1. Introduction
In accordance with the definition of the International Union of Pure and Applied Chemistry (IUPAC), porous materials are continuous solid network materials that are filled through voids.Porous materials possess ordered pore structure and large surface area, which are of significance in many fields, including catalysis, adsorption, and energy storage. Mesoporous materials typically exhibit pore sizes between 2 and 50 nm, while microporous materials have pore sizes less than 2 nm. The synthesis of these materials is undergoing a period of evolution, with the advent of sol-gel methods for the production of mesoporous materials and ionothermal synthesis method for microporous materials, which allow for precise control of the structures of the materials, thereby enabling their adaptation to specific application requirements. Besides, the characterization of these materials is of critical importance for the understanding of their properties and potential applications, and commonly employed characterization techniques include X-ray diffraction, nitrogen adsorption, and transmission electron microscopy, providing comprehensive data regarding the crystal structure, surface area, and pore distribution of the material. Furthermore, mesoporous and microporous materials have a wide range of applications, exerting a profound impact on numerous fields. Therefore, the paper aims to provide a comprehensive overview of the synthesis methods. Moreover, it examines the latest developments, highlighting the key role of these materials in driving technological advances and addressing global challenges such as energy storage and environmental protection.
2. Typical Synthesis of Mesoporous and Microporous Materials
The Sol-Gel and Ionothermal Synthesis methods have attracted much attention as potential routes for synthesizing mesoporous and microporous materials due to their respective advantages. Therefore, they are selected for detailed analysis.
2.1. Typical Synthesis of Mesoporous: Sol-gel Synthesis Method
The Sol-Gel synthesis, a solution chemical synthesis method commonly used for the preparation of mesoporous materials, forms a homogeneous gel system in solution through a sol-to-gel transition, resulting in a material with a mesoporous structure. The core steps of the method include dissolving metal precursors, adding surfactants or templating agents, controlling the pH and temperature of the solution. When appropriate conditions satisfies, the precursors in the solution gradually aggregate to form a gel with regular distribution of templating molecules inside, which is subsequently dried and calcined to form the final mesoporous material. This method features by ease of operation, low cost, and strong control over the structure of the material. By adjusting the conditions and adding different templating agents, precise control of the pore size, structure, and surface properties of mesoporous materials can be achieved. Therefore, the solution-phase synthesis method is widely used for mesoporous materials. In this method, appropriate self-assembled template molecules play a key role in determining the final pore size and homogeneity. However, preparing mesoporous material in the 10-30 nm range is often challenging due to the lack of suitable templates, and the difficulty in arranging the template materials in a well-defined manner often leads to poor spatial orientation of the pores and wide size distributions [1]. The precise control of biomolecules at the nanoscale or even in situ enables manipulation of their microstructure, making them promising template materials. Among them, protein cages, which are characterized by a hollow interior and can be assembled in a controlled manner with an intrinsic size range of 10-50 nm in diameter, have been demonstrated to be a promising synthetic material due to the versatility of protein cage assembly [2]. Meanwhile, silica is often chosen as an ideal material for protein template systems due to the robustness, biocompatibility and mild synthesis conditions, which allow for the preservation of protein shape and function [3].
Figure 1 depicts a pathway to synthesize mesoporous silica using ferritin crystal as template, where the empty spherical ferritin cages apoferritin (aFT), in which an empty spherical ferritin cage apoferritin (aFT) with an outer diameter of 12 nm and an inner cavity diameter of 8 nm was utilized as the protein unit [4]. Apoferritin spontaneously assembles into a lattice by electrostatic self-assembly with cationic particles Cd2+ released in agarose gels. The size and spacing of the intermediary particles finally determine the pore structure. The crystals then bind to clusters of pre-hydrolyzed cations derived from N-[3-(trimethoxysilyl)propyl]-N,N,N-trimethylammonium chloride (TMAPS) and tetraethoxysilane (TEOS) precursors. Through the formation of strong electrostatic, a bond between apoferritin crystals and the cationic clusters is created. And silica encapsulating the crystals (aFT-crystals-silica) can be formed. In the end, the apoferritin is removed by calcination process at 500℃ for 4 hours, resulting in mesoporous silica with hypoferritin pore regularity [5].
