1. Introduction
With rising global energy demands and environmental issues from fossil fuel use, clean, renewable energy solutions are urgently needed. Traditional pollutant treatments have limitations and potential secondary pollution. Microbial Fuel Cells (MFCs) convert organic matter into electricity via microbial metabolism, offering low pollution, low noise, and renewability. This paper reviews MFC principles, influencing factors, and coupling technologies. We explore MFCs' potential in addressing energy and environmental challenges, technical hurdles, and future development directions. Understanding MFCs' capabilities and limitations can promote their practical application, supporting a greener energy mix and sustainable environmental management.
2. Microbial fuel cell technology
2.1. How microbial fuel cells work
Microbial Fuel Cells (MFCs) convert organic pollutants into electricity through microbial metabolic activity. MFCs couple electrochemical and microbial processes. (1) In the anode (anaerobic environment), microbes oxidize organic pollutants, releasing electrons and protons. (2) Protons move to the cathode chamber through a Proton Exchange Membrane (PEM). (3) Electrons travel through an external circuit from the anode to the cathode. (4) At the cathode (aerobic environment), electrons and protons react with oxygen to form water, completing the circuit and generating electricity. When glucose is the anode substrate and O2 is the cathode electron acceptor, the electrode reaction formula is as follows:
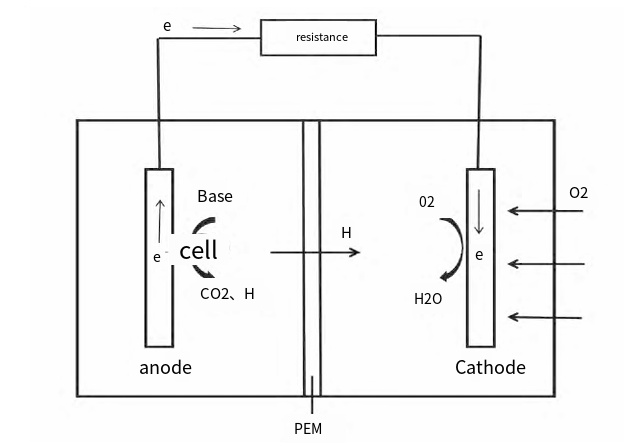
Figure 1. Schematic diagram of the working principle of MFC
Anode reaction: C6H12 O6+6H2O→6CO2+24H++24e− (1)
(E0) = 0.014 V
Cathode reaction: 6O2+24H++24e−→12H2O (2)
(E0 =1.23V)
Total response: C6H12O6+6O2→6CO2+6H2O (3)
Where 𝐸0is the electromotive force. MFCs require the smooth completion of each step: metabolic activities of electrogenic microorganisms, electron transfer to the anode, proton transfer through the diaphragm to the cathode, and the reaction of protons and electrons with the electron acceptor in the cathode. MFCs consist of a cathode, anode, microorganisms, electrolyte, and external circuit. They can be divided into two-chamber and single-chamber configurations based on the presence of a proton exchange membrane between the anode and cathode chambers.
2.2. Two-compartment microbial fuel cell
A two-compartment microbial fuel cell (MFC) consists of an anode chamber and a cathode chamber, where nitrates are removed by denitrification at the cathode. Clauwaert et al. [1] showed that an MFC with separate carbonization and denitrification units could achieve nitrate removal efficiencies of about 0.146 kg/(m³·d). The circular bicellular MFC developed by Virdis et al. [2] achieved efficient removal of COD and nitrate with rates of about 2 kg/(m³·d) and 0.41 kg/(m³·d), respectively. Xie et al. [3] built a coupling system of aerobic and anaerobic biocathode MFCs, improving ammonia and total nitrogen removal to 97.4% and 97.3%. Zhang et al. [4] created a dual-cathode MFC, but it only treated about 76% of nitrogen in wastewater. Lee et al. [5] constructed a dual-cathode MFC with removal rates for ammonia and nitrate nitrogen at 97.9% and 89.9%, respectively. These studies indicate that multi-compartment MFCs can improve nitrogen removal efficiency. Therefore, a two-compartment MFC may still be a better model for wastewater treatment and energy recovery.
2.3. Single-compartment microbial fuel cell
A single-compartment MFC places the anode and cathode in the same chamber. It uses an air cathode biofilm for nitrification, and the nitrogen removal rate is increased by enlarging the air cathode area to enhance air permeability. In a single-compartment MFC, microorganisms oxidize the fuel at the anode, transferring electrons to the cathode through an external circuit and protons to the cathode through a proton exchange membrane or directly in a membrane-free type. The cathode is exposed to air, using oxygen as the electron acceptor.
