1. Introduction
Lithium metal batteries (LMBs) can exhibit a higher theoretical energy density (over 3860 mAh/g) as compared to the Li ion batteries [1]. However, the current lithium metal anode has serious problems, including uneven Li deposition on the anode and the accompanying severe Li dendrite growth, which leads to rapid capacity decay and safety issues such as short circuits and explosions of lithium batteries. Solid electrolyte interface (SEI) can be used to affect the regulation of Li deposition. Recent work has showed that the SEI layer serves multiple roles in the capacity and stability of batteries [2]. The formation reaction of the solid electrolyte interphase is one of the major side reactions that limiting the number of lithium ions available for cyclic charging. When the battery is charged and discharged for the first time, the parasitic electrochemical reactions take place synchronously. These reactions will decompose the electrolyte, consume the cyclable lithium ions and thereby result in capacity fade of batteries. The SEI also brings some positive effects, including separating the active material and the electrolyte, limiting their side-reactions, reducing the dead lithium formed during charging and suppressing the dendrites [3]. Every battery system will suffer from a common problem of growth of dendrites, which inhibit wide scale commercial application of rechargeable cells. Dendrites penetrating the separator and reaching the cathode will result in a short circuit, which might lead to thermal runaway, burning, or even explosions. Also, SEI plays an important role in promoting uniform metal deposition. However, several challenges need to be addressed to improve the stability and safety of SEI layers [4, 5]. These include the thickness and mechanical properties which is associated with the crack on the surface of anode, the grain boundaries (GBs) distribution in the SEI layer, stability in high/low temperature.
In this research, the relationship between the physical, chemical structure of the solid electrolyte interphase and how the structure of SEI layer improves the lithium metal battery will be described, in order to achieve a high energy density and long revisable lifespan. Also, some difference design cases of the SEI layers on the anodes are discussed, with a focus on the brand-new structural understandings discovered by cutting-edge analytical methods. In this research, many methods for enhancing SEI stability are discussed, such as merging a native SEI with a membrane to create a hybrid composite. Exciting and counterintuitive facts are highlighted, such as how the SEI structure and stability are significantly influenced by the metal anode's roughness.
2. SEI background
During the first charging and discharging process of a lithium ion/metal battery, the organic electrolytes are thermodynamically unstable at high or low Li/Li+ potentials. Therefore, the electrolyte undergoes a reduction reaction on the surface of the anode, producing a large amount of organic or inorganic lithium salts that deposit on the anode surface, forming a dense passivation film called SEI layers. The SEI layer is insoluble in the organic electrolyte between the two electrodes, so it can stably exist in organic electrolyte solutions. Solvent molecules can pass through this layer to passivate the film, resulting in an increase in electrochemical performance of the prepared battery. However, SEI is also a major source of performance degradation and safety risks for metal battery systems, as it may become unstable and lead to the formation of dendrites during long-term use, leading to battery short circuits and thermal runaway.
The surface stabilization mechanism of Li metal submerged in liquid electrolyte was initially studied by Dey in the 1970s. They discovered that the reason of stable liquid electrolyte Li-metal batteries was a surface protective coating made primarily of electrolyte breakdown products. And now one of the most widely accepted models for the SEI is the multilayered model, which was proposed by Aurbach in 1999. According to their theory, the SEI has a multilayer structure. The inorganic salt composition in the SEI layers of lithium metal battery is typically LiF, LiCl, Li2O, and a large content of Li2CO3. These inorganic salts stack together to form SEI layers. During the formation of the SEI layer, all solution components undergoing low selectivity reduction processes when a new anode is introduced to the solution. Solution specie with higher selectivity deposits on the surface of the active materials in selective reactions, thus leading to a decrease in the concentration of this solution specie and its selectivity. They cover the active surface to prevent other solution species from entering, thus forming a multi-layer structure of SEI layers
However, recent studies have challenged the multilayered model and suggested that the SEI may have a more mosaic-like structure. For example, SEM and TEM studies have revealed that the SEI can have a highly porous and interconnected structure, with nanoscale grains and voids that can facilitate ion transport and accommodate volume changes during cycling. Other studies have suggested that the SEI can have a gradient-like structure, with different layers of organic and inorganic components that vary in thickness and composition.
3. Importance of SEI and why artificial
SEI layers are needed in metal battery systems to improve the stability and safety of the SEI and prevent the formation of dendrites. In lithium metal batteries system, dendrites are inorganic lithium salts with needle-like structures. Lithium dendrite growth can bring many negative effects to the battery, which greatly reduces the cost-efficiency of the LMBs and largely restricts its practical applications. When the dendrite grows large enough to reach the other electrode, the battery will self-discharge and short-circuit, leading to thermal runaway and even explosion.