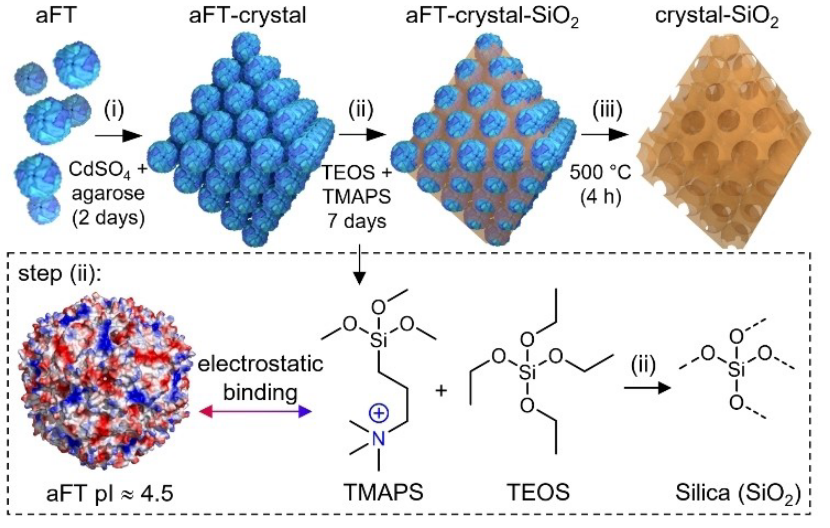
Figure 1. Synthesis of Mesoporous Silica by Ferritin as Templating Molecule [5]
2.2. Typical Synthesis of Microporous Materials: Ionothermal Synthesis Method
Ionothermal Synthesis, a method proposed by Morris et al for the preparation of aluminum phosphate molecular-sieve analogues, employs eutectic mixture (EUs) or ionic liquid as solvent and structure directing agents (SDAs) during the synthesis process, featured by the removal of competition between the template-framework and solvent-framework interactions present in hydrothermal or solvothermal synthesis. This approach has received increasing attention due to the potential in the discovery in new materials such as metal phosphates and metal–organic frameworks [6].
Organohalide salts and hydrogen bond donors (such as amides, amines, carboxylic acids and alcohols), as a class of eutectic mixture, and the imidazole-based ionic liquids, as solvents and potential SDA, are two template materials commonly used in ionothermal synthesis. In contrast to conventional hydrothermal or solvothermal synthesis, ionothermal synthesis does not require high temperature or pressure, which is helpful to avoid thermal instability of the material structure and solvent volatilization problems. In some previous studies, the synthesis of four different aluminium phosphate molecular sieve materials using 1-methyl-3-ethylimidazolium bromide ionic liquid (EMIBr) was first reported by Cooper et al [7]; nine aluminium phosphate zeolites were synthesized for the first time using eutectic mixture composed of ammonium halides and urea and its derivatives by Parnham et al [8]. In this method, quaternary ammonium salts are very attractive structure guiding agents as they can be easily prepared in a variety of shapes and sizes.
3. Characterization Methods for Mesoporous and Microporous Materials
3.1. X-Ray Diffraction (XRD)
X-ray diffraction is a valuable tool in the study of mesoporous and microporous materials, as it enables the determination of the crystal structure and the detection of crystallinity. In an X-ray diffraction experiment, a material is subjected to high-energy X-rays, which are diffracted by atoms or molecules in the crystal lattice to form a diffraction pattern. The analysis of the position and intensity of diffraction peaks in the diffraction pattern allows the type of crystal structure (e.g. cubic, hexagonal, etc.) and lattice constants to be determined, thereby enabling the crystal structure of the material to be inferred [9]. Furthermore, XRD provides both qualitative and quantitative information about the crystallinity of the material, including crystallinity and grain size. In the case of mesoporous and microporous materials, the application of X-ray diffraction is primarily concerned with the analysis of the pore structure. The analysis of changes in the shape and position of diffraction peaks allows the size, distribution, and arrangement of pores to be deduced, thus enabling the assessment of pore properties and surface characteristics of the material [10]. For example, Zhao et al. successfully synthesized mesoporous silica SBA-15 with adjustable periodic 50-300 Angstrom pores by template composed of amphiphilic triblock copolymer polyethylene oxide-polypropylene oxide-polyethylene oxide (PEO20-PPO70-PEO20). The small-angle diffraction pattern shows four distinct peaks (100), (110), (200), and (210), shown in part A figure 2, which coincides with the hexagonal symmetry of p6mm [11].