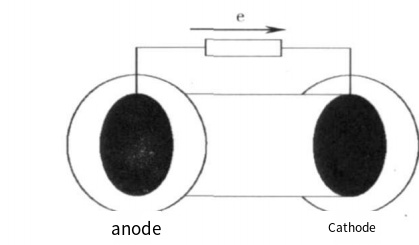
Figure 2. Diagram of a common single-compartment MFC
Single-compartment MFCs can be "2-in-1," "3-in-1," or non-membrane types. The "2-in-1" type presses the cathode and proton membrane together, with the anode separated by an anode solution, reducing oxygen's impact on the anode. Zhang et al. [6] used this type, achieving a maximum power density of 760 mW/m² with an anode made from graphite particles and PTFE latex. The "3-in-1" type presses the anode, proton membrane, and cathode together to reduce internal resistance. Cao Xiaoxin et al. [7] achieved an internal resistance of 10-30Ω and a power density of 300 mW/m² with a coulomb efficiency of 50%. This design significantly improves output power.
3. Factors affecting MFC
3.1. Anode material
The choice of anode material has a significant impact on the performance of MFC. The ideal anode material should have good biocompatibility, high electrical conductivity, adequate mechanical strength and chemical stability. Studies have shown that the electron transfer efficiency and microbial attachment ability of the anode can be improved by using carbon-based materials, metals or metal oxides, as well as composite materials.
3.1.1. Metallic Materials
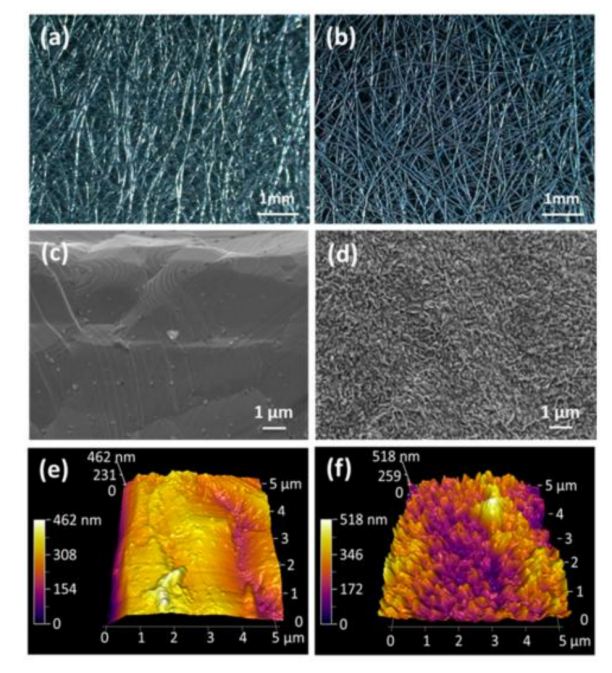
Precious metals like palladium and gold are excellent in electrochemistry but are costly. Non-precious metals and their oxide nanoparticles offer similar catalytic activity at a lower cost, reducing electrical resistance and enhancing microbial adhesion to electrodes. Metals like stainless steel have poor biocompatibility, which can be improved by thermal deposition, surface oxidation, and physical adsorption. Lamp et al. [8] used thermal deposition to create a nano carbon-modified stainless steel bioanode (CNS-M), achieving a power density of 187 mW/m², significantly higher than the original 2.9 mW/m² of stainless steel. Guo et al. [9] used flame oxidation to generate iron oxide nanoparticles on stainless steel felt, increasing the bioanode area current density to 1.92 mA/cm² and volume current density to 27.42 mA/cm³, much higher than unmodified stainless steel and carbon felt. Zheng et al. [10] improved the biocompatibility of stainless steel mesh (SSM) by physically adsorbing a 0.3 mm carbon black layer, resulting in higher current densities than unmodified stainless steel and ordinary graphite felt.
