastewater is becoming better and efficient. Although there might some advantages or disadvantages for different kinds of tertiary wastewater treatment, but we ultimately believe that as people can use them more and more proficient and effective, the wastewater treatment can be finally better and better, which will boost civilization for a future better life.
References
[1]. Metcalf & Eddy (2014). Wastewater engineering: treatment and resource recovery. George Tchobanoglous, H. David Stensel, Ryujiro Tsuchihashi, Franklin L. Burton, Mohammad Abu-Orf, Gregory Bowden (Fifth ed.). New York, NY. ISBN 978-0-07-340118-8. OCLC 858915999.
[2]. Wastewater engineering: treatment and reuse. George Tchobanoglous, Franklin L. Burton, H. David Stensel, Metcalf & Eddy (4th ed.). Boston: McGraw-Hill. 2003. ISBN 0-07-041878-0. OCLC 48053912.
[3]. Sadiye Kosar, Onur Isik, Busra Cicekalan, Hazal Gulhan, Ece Sagir Kurt, Ezgi Atli, Safak Basa, Hale Ozgun, Ismail Koyuncu, Mark C.M. van Loosdrecht, Mustafa Evren Ersahin, Impact of primary sedimentation on granulation and treatment performance of municipal wastewater by aerobic granular sludge process, Journal of Environmental Management, Volume 315, 2022, 115191, ISSN 0301-4797, https://doi.org/10.1016/j.jenvman.2022.115191.
[4]. Baker, R 2012, Microfiltration, in Membrane Technology and Applications, 3rd edn, John Wiley & Sons Ltd, California p. 303-324
[5]. Tan, X., & Rodrigue, D. (2019). A Review on Porous Polymeric Membrane Preparation. Part I: Production Techniques with Polysulfone and Poly (Vinylidene Fluoride). Polymers, 11.
[6]. Zagklis DP, Bampos G. Tertiary Wastewater Treatment Technologies: A Review of Technical, Economic, and Life Cycle Aspects. Processes. 2022; 10(11):2304. https://doi.org/10.3390/pr10112304
[7]. Grégorio Crini, Eric Lichtfouse. Advantages and disadvantages of techniques used for wastewater treatment. Environmental Chemistry Letters (2019) 17:145–155 https:// doi.org/10.1007/s10311-018-0785-9
[8]. By Supriya N, Chlorination in Wastewater Treatment from: https://Chlorination in Wastewater Treatment - Meaning, Factors Affecting & Process -Biology Reader
[9]. Dominic O’Donnell on July 26, 2021,Wastewater Chlorination: Everything You Need To Know from: https://Wastewater Chlorination: Everything You Need To Know - Sensorex Liquid Analysis Technology
[10]. Advantages and Disadvantages of Chlorination of Water from: https://10 Benefits and Drawbacks of Chlorination of Water - Tech Quintal
[11]. Pérez-Moya, M.; Graells, M.; Castells, G.; Amigó, J.; Ortega, E.; Buhigas, G.; Pérez, L.M.; Mansilla, H.D. Characterization of the degradation performance of the sulfamethazine antibiotic by photo-Fenton process. Water Res. 2010, 44, 2533–2540 [Google Scholar] [CrossRef]
[12]. Trovó, A.G.; Pupo Nogueira, R.F.; Agüera, A.; Fernandez-Alba, A.R.; Malato, S. Degradation of the antibiotic amoxicillin by photo-Fenton process—Chemical and toxicological assessment. Water Res. 2011, 45, 1394–1402 [Google Scholar] [CrossRef] [PubMed] [Green Version]
[13]. Miralles-Cuevas, S.; Oller, I.; Ruiz Aguirre, A.; Sánchez Pérez, J.A.; Malato Rodríguez, S. Removal of pharmaceuticals at microg L−1 by combined nanofiltration and mild solar photo-Fenton. Chem. Eng. J. 2014, 239, 68–74 [Google Scholar] [CrossRef]
[14]. Elmolla, E.S.; Chaudhuri, M. Combined photo-Fenton–SBR process for antibiotic wastewater treatment. J. Hazard. Mater. 2011, 192, 1418–1426 [Google Scholar] [CrossRef]
[15]. Rodríguez, R.; Espada, J.J.; Pariente, M.I.; Melero, J.A.; Martínez, F.; Molina, R. Comparative life cycle assessment (LCA) study of heterogeneous and homogenous Fenton processes for the treatment of pharmaceutical wastewater. J. Clean. Prod. 2016, 124, 21–29 [Google Scholar] [CrossRef]
[16]. Gallego-Schmid, A.; Tarpani, R.R.Z.; Miralles-Cuevas, S.; Cabrera-Reina, A.; Malato, S.; Azapagic, A. Environmental assessment of solar photo-Fenton processes in combination with nanofiltration for the removal of micro-contaminants from real wastewaters. Sci. Total Environ. 2019, 650, 2210–2220 [Google Scholar] [CrossRef].