In the actual use of batteries, electric driving force can cause uneven ion distribution and diffusion, resulting in unevenness and tips on the electrode surface. The tip has always been considered an active site during the deposition process. The charges tend to accumulate at the tip, and this local accumulation makes it easier for lithium ions to deposit. This process is the main factor controlling the growth of Li dendrites. The "Sand’s time" is a widely accepted equation for calculating when dendrites begin to grow [6]. Due to the relationship between the distance between electrodes and the initial Li salt concentration, the formation of dendrites should have a critical ion concentration gradient. At the tip of the electrode surface, an excessively positive charge provides a strong electric field, which then triggers the Li dendrites formation. The current density determines the speed of dendrite growth, and the electric field is the main driving factor in the early stage of dendrite formation.
Compared with the SEI created by the battery itself, the artificial SEI film exhibits more controllable thickness, chemical composition and better ionic conductivity, enabling designing of a better performance of the lithium metal anodes. Artificial SEI layers can be created through many different methods, including adding additives to the electrolyte, the deposition of a secondary layer on top of the native SEI, or the use of a hybrid composite membrane that combines the artificial membrane with the SEI that is created by the battery. These artificial SEI layers can help to stabilize the lithium deposition and prevent dendrite growth by providing a more uniform and stable surface for ion transport and reducing side reactions caused by direct contact between metal anodes and electrolytes. The artificial SEI layers can also improve the performance of metal battery systems by increasing the efficiency of ion transport. For example, the application of artificial SEI layers can be used to improve performance of the LMBs, such as cycling stability and Coulombic efficiency, which are known to be highly reactive and prone to dendrite growth.
4. Characteristics of SEI
Ali and co-workers [7] reported that the SEI layer can be used to reduce the fracture probability on the surface of lithium-ion battery electrodes. In their model, the fracture probability of lithium-ion battery electrode particles can be affected by the SEI layer. Tensile tension inside the active particles transforms into compressive stress when the SEI layer thickens, lowering the likelihood of particle breakage. However, the SEI restriction is broken once the SEI layer fractures, increasing the likelihood that a particle fracture would follow. The stress and fracture likelihood inside the SEI layer and anode particles are examined in this article while accounting for the combined effects of the declining state of charge and the thickening of the SEI layer after a number of cycles.
They create the 2D core-shell particle model to illustrate the behaviours of electrode particles during cycling. The active material is in the core of the model's circular core-shell structures, while the SEI layer surround on the active material surface as the shell. In order to mimic the reduction of lithium ions caused by SEI formation at the negative electrode and the mechanical behaviours of the electrode particles, the model is a combination of a 1D electrochemical model with a 2D particle model. A generic projection approach is used to apply the lithium current density. Their model shows internal particle and SEI layer stresses after 2000 cycles. After 500 cycles, Figure 1 shows how the internal tangential stress of the particles changes from stretching to compression, and the compression behaviours becomes stronger over time.