| Figure 3. (a, b) optical microscope image; (c, d) scanning electron microscope; (e, f) atomic force microscope (AFM). The left is the untreated stainless steel felt, and the right is the stainless steel felt after flame oxidation |
Figure 4. Bioelectrocatalytic current generation at untreated (a) and f lame oxidized (b) SS felt electrodes. (Chronoamperometry, anodic potential set at − 0.2V vs Ag/AgCl)
3.1.2. Non-metallic materials
Traditional carbon materials have high stability and conductivity but large resistance limits their use in microbial fuel cells (SMFC). Metal materials offer better conductivity but are generally unsuitable for SMFCs due to corrosion and poor bacterial adhesion. Reimers et al. [11] first used platinum mesh as a cathode, finding it effective for oxygen reduction but poor in conductivity, impacting power output. Research now focuses on new carbon materials with high surface area and conductivity, like carbon nanotubes and graphene. Thepsuprungsikal et al. [12] found that multi-walled carbon nanotubes with carboxyl groups achieved the highest current density and voltage due to their structure, strength, surface area, thermal stability, and reactivity. However, their lower conductance limits their use in SMFCs [13]. Zhang Yelong found that adhesive-based carbon fiber, with its surface grooves, enhanced electrode anti-polarization and power density [14], improving generation capacity to 673.2 mW/m², 2.4 times higher than unmodified batteries. Graphene, with strong conductivity and large surface area, is a recent focus for enhancing electron transfer and electricity generation in SMFCs.
3.2. Cathode material
The cathode is crucial for MFC efficiency, and can be biological or non-biological. Biological cathodes use microorganisms as electron acceptors, avoiding catalyst poisoning and reducing costs. Non-biological cathodes typically use oxygen (O2) due to its availability and low cost. However, the slow oxygen reduction reaction (ORR) limits efficiency, necessitating catalysts to speed up the process. While platinum (Pt) was initially used, its high cost led to the exploration of alternatives like metal-containing/metal oxide types, metal-doped nitrogen carbon materials (M-N-C), and heteroatom-doped carbon materials. Jiang et al. [15] synthesized NiFe-LDH@Co3O4 as a catalyst for air cathodes, achieving a maximum power density of 467.35 mW/m², comparable to Pt. Liu et al. [16] prepared nanometer manganese oxide (Mn₅O₈₄₃₂ₓ) via electrodeposition, achieving a maximum power density of 772.8 mW/m³. Modified cathodes generally exhibit lower internal and electron transfer resistance, stronger oxygen adsorption, and faster oxygen diffusion, enhancing ORR and overall MFC performance.
3.3. MFC coupled photocatalysis
MFCs can be coupled with photocatalysis technology to degrade pollutants and generate electricity using microbial metabolism and light energy. In the anode chamber, biological anodes release protons and electrons, which transfer to the cathode through the electrolyte and membrane, while electrons move through an external circuit. In the cathode chamber, light generates electrons and holes on the cathode surface. These holes combine with electrons from the anode to form a current, while the electrons migrate to catalytic sites on the cathode to reduce contaminants, enhancing electricity generation. Adding activated carbon in the MFC electrode chamber enriches microorganisms at the anode, improving wastewater treatment and electricity generation. Photocatalysis achieves rapid and complete decomposition. Zhang et al. [17] loaded nano titanium dioxide on a biological carrier, finding that combined photocatalysis and microbial degradation increased total phosphorus degradation efficiency to about 92%. For nitrifying bacteria and cocci, this efficiency improved from 50% to about 90%.Lu et al. [18] constructed a single-cycle C-PEC-MFC and a double-cycle CC-PEC-MFC to reduce ethyl acetate and isopropyl alcohol concentrations. Using Ag/AgBr/TiO₂-ZnO photocatalysts, the single-cycle system effectively degraded pollutants and improved electricity generation. The double-cycle system quickly reduced pollutant concentration by circulating the dissolved solution through the EC-MFC system.