[17]. Rakshit Ameta, Anil K. Chohadia, Abhilasha Jain, Pinki B. Punjabi, Chapter 3 - Fenton and Photo-Fenton Processes, Editor(s): Suresh C. Ameta, Rakshit Ameta, Advanced Oxidation Processes for Waste Water Treatment, Academic Press, 2018, Pages 49-87
Cite this article
Chang,J.;He,Z.;Shen,Q.;Xu,S. (2024). Exploration of the importance of tertiary treatment in domestic wastewater treatment. Applied and Computational Engineering,84,173-178.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Metcalf & Eddy (2014). Wastewater engineering: treatment and resource recovery. George Tchobanoglous, H. David Stensel, Ryujiro Tsuchihashi, Franklin L. Burton, Mohammad Abu-Orf, Gregory Bowden (Fifth ed.). New York, NY. ISBN 978-0-07-340118-8. OCLC 858915999.
[2]. Wastewater engineering: treatment and reuse. George Tchobanoglous, Franklin L. Burton, H. David Stensel, Metcalf & Eddy (4th ed.). Boston: McGraw-Hill. 2003. ISBN 0-07-041878-0. OCLC 48053912.
[3]. Sadiye Kosar, Onur Isik, Busra Cicekalan, Hazal Gulhan, Ece Sagir Kurt, Ezgi Atli, Safak Basa, Hale Ozgun, Ismail Koyuncu, Mark C.M. van Loosdrecht, Mustafa Evren Ersahin, Impact of primary sedimentation on granulation and treatment performance of municipal wastewater by aerobic granular sludge process, Journal of Environmental Management, Volume 315, 2022, 115191, ISSN 0301-4797, https://doi.org/10.1016/j.jenvman.2022.115191.
[4]. Baker, R 2012, Microfiltration, in Membrane Technology and Applications, 3rd edn, John Wiley & Sons Ltd, California p. 303-324
[5]. Tan, X., & Rodrigue, D. (2019). A Review on Porous Polymeric Membrane Preparation. Part I: Production Techniques with Polysulfone and Poly (Vinylidene Fluoride). Polymers, 11.
[6]. Zagklis DP, Bampos G. Tertiary Wastewater Treatment Technologies: A Review of Technical, Economic, and Life Cycle Aspects. Processes. 2022; 10(11):2304. https://doi.org/10.3390/pr10112304
[7]. Grégorio Crini, Eric Lichtfouse. Advantages and disadvantages of techniques used for wastewater treatment. Environmental Chemistry Letters (2019) 17:145–155 https:// doi.org/10.1007/s10311-018-0785-9
[8]. By Supriya N, Chlorination in Wastewater Treatment from: https://Chlorination in Wastewater Treatment - Meaning, Factors Affecting & Process -Biology Reader
[9]. Dominic O’Donnell on July 26, 2021,Wastewater Chlorination: Everything You Need To Know from: https://Wastewater Chlorination: Everything You Need To Know - Sensorex Liquid Analysis Technology
[10]. Advantages and Disadvantages of Chlorination of Water from: https://10 Benefits and Drawbacks of Chlorination of Water - Tech Quintal
[11]. Pérez-Moya, M.; Graells, M.; Castells, G.; Amigó, J.; Ortega, E.; Buhigas, G.; Pérez, L.M.; Mansilla, H.D. Characterization of the degradation performance of the sulfamethazine antibiotic by photo-Fenton process. Water Res. 2010, 44, 2533–2540 [Google Scholar] [CrossRef]
[12]. Trovó, A.G.; Pupo Nogueira, R.F.; Agüera, A.; Fernandez-Alba, A.R.; Malato, S. Degradation of the antibiotic amoxicillin by photo-Fenton process—Chemical and toxicological assessment. Water Res. 2011, 45, 1394–1402 [Google Scholar] [CrossRef] [PubMed] [Green Version]
[13]. Miralles-Cuevas, S.; Oller, I.; Ruiz Aguirre, A.; Sánchez Pérez, J.A.; Malato Rodríguez, S. Removal of pharmaceuticals at microg L−1 by combined nanofiltration and mild solar photo-Fenton. Chem. Eng. J. 2014, 239, 68–74 [Google Scholar] [CrossRef]
[14]. Elmolla, E.S.; Chaudhuri, M. Combined photo-Fenton–SBR process for antibiotic wastewater treatment. J. Hazard. Mater. 2011, 192, 1418–1426 [Google Scholar] [CrossRef]
[15]. Rodríguez, R.; Espada, J.J.; Pariente, M.I.; Melero, J.A.; Martínez, F.; Molina, R. Comparative life cycle assessment (LCA) study of heterogeneous and homogenous Fenton processes for the treatment of pharmaceutical wastewater. J. Clean. Prod. 2016, 124, 21–29 [Google Scholar] [CrossRef]
[16]. Gallego-Schmid, A.; Tarpani, R.R.Z.; Miralles-Cuevas, S.; Cabrera-Reina, A.; Malato, S.; Azapagic, A. Environmental assessment of solar photo-Fenton processes in combination with nanofiltration for the removal of micro-contaminants from real wastewaters. Sci. Total Environ. 2019, 650, 2210–2220 [Google Scholar] [CrossRef].
[17]. Rakshit Ameta, Anil K. Chohadia, Abhilasha Jain, Pinki B. Punjabi, Chapter 3 - Fenton and Photo-Fenton Processes, Editor(s): Suresh C. Ameta, Rakshit Ameta, Advanced Oxidation Processes for Waste Water Treatment, Academic Press, 2018, Pages 49-87