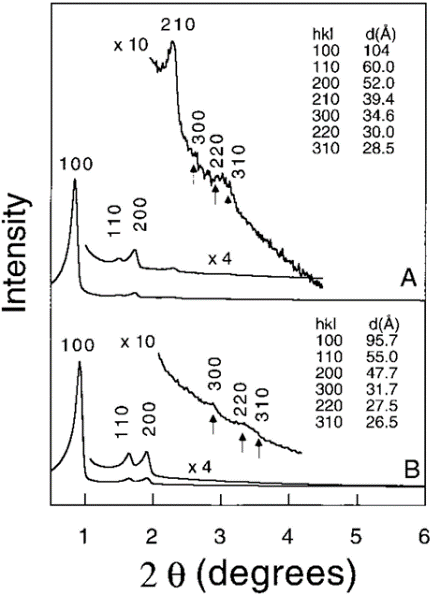
Figure 2. Powder XRD Diffraction Patterns of (A): before Calcination and (B): Calcinated Mesoporous Silica (SBA-15) [11]
In addition, part B figure 2 shows that the scattering reflection peaks (300), (220), and (310) are three distinct small peaks distributed in the 2θ range of 2-4 \( ° \) , suggesting that the synthesized SBA-15 has a high degree of hexagonal mesoscopic organization. In addition, the intensity of the (100) peak reflects a d-spacing of up to 104 Å. From the comparison of A and B, the p6mm morphology is well preserved after the calcination treatment, which confirms that SBA-15 is thermally stable, although a slight shift of its position to a larger angle, d(100) = 95.7 Å, is observed after 6 h of calcination at 500 °C. The p6mm morphology is well preserved after the calcination treatment, which confirms that SBA-15 is thermally stable [11].
3.2. Nitrogen Gas Adsorption
Nitrogen adsorption is employed to quantify the specific surface area and pore structure of materials, which serves as a pivotal tool for investigating the pore structure and surface properties of mesoporous and microporous material, providing crucial pore information that contributes to the comprehension of the properties of these materials. According to the adsorption isotherm, a curve of adsorption versus relative pressure can be obtained, so as to calculate the parameters such as the specific surface area [12]. The shape of the adsorption isotherm can be analyzed to obtain information about the pore size distribution of the material, including the size and distribution of micro- and mesopores [13]. Finally, the pore volume is defined as the volume of pores per unit volume and can be calculated by determining the intercept and slope of the adsorption isotherm, which reflects the degree of openness of the pore structure and the bulk size of the material [13].
4. Applications of Mesoporous and Microporous Materials
The application of porous materials in electrochemical capacitors and photocatalytic degradation of heavy metal ions exemplifies their pivotal role and expansive potential in the nascent fields of new energy and environmental engineering, which is of great significance in promoting the development of new energy and environmental protection technology.
4.1. Applications in Electrochemical Capacitors
The high performance electrode active materials for electrochemical capacitors, especially for electrochemical double layer capacitors (EDLCs), is the key to achieving efficient energy harvesting. The active material for the EDLCs must fulfill specifications of high specific surface area (SSA) and electronic conductivity [14] to achieve the high capacitance performance. Therefore, microporous and mesoporous material are ideal at this senario. At present, commonly used materials include, activated, templated and carbide-derived carbons, carbon fabrics, fibers, nanotubes, onions and nano-horns, of which, the activated carbons are the most frequently used material due to its low cost and high SSA led by high intrinsic porosity, microporous (<2 nm in size), mesoporous (2-50 nm in size), and macro-porous (>50 nm) network within the carbon grains after activation process from carbon-rich organic precursors. Based on these unique properties, the bilayer capacitance can reach 100-120 F/g in organic electrolytes and exceed 150-300 F/g in aqueous electrolytes [14].