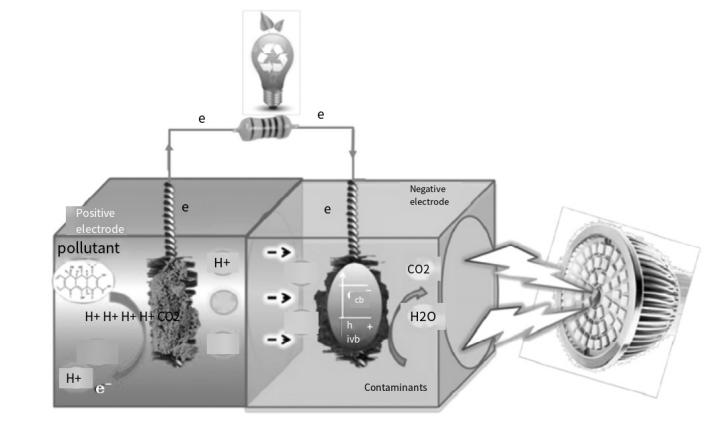
Figure 5. photocatalytic cathode-bianodic coupling system
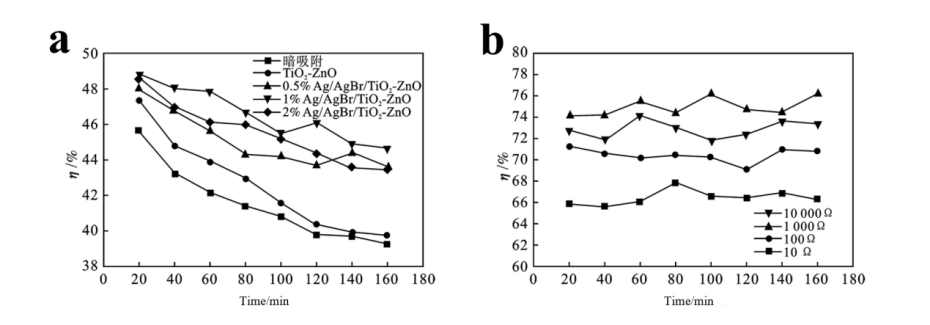
Figure 6. (a) Effect of Ag/AgBr content on photocatalytic degradation of EA; (b) Effects of different external resistors on EA degradation in PEC-MFC
3.4. MFC coupled electrofenton technique
Electrofenton technology, known for its strong oxidation capacity and low energy consumption, has gained attention in recent years [19]. Combining MFC (microbial fuel cell) with Electrofenton technology forms a new process that uses electricity from the MFC to drive the Electrofenton reaction, controlling contaminant degradation at the MFC cathode. This combined process saves energy costs and expands the MFC's application range compared to traditional Electrofenton systems. Electrofenton coupled with MFCs works by transmitting electrons from the anode to the aerobic cathode, producing hydrogen peroxide (H₂O₂). H₂O₂ reacts with Fe²⁺ added to the system to degrade pollutants. Feng et al. [20] improved MFC performance by using a PPy/AQDS conductive film, significantly accelerating Orange II dye degradation in the cathode chamber and enhancing the oxidative destruction ability of the Electrofenton system. This system is also being explored for degrading pollutants like nitro-aromatic and chlorinated aromatic compounds, which is important for practical wastewater treatment. The catalyst load of 0.4 mg/cm² enabled a 91% degradation rate, reducing COD to 84 mg/L [21]. LeiFu et al. [22] studied the degradation of the azo dye Mycetin, achieving a COD removal rate of 76.43% with 0.5 mmol/L Fe³⁺ and a maximum output power density of 28.3 W/m³. Zhao et al. [23] found that the MFC-Fenton system efficiently removes mesomethylsulfoxone through microbial degradation and Fenton oxidation, achieving removal rates of 0.83 mg·L⁻¹·h⁻¹ and 1.39 mg·L⁻¹·h⁻¹, respectively, under optimized conditions.
A Microbial Electrolysis Cell (MEC) is a process that requires the consumption of electrical energy. When a MEC is combined with an MFC (microbial fuel cell), the electrical energy produced by the MFC can be used as a power source for the MEC. The system mainly uses wastewater to generate electrical energy to produce hydrogen or methane from the MEC. MinSun [24] et al. investigated the coupling of an electrically assisted microbial fuel cell (MFC) to a hydrogen-producing microbial electrolysis cell (MEC) and controlled hydrogen production in a MEC-MFC coupled system by adjusting the power input on the MEC. Studies have shown that hydrogen production increases significantly when MFCS are connected in series. In contrast, using a parallel connection slightly reduces hydrogen production. Connecting multiple power-assisted MFCS in series has the potential to improve the hydrogen production capacity of MECs in organic waste. In YangLi [25] et al. 's study, the decolorization ability of the MFC-MEC coupled system for azo dyes was validated. The experimental results showed that compared with the MFC system alone, the decolorization rate of the MFC-MEC coupled system was increased from 36.52% to 75.28%, and the decolorization effect of the azo dyes was significantly enhanced in the coupled system by consuming more acetate and K3Fe(CN)6 and MFC in-situ power generation. At the same time, the cathode pH value in the range of 7.0 to 10.3 has little effect on the performance of the reactor in the coupled system. Danjing [26] Wu et al. used an MFC-MEC coupled system for self-driven operation to recover metallic cobalt in a solution containing Co(Ⅱ). Co(Ⅱ) can be recovered in the cathode chamber of the MEC without the need for other electrical energy. Experiments have proved that when MEC uses titanium plate, stainless steel, carbon paper as the cathode material, carbon paper as the cathode electrode material can obtain higher loop current, which is conducive to the reduction of Co(Ⅱ), so as to obtain better recovery effect. When the pH of the cathode solution is 3, the anode coulomb efficiency is 20.5%, the electrode spacing is 16 cm, and the specific recovery rate of Co is 0.85 g Co/g COD, the removal rate of the cathode Co(Ⅱ) can reach 97.4%. The MFC-MEC system constructed by pan et al. is beneficial to the treatment of heavy metal wastewater containing cadmium, and the removal rate of Cr6+ can reach more than 80%.