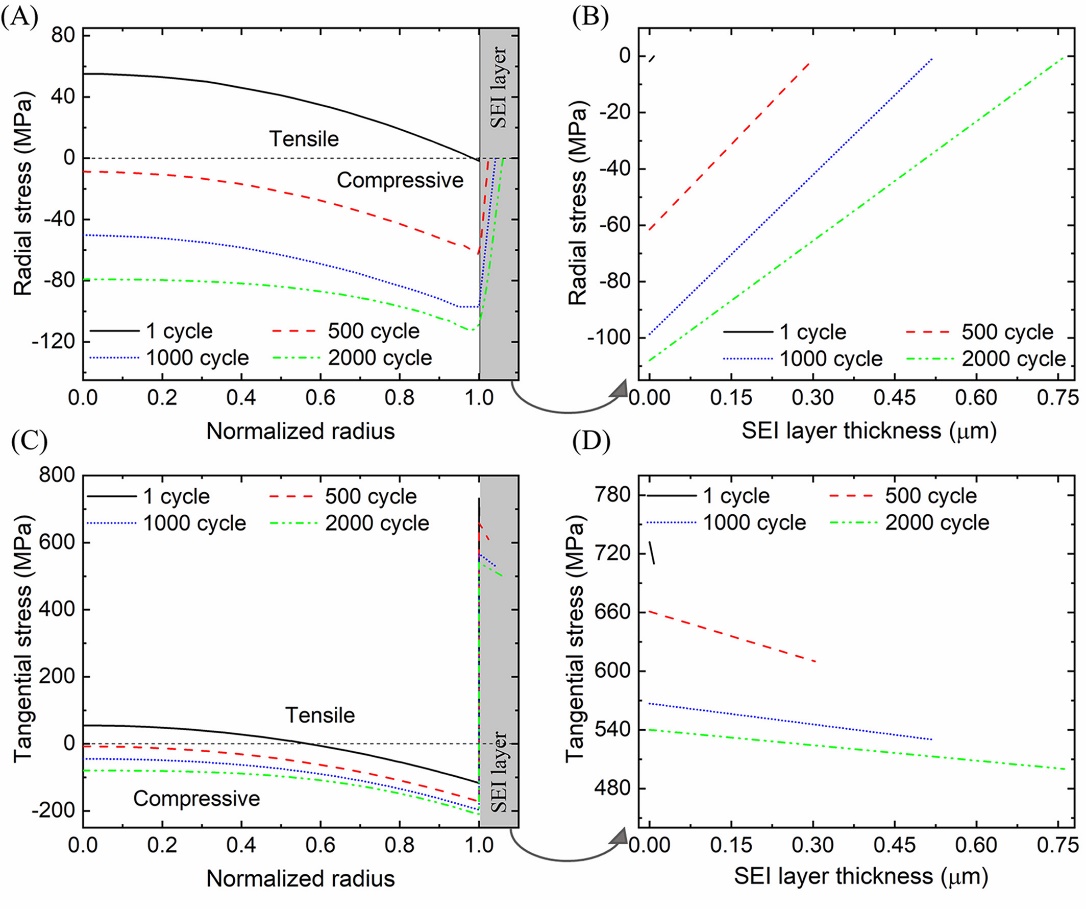
Figure 1. Radial and tangential stresses inside particles and SEI shells [7].
They also studied the relationship between the particle size and the SEI layer fracture possibility. They simulated a heterogeneous electrode model and found that smaller particles experienced greater tensile stress within the SEI layer compared to larger particles. Because the smaller the particle size, the larger the surface area ratio, and a larger specific surface area means that the surface is more susceptible to stretching or compression when volume changes. Moreover, they also found that adjusting the particle size helps reduce the fracture probability of the SEI layer.
The defects of the SEI layer can provide channels for lithium-ion transport, thereby increasing the diffusion rate of Li+ ions in the SEI layer. And a positively charged space charge layer will also be formed in the grain boundary, thereby reducing electron passage and battery self-discharge. Min Feng and co-workers [8] found that the electronic properties of grain boundaries (GBs) and surfaces in the SEI of Lithium batteries are closely related to the bandgap of the SEI components. The result shows that bandgaps are reduced in all the GB structures compared with their corresponding single crystals. In addition, they discovered that, with the exception of LiF (111) and Li2S (111), the majority of surface bandgaps are smaller than bulk bandgaps. Surface orientations affect the surface bandgap. They provided an analytical approach to easily anticipate the GB bandgaps, which may help multiscale simulations, and they indicated that ordered GBs and sharp interfaces are favoured for designing the electrically passivating SEI.
Another major factor affecting the performance of SEI layer is temperature. At low temperatures, the resistance of the SEI film increases, causing the negative electrode potential to shift towards a lower potential, resulting in greater polarization, making it easier for lithium to precipitate. The precipitation of lithium metal also maintains the surface potential of the electrode at a lower level, allowing the organic electrolyte to continue to decompose and form the SEI film. Lithium precipitation and electrolyte decomposition form a vicious cycle, resulting in fewer and fewer active Li ions in the battery. When the temperature of the battery increases, chemical reactions occur between the SEI film, electrolyte, and electrode materials, leading to changes in its composition and thickness. These reactions also release a large amount of heat, causing internal heating and even ignition and explosion of the battery.
5. Application of SEI in lithium battery
The optimal SEI layer will have the following characteristics. The first is the high conductivity of lithium ions, but also the high resistance of electrons. It should also maintain a certain thickness on the entire SEI layer, and high mechanical toughness, allowing for volume changes in anodes caused by charging. Finally, the SEI layer should overcome the performance issues at high and low temperatures, high and low voltages. Whether using ion insertion anodes or not, stable SEI is a recognized requirement for safe battery operation. If the development of SEI is moderate and uniform, rather than rapid and uneven, it is more likely to form dendritic structures. Spatial average SEI chemistry is the main theme of many publications. Also, recent developments have proposed the concept of specific locations in SEI analysis, presenting that compared historically recognized, SEI layers have a much more dynamic structure while in long-term use.