4.2. Applications in Photocatalytic Degradation
Rare-earth doped metal-organic frameworks (Re-MOFs [15]) represent an example of microporous materials that combine the advantages of high specific surface area, adjustability of structure and function, and high thermal and chemical stability. These materials have important applications in photocatalytic degradation of antibiotics and heavy metal ions, and photoreduction of carbon dioxide and photocatalytic hydrogen production, which can contribute to the resolution of environmental issues.
Heavy metal ions produced by industry and other sectors present a significant threat to humans, animals, and the natural environment due to their high toxicity, difficulty in natural degradation, and the stability of the water environment. The utilization of Re-MOFs materials to eliminate heavy metal ions from aquatic environments has been demonstrated to be an efficacious approach. The application of Sm-MOFs with free amino groups [14] enables the detection and removal of Hg2+ from aqueous environments. In an aqueous environment, the Hg2+ will be captured by the organic ligands of the MOFs structure through a cooperative interaction between the N atoms and the Hg2+ ions. The photoelectrons generated from Sm will be quenched by the Hg2+ during the charge transfer and emission process under ultraviolet (UV) light, inducing fluorescence emission of Sm3+ ions from Sm-MOFs.
5. Conclusion
The analysis in this paper demonstrates the importance and potential of mesoporous and microporous materials in a number of fields, due to their unique structural properties and wide range of applications. The investigation of their typical synthesis methods and diverse characterization techniques has led to an in-depth understanding of the key role of these materials in catalysis, adsorption, energy storage, and environmental governance. With regard to the synthesis of these materials, the sol-gel method represents an effective approach for the preparation of mesoporous materials, while ionothermal synthesis provides microporous materials with highly ordered structural features. The application of characterization techniques, including X-ray diffraction, nitrogen adsorption, and transmission electron microscopy, provides further insight into the crystal structure, pore size distribution, and surface properties of these materials. In terms of applications, mesoporous materials show significant advantages in electrochemical capacitors, especially in electrochemical double layer capacitors for efficient energy storage and rapid energy release. Moreover, they play a pivotal role in photocatalysis, catalyst carriers, and environmental governance, offering innovative solutions to energy efficiency and environmental pollution problems. In the future, the synthesis techniques should be optimized and more application scenarios and material design strategies should be explored to further enhance the application value and sustainability of mesoporous and microporous materials in the development of modern science and technology.
References
[1]. Hoffmann, F., Cornelius, M., et al. (2006) Silica-Based Mesoporous Organic-Inorganic Hybrid Materials. A Journal of the German Chemical Society, 45(20): 3216-3251.
[2]. Lach, M., et al (2018) Free-Standing Metal Oxide Nanoparticle Superlattices Constructed with Engineered Protein Containers Show in Crystallo Catalytic Acticity. Dalton Trans., 47: 10382-10387.
[3]. Ellerby, L.M., et al. (1992) Encapsulation of Proteins in Transparent Porous Silicate Glasses Prepared by the Sol-Gel Method. Science, 255(5048): 1113-1115.
[4]. Fujikawa, S., Muto, E., et al. (2007) Embedding of Individual Ferritin Molecules in Large, Self-Supporting Silica Nanofilms. Langmuir, 23(8): 4629-4633.
[5]. Korpi, A., and Kostiainen, M.A. (2021) Sol‐gel Synthesis of Mesoporous Silica Using a Protein Crystal Template. ChemNanoMat, 8(4): 1-6.
[6]. Lei, L., Li, X., et al. (2009) Template Control in Ionothermal Synthesis of Aluminophosphate Microporous Materials. Dalton Transactions. 47(47): 10418-10421.
[7]. Cooper, E.R., Andrews, C.D., Wheafley, P.S., el a1. (2004) Ionic Liquids and Euteetie Mixtures as Solvent and Template in Synthesis of Zeolite Analogues. Nature, 430: 1012-1016.
[8]. Parnham, E.R., et al. (2006) Ionothermal Materials Synthesis Using Unstable Deep-Eutectic Solvents as Template-Delivery Agents. Angewandte Chemie International Edition, 45(30): 4875-5025.