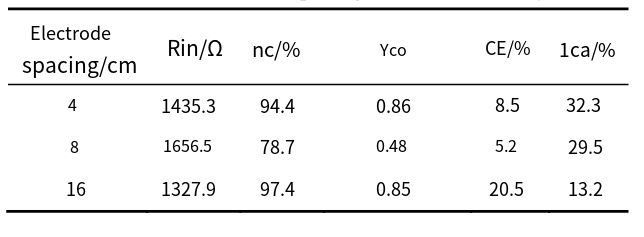
Figure 7. Effect of electrode spacing on cobalt recovery
3.5. MFC coupled constructed wetland technology
MFC and the CW interaction of artificial wetland, soil microbial cells (constructed wetland coupled with microbial arg, cell, the CW - MFC) technology is adopted the electrochemical process of processing technology. In the CW-MFC (Composite Wetland Microbial Fuel cell) system, microorganisms in the anode region use the root exudates of aquatic plants and organic matter in sewage as a carbon source. During oxidative decomposition, they produce carbon dioxide (CO2), proton donors (H+), and cations. These cations are transferred to the anode through a REDOX medium, such as nanowires, to form an electric current. At the same time, the proton donor is transported by flow to the cathode region. In the cathode region, the transferred cation combines with the external circuit and with the electron acceptor (usually oxygen), which undergoes a reduction reaction and eventually produces water. The whole process converts the biomass energy in the organic matter into energy. The reaction equation is shown below, and the schematic diagram of the principle is shown in Figure 2.
For the anode:
(CH2O)n+nH2O→nCO2+4ne-+4nH+
Cathode:
4e-+O2 +4H+→2H2 O
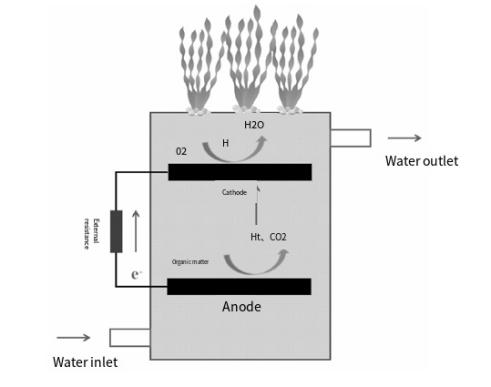
Figure 8. Schematic diagram of how CW-MFC works
4. Conclusion and outlook
The CW-MFC system, based on Plant Microbial Fuel Cell (PlantMFC) technology, differs in material sources: PlantMFC uses carbon sources from plant root secretions, while CW-MFC uses organic matter in sewage. In 2012, Yadav et al. first used CW-MFC to treat azo dye wastewater, showing better treatment and current generation than traditional CW systems. Fang et al. achieved a 91.2% chroma removal rate and 85.7% COD removal rate for complex dye wastewater. Studies on various wastewater types, including pig, synthetic (glucose and sodium acetate), domestic, oil-containing, and antibiotic wastewater, have shown good treatment effects. In CW-MFC, as wastewater concentration increases, output power and COD removal rates first increase, then decrease, with the highest COD removal rate not aligning with the maximum output power density. The upflow design of CW-MFC, with the surface as the cathode and deep as the anode, ensures optimal dissolved oxygen levels, enhancing REDOX potential, sewage treatment efficiency, and power generation performance.
In recent years, MFCs have emerged as a clean energy technology that converts organic matter into electricity through microbial metabolism. They offer low pollution, low noise, and renewability, showing high efficiency in treating organic wastewater and recovering energy from biodegradation, which is valuable for sewage treatment and energy recovery.
Despite their potential, MFC technology faces challenges such as improving energy conversion efficiency, reducing costs, and enhancing system stability. To further advance MFCs in wastewater treatment and recycling, breakthroughs are needed in these areas.
(1) Anode and cathode material innovation: Enhancing electron transfer efficiency and microbial attachment by using advanced materials like carbon-based substances, metals, metal oxides, and composites can improve MFC performance.
(2) Exploration of coupling systems: Combining MFCs with technologies such as photocatalysis, electrofenton, MEC, and constructed wetlands has shown promise but needs further refinement. Future research could explore coupling with membrane bioreactors, electrolysis, and ultrasonic technology for better energy conversion and contaminant removal.