Zhou and co-workers [9] reported that some hybrid polymer can be good choices for SEI layer. In their research, polymethyl methacrylate (PMMA) and poly (vinylidene fluoride) (PVDF) hybrid polymer were used to synthesized the SEI layer. These two kinds of polymer both have good mechanical strength which makes less cracks during the charging and discharging, but they play different roles in the lithium-ion transport. PVDF can form a thin LiF layer on the lithium surface and extend the cycling lifespan of the anode. And for PMMA, it can generate massive Li-O bond with lithium ion, which is very important in the transport of lithium ions. As a result, the formed double-network structure can provide a solution for high-performance and high cycle times lithium metal battery anodes. This robust and flexible polymer structure is also a good solution for suppressing dendrite growth because of its flexibility and elasticity, which can adjust volume expansion during the cyclic process.
In addition, Lin and colleagues [10] revealed a practical method to obtain a high-quality SEI, and the mechanical properties of this type of SEI was further analyzed. The result shows that Li2O, LiF and S-based species make an inorganic-rich SEI, with an average elastic modulus of about 9.02 GPa. While the average elastic modulus of lithium metal is about 4.09 GPa.
6. Conclusion
The SEI layer is an important component of the LMBs. Improving the performance of SEI can effectively solve the battery performance and safety issues. The focus of this research is on the relationship between the different characteristics of membranes and their performance, as well as some examples of constructing artificial SEI layers to stabilize metal anodes during repeated battery charging and discharging. The most recent advancements in methods for analysing the SEI structure and the improved comprehension of the SEI data are also mentioned in this research. While highlighting the particular unanswered concerns of dendritic science, it can stress new research paths, which will be most productive for future study throughout the text. It can give some case studies that demonstrate the many strategies authors have used to improve cycle stability while emphasizing new advancements in membranes for Li anodes. Unexpected results are examined, such as how dendritic development on the metal anode the membrane is supposed to protect is influenced by the likelihood of stable vs unstable SEI generation on the membranes themselves.
References
[1]. Pu K C, Zhang X, Qu X L, et al. 2020 Rare Metals 39 616-635
[2]. Lin R, He Y, Wang C, et al. 2022 Nature Nanotechnology 17(7) 768-776
[3]. Ma M, Cai H, Xu C, et al. 2021 Advanced Functional Materials 31(25) 2100278
[4]. Verma P, Maire P, Novák P. 2010 Electrochimica Acta 55(22) 6332-6341
[5]. Wang H, Zhai D, Kang F. 2020 Energy & Environmental Science 13(12) 4583-4608
[6]. Sand H J S. III. 1901 The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science 1(1) 45-79
[7]. Ali Y, Iqbal N, Lee S. 2021 International Journal of Energy Research 45(4) 5293-5308
[8]. Feng M, Pan J, Qi Y. 2021 The Journal of Physical Chemistry C 125(29) 15821-15829
[9]. Zhou Z, Feng Y, Wang J, et al. 2020 Chemical Engineering Journal 396 125254
[10]. Lin Y, Chen J, Zhang H, et al. 2023 Journal of Energy Chemistry 80 207-214
Cite this article
Tan,J. (2024). Application of artificial SEI layers for lithium metal battery anodes. Applied and Computational Engineering,85,72-77.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 4th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Pu K C, Zhang X, Qu X L, et al. 2020 Rare Metals 39 616-635
[2]. Lin R, He Y, Wang C, et al. 2022 Nature Nanotechnology 17(7) 768-776
[3]. Ma M, Cai H, Xu C, et al. 2021 Advanced Functional Materials 31(25) 2100278
[4]. Verma P, Maire P, Novák P. 2010 Electrochimica Acta 55(22) 6332-6341
[5]. Wang H, Zhai D, Kang F. 2020 Energy & Environmental Science 13(12) 4583-4608
[6]. Sand H J S. III. 1901 The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science 1(1) 45-79
[7]. Ali Y, Iqbal N, Lee S. 2021 International Journal of Energy Research 45(4) 5293-5308
[8]. Feng M, Pan J, Qi Y. 2021 The Journal of Physical Chemistry C 125(29) 15821-15829
[9]. Zhou Z, Feng Y, Wang J, et al. 2020 Chemical Engineering Journal 396 125254
[10]. Lin Y, Chen J, Zhang H, et al. 2023 Journal of Energy Chemistry 80 207-214