[9]. Vainio, U. (2015) Small-angle X-ray Scattering. X-Ray Diffraction, 89-127.
[10]. Flodström, K. et al. (2004a) In situ synchrotron small-angle X-ray scattering/X-ray diffraction study of the formation of SBA-15 mesoporous silica, Langmuir, 20(12): 885-4891.
[11]. Zhao, D. et al. (1998) Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores’, Science, 279(5350): 548-552.
[12]. Jaroniec, M., and Fulvio, P.F. (2013) Standard Nitrogen Adsorption Data for α-alumina and Their Use for Characterization of Mesoporous Alumina-based Materials, Adsorption, 19(2-4): 475-481.
[13]. Simon, P. and Gogotsi, Y. (2008) Materials for Electrochemical Capacitors. Nature Materials, 7: 845-853.
[14]. Le, S.K., Jin, Q.J., et al. (2024) Rare Earth Element-Modified MOF Materials: Synthesis and Photocatalytic Applications in Environmental Remediation. Rare Metals, 43(4): 1390-1406.
Cite this article
Wang,P. (2024). Synthesis methods and applications of mesoporous and microporous materials. Applied and Computational Engineering,89,29-34.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Functional Materials and Civil Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Hoffmann, F., Cornelius, M., et al. (2006) Silica-Based Mesoporous Organic-Inorganic Hybrid Materials. A Journal of the German Chemical Society, 45(20): 3216-3251.
[2]. Lach, M., et al (2018) Free-Standing Metal Oxide Nanoparticle Superlattices Constructed with Engineered Protein Containers Show in Crystallo Catalytic Acticity. Dalton Trans., 47: 10382-10387.
[3]. Ellerby, L.M., et al. (1992) Encapsulation of Proteins in Transparent Porous Silicate Glasses Prepared by the Sol-Gel Method. Science, 255(5048): 1113-1115.
[4]. Fujikawa, S., Muto, E., et al. (2007) Embedding of Individual Ferritin Molecules in Large, Self-Supporting Silica Nanofilms. Langmuir, 23(8): 4629-4633.
[5]. Korpi, A., and Kostiainen, M.A. (2021) Sol‐gel Synthesis of Mesoporous Silica Using a Protein Crystal Template. ChemNanoMat, 8(4): 1-6.
[6]. Lei, L., Li, X., et al. (2009) Template Control in Ionothermal Synthesis of Aluminophosphate Microporous Materials. Dalton Transactions. 47(47): 10418-10421.
[7]. Cooper, E.R., Andrews, C.D., Wheafley, P.S., el a1. (2004) Ionic Liquids and Euteetie Mixtures as Solvent and Template in Synthesis of Zeolite Analogues. Nature, 430: 1012-1016.
[8]. Parnham, E.R., et al. (2006) Ionothermal Materials Synthesis Using Unstable Deep-Eutectic Solvents as Template-Delivery Agents. Angewandte Chemie International Edition, 45(30): 4875-5025.
[9]. Vainio, U. (2015) Small-angle X-ray Scattering. X-Ray Diffraction, 89-127.
[10]. Flodström, K. et al. (2004a) In situ synchrotron small-angle X-ray scattering/X-ray diffraction study of the formation of SBA-15 mesoporous silica, Langmuir, 20(12): 885-4891.
[11]. Zhao, D. et al. (1998) Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores’, Science, 279(5350): 548-552.
[12]. Jaroniec, M., and Fulvio, P.F. (2013) Standard Nitrogen Adsorption Data for α-alumina and Their Use for Characterization of Mesoporous Alumina-based Materials, Adsorption, 19(2-4): 475-481.
[13]. Simon, P. and Gogotsi, Y. (2008) Materials for Electrochemical Capacitors. Nature Materials, 7: 845-853.
[14]. Le, S.K., Jin, Q.J., et al. (2024) Rare Earth Element-Modified MOF Materials: Synthesis and Photocatalytic Applications in Environmental Remediation. Rare Metals, 43(4): 1390-1406.