(3) System optimization: Improving MFC design and operational parameters can enhance capacitance conductivity and system stability, leading to more efficient and reliable performance.
References
[1]. Clauwaert, P.; Rabaey, K.; Aelterman, P.; De Schamphelaire, L.; Pham, T. H.; Boeckx, P.; Boon, N.; Verstraete, W., Biological Denitrification in Microbial Fuel Cells. Environmental Science & Technology 2007, 41 (9), 3354-3360.
[2]. Virdis, B.; Read, S. T.; Rabaey, K.; Rozendal, R. A.; Yuan, Z.; Keller, J., Biofilm stratification during simultaneous nitrification and denitrification (SND) at a biocathode. Bioresource Technology 2011, 102 (1), 334-341.
[3]. Zhang Yilei; Cui Kangping; Sun Shiqun; Cao Xiao-Yan, Enhanced denitrification of single-chamber stainless steel anode microbial fuel cell %J Guangdong Chemical Industry. 2012, 39 (02), 4-5+7.
[4]. Tang Yulan; Wang Jiao; Zhao Jingtao; Yu Yan; Sun Yi; Jinxiang Fu, effects of substrate concentration on performance and growth of microbial fuel cells. J Environmental Engineering, 2013, 7 (01), 137-142.
[5]. Lee, K.-y.; Ryu, W.-s.; Cho, S.-i.; Lim, K.-h., Comparative study on power generation of dual-cathode microbial fuel cell according to polarization methods. Water Research 2015, 84, 43-48.
[6]. Wang F.Culture and characteristics of aerobic granular sludge in SBAR. Ph. D., Dalian University of Technology, 2005.
[7]. Hui-xia LAN; Chen Zhonghao; Chen Yuancai; Chen Rong, Culture of aerobic granular sludge under micro-aerobic conditions %J Journal of South China University of Technology (Natural Science Edition). 2005, (07), 37-41+46.
[8]. Lamp, J.; Guest, J.; Naha, S.; Radavich, K.; Love, N.; Ellis, M.; Puri, I., Flame synthesis of carbon nanostructures on stainless steel anodes for use in microbial fuel cells. Lancet 2011, 196, 5829-5834.
[9]. Guo, K.; Donose, B. C.; Soeriyadi, A. H.; Prevoteau, A.; Patil, S. A.; Freguia, S.; Gooding, J. J.; Rabaey, K., Flame Oxidation of Stainless Steel Felt Enhances Anodic Biofilm Formation and Current Output in Bioelectrochemical Systems. Environmental Science & Technology 2014, 48 (12), 7151-7156.
[10]. Zeng, F.; Wu, Y.; Bo, L.; Zhang, L.; Liu, W.; Zhu, Y., Coupling of electricity generation and denitrification in three-phase single-chamber MFCs in high-salt conditions. Bioelectrochemistry (Amsterdam, Netherlands) 2020, 133, 107481.
[11]. Reimers, C. E.; Tender, L. M.; Fertig, S.; Wang, W., Harvesting Energy from the Marine Sediment−Water Interface. Environmental Science & Technology 2001, 35 (1), 192-195.
[12]. Thepsuparungsikul, N.; Ng, T. C.; Lefebvre, O.; Ng, H. Y., Different types of carbon nanotube-based anodes to improve microbial fuel cell performance. Water science and technology : a journal of the International Association on Water Pollution Research 2014, 69 (9), 1900-10.
[13]. Song, T.-s.; Tan, W.-m.; Wu, X.-y.; Zhou, C. C., Effect of graphite felt and activated carbon fiber felt on performance of freshwater sediment microbial fuel cell. 2012, 87 (10), 1436-1440.
[14]. Zhang Y L. Application of carbon materials in submarine sediment fuel cells. M.s., Ocean University of China, 2014.
[15]. Jiang, L.; Chen, J.; Han, D.; Chang, S.-I.; Yang, R.; An, Y.; Liu, Y.; Chen, F. J. J. o. P. S., Potential of core-shell NiFe layered double hydroxide@Co3O4 nanostructures as cathode catalysts for oxygen reduction reaction in microbial fuel cells. 2020, 453, 227877.
[16]. Zhao, Y.; Zhan, X.; Wang, H.; Yu, J.; Sun, Y.; Chen, L.; He, M.; Liu, J.; Shi, H., MOFs-derived MnOx@C nanosheets for peroxymonosulfate activation: Synergistic effect and mechanism. Chemical Engineering Journal 2022, 433, 133806.
[17]. Zhang, J.; Wang, Z.; Chu, L.; Chen, R.; Zhang, C.; Toan, S.; Bagley, D. M.; Sun, J.; Dong, S.; Fan, M., Unified photoelectrocatalytic microbial fuel cell harnessing 3D binder-free photocathode for simultaneous power generation and dual pollutant removal. Journal of Power Sources 2021, 481, 229133.
[18]. Lu Xuguang; Liu Lifen; Han J Q, a novel photocatalytic coupled microbial fuel cell for the degradation of VOCs %J Applied Chemical Engineering. 2022, 51 (05), 1256-1260.
[19]. Qi R Y. Theoretical and experimental study on microbial degradation of indoor formaldehyde pollutants. M.s., Tianjin University, 2009.
[20]. Feng, C.; Li, F.; Liu, H.; Lang, X.; Fan, S. J. E. A., A dual-chamber microbial fuel cell with conductive film-modified anode and cathode and its application for the neutral electro-Fenton process. 2010, 55, 2048-2054.
[21]. M.s., Beijing University of Chemical Technology, 2021.
[22]. Fu, L.; You, S.; Zhang, G.; Yang, F.; Fang, X. J. C. E. J., Degradation of azo dyes using in-situ Fenton reaction incorporated into H2O2-producing microbial fuel cell. 2010, 160, 164-169.
[23]. Zhao, H.-H.; Zhang, Q. J. B. t., Performance of electro-Fenton process coupling with microbial fuel cell for simultaneous removal of herbicide mesotrione. 2021, 319, 124244.
[24]. Sun, M.; Sheng, G.-P.; Mu, Z.-X.; Liu, X.-W.; Chen, Y.-Z.; Wang, H.-L.; Yu, H.-Q., Manipulating the hydrogen production from acetate in a microbial electrolysis cell–microbial fuel cell-coupled system. Journal of Power Sources 2009, 191 (2), 338-343.
[25]. Li, Y.; Yang, H.-Y.; Shen, J.-Y.; Mu, Y.; Yu, H.-Q., Enhancement of azo dye decolourization in a MFC–MEC coupled system. Bioresource Technology 2016, 202, 93-100.
[26]. Wu, Tan-ching; Lulu Pan; Liu Wei-Ping, Cobalt recovery by MFC-MEC bioelectrochemical coupling system, J Chinese Journal of Nonferrous Metals, 2019, 29 (07), 1536-1542.
Cite this article
Cao,B. (2024). Application and prospect analysis of microbial fuel cell technology. Applied and Computational Engineering,70,206-214.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Functional Materials and Civil Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Clauwaert, P.; Rabaey, K.; Aelterman, P.; De Schamphelaire, L.; Pham, T. H.; Boeckx, P.; Boon, N.; Verstraete, W., Biological Denitrification in Microbial Fuel Cells. Environmental Science & Technology 2007, 41 (9), 3354-3360.
[2]. Virdis, B.; Read, S. T.; Rabaey, K.; Rozendal, R. A.; Yuan, Z.; Keller, J., Biofilm stratification during simultaneous nitrification and denitrification (SND) at a biocathode. Bioresource Technology 2011, 102 (1), 334-341.
[3]. Zhang Yilei; Cui Kangping; Sun Shiqun; Cao Xiao-Yan, Enhanced denitrification of single-chamber stainless steel anode microbial fuel cell %J Guangdong Chemical Industry. 2012, 39 (02), 4-5+7.
[4]. Tang Yulan; Wang Jiao; Zhao Jingtao; Yu Yan; Sun Yi; Jinxiang Fu, effects of substrate concentration on performance and growth of microbial fuel cells. J Environmental Engineering, 2013, 7 (01), 137-142.
[5]. Lee, K.-y.; Ryu, W.-s.; Cho, S.-i.; Lim, K.-h., Comparative study on power generation of dual-cathode microbial fuel cell according to polarization methods. Water Research 2015, 84, 43-48.
[6]. Wang F.Culture and characteristics of aerobic granular sludge in SBAR. Ph. D., Dalian University of Technology, 2005.
[7]. Hui-xia LAN; Chen Zhonghao; Chen Yuancai; Chen Rong, Culture of aerobic granular sludge under micro-aerobic conditions %J Journal of South China University of Technology (Natural Science Edition). 2005, (07), 37-41+46.
[8]. Lamp, J.; Guest, J.; Naha, S.; Radavich, K.; Love, N.; Ellis, M.; Puri, I., Flame synthesis of carbon nanostructures on stainless steel anodes for use in microbial fuel cells. Lancet 2011, 196, 5829-5834.
[9]. Guo, K.; Donose, B. C.; Soeriyadi, A. H.; Prevoteau, A.; Patil, S. A.; Freguia, S.; Gooding, J. J.; Rabaey, K., Flame Oxidation of Stainless Steel Felt Enhances Anodic Biofilm Formation and Current Output in Bioelectrochemical Systems. Environmental Science & Technology 2014, 48 (12), 7151-7156.
[10]. Zeng, F.; Wu, Y.; Bo, L.; Zhang, L.; Liu, W.; Zhu, Y., Coupling of electricity generation and denitrification in three-phase single-chamber MFCs in high-salt conditions. Bioelectrochemistry (Amsterdam, Netherlands) 2020, 133, 107481.
[11]. Reimers, C. E.; Tender, L. M.; Fertig, S.; Wang, W., Harvesting Energy from the Marine Sediment−Water Interface. Environmental Science & Technology 2001, 35 (1), 192-195.
[12]. Thepsuparungsikul, N.; Ng, T. C.; Lefebvre, O.; Ng, H. Y., Different types of carbon nanotube-based anodes to improve microbial fuel cell performance. Water science and technology : a journal of the International Association on Water Pollution Research 2014, 69 (9), 1900-10.
[13]. Song, T.-s.; Tan, W.-m.; Wu, X.-y.; Zhou, C. C., Effect of graphite felt and activated carbon fiber felt on performance of freshwater sediment microbial fuel cell. 2012, 87 (10), 1436-1440.
[14]. Zhang Y L. Application of carbon materials in submarine sediment fuel cells. M.s., Ocean University of China, 2014.
[15]. Jiang, L.; Chen, J.; Han, D.; Chang, S.-I.; Yang, R.; An, Y.; Liu, Y.; Chen, F. J. J. o. P. S., Potential of core-shell NiFe layered double hydroxide@Co3O4 nanostructures as cathode catalysts for oxygen reduction reaction in microbial fuel cells. 2020, 453, 227877.
[16]. Zhao, Y.; Zhan, X.; Wang, H.; Yu, J.; Sun, Y.; Chen, L.; He, M.; Liu, J.; Shi, H., MOFs-derived MnOx@C nanosheets for peroxymonosulfate activation: Synergistic effect and mechanism. Chemical Engineering Journal 2022, 433, 133806.
[17]. Zhang, J.; Wang, Z.; Chu, L.; Chen, R.; Zhang, C.; Toan, S.; Bagley, D. M.; Sun, J.; Dong, S.; Fan, M., Unified photoelectrocatalytic microbial fuel cell harnessing 3D binder-free photocathode for simultaneous power generation and dual pollutant removal. Journal of Power Sources 2021, 481, 229133.
[18]. Lu Xuguang; Liu Lifen; Han J Q, a novel photocatalytic coupled microbial fuel cell for the degradation of VOCs %J Applied Chemical Engineering. 2022, 51 (05), 1256-1260.
[19]. Qi R Y. Theoretical and experimental study on microbial degradation of indoor formaldehyde pollutants. M.s., Tianjin University, 2009.
[20]. Feng, C.; Li, F.; Liu, H.; Lang, X.; Fan, S. J. E. A., A dual-chamber microbial fuel cell with conductive film-modified anode and cathode and its application for the neutral electro-Fenton process. 2010, 55, 2048-2054.
[21]. M.s., Beijing University of Chemical Technology, 2021.
[22]. Fu, L.; You, S.; Zhang, G.; Yang, F.; Fang, X. J. C. E. J., Degradation of azo dyes using in-situ Fenton reaction incorporated into H2O2-producing microbial fuel cell. 2010, 160, 164-169.
[23]. Zhao, H.-H.; Zhang, Q. J. B. t., Performance of electro-Fenton process coupling with microbial fuel cell for simultaneous removal of herbicide mesotrione. 2021, 319, 124244.
[24]. Sun, M.; Sheng, G.-P.; Mu, Z.-X.; Liu, X.-W.; Chen, Y.-Z.; Wang, H.-L.; Yu, H.-Q., Manipulating the hydrogen production from acetate in a microbial electrolysis cell–microbial fuel cell-coupled system. Journal of Power Sources 2009, 191 (2), 338-343.
[25]. Li, Y.; Yang, H.-Y.; Shen, J.-Y.; Mu, Y.; Yu, H.-Q., Enhancement of azo dye decolourization in a MFC–MEC coupled system. Bioresource Technology 2016, 202, 93-100.
[26]. Wu, Tan-ching; Lulu Pan; Liu Wei-Ping, Cobalt recovery by MFC-MEC bioelectrochemical coupling system, J Chinese Journal of Nonferrous Metals, 2019, 29 (07), 1536-1542.