1. Introduction
Lithium-ion batteries are currently commonly used energy storage devices, which are widely used in various industries. Due to the lack of lithium resources and relatively abundant sodium resources, sodium-ion batteries (SIBs) have been widely concerned by researchers at home and abroad. The anode material of lithium-ion battery is generally graphite, and sodium is difficult to be embedded in the limited space of graphite due to its large ionic radius (0.102nm) [1]. In addition, the high activity of sodium leads to the irregular growth of sodium dendrites, and the large atomic radius makes the electrode reaction slow in terms of kinetics, resulting in poor electrochemical performance[2]. Therefore, we need to look for anode materials with high capacity and long cycle life.
Among the negative electrode materials of sodium-ion batteries, Sn-based materials are widely regarded as one of the most promising anode materials, with high theoretical capacity, excellent safety and good environmental compatibility[3]. With the progress of the research, Sn-based materials also showed some shortcomings[4]: (1) During the process of embedding or extraction of Na+, there was a serious volume expansion problem, and the volume expansion rate was as high as 420%. (2) The material's own electrical conductivity and ionic conductivity are poor, resulting in a slow kinetic reaction, which reduces the high rate performance. (3) The solid electrolyte interface phase is repeatedly generated on the exposed new surface, and the initial reaction is poor in reversibility, resulting in a large amount of Na+ consumption of the cathode and electrolyte, and low coulomb efficiency. In order to reduce the negative impact of Sn-based materials, four methods have been mainly adopted to improve the structure of nanostructures[5], carbon composite structures[6], alloying [7]and structural design[8].
In this paper, the principle and characteristics of electrode reactions of various Sn-based anode materials in SIBs are introduced first, and then the latest research progress of improving methods of Sn-based materials is reviewed, and the mechanism and effect of tin-based materials are analyzed, and the application of different Sn-based materials in sodium ion batteries is described. Finally, the development of Sn-based materials is summarized and prospected.
2. Reaction of Sn-based materials in SIBs
Sn-based negative electrode has a large storage of sodium ions, which has significant potential in improving battery energy density[9], and has an important position in modern battery technology and a wide range of application prospects. The following will introduce the electrode reaction conversion process/charge and discharge process of various types of Sn-based negative electrode materials in sodium ion batteries.
2.1. Metal Sn and alloys
Sn is a kind of metal material with abundant resources and low cost. The metal Sn is a good material for use as a negative electrode for sodium-ion batteries, with a theoretical capacity of up to 847mAh g−1[10]. When the alloy formed by Sn and Na is saturated, Na15Sn4 is the main one, and several amorphous phases are formed in the reaction. In the process of reacting with sodium, Sn successively forms NaSn3、NaSn2、a-Na1.2Sn、expanded Na1+xSn2、Na4.4Sn2、Na4.75Sn2、Na15Sn4 and Na15+xSn4. From the final product Na15Sn4, we can know that One Sn can store up to 4.75 sodium. When the ratio of the two in the alloy reaches 15:4, a part of Na will continue to react with Sn to form Na15+xSn4 to store additional sodium[11].
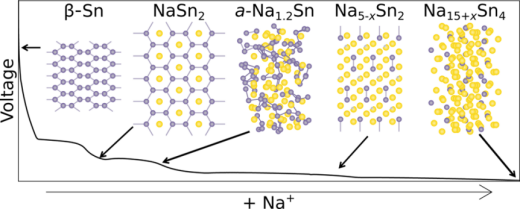
Figure 1: Structure diagram of each phase in the reaction process of Sn and sodium[11].
In addition to Sn, the transition metal alloy materials of Sn, such as Sn-copper alloy and Sn-iron alloy, all exhibit high sodium reserves, good cycle stability and long service life[12]. The metal Sn provides a high storage capacity for the battery, and the transition metal alloying element provides a stable metal skeleton for Sn, which is conducive to the embedding and removal of Na+. The transition metal elements forming the alloy have certain ductility and have a buffering effect on the volume expansion caused by the Na+ embedding and removal process[13]. In addition, there are two kinds of Sn, Na active Sn and Na inactive Sn, in many Sn alloys, in which the inactive Sn acts as an electron conduction path, and the good conductivity of transition metal elements is conducive to amplifying current and improving energy utilization[14].
2.2. Compounds of Sn
2.2.1. SnO2
Compared with traditional carbon anode materials, Sn dioxide (SnO2) has the advantages of high theoretical specific capacity (782mAh g−1), low cost, simple preparation, etc., which helps to improve the battery life[7]. SnO2 can effectively embed and release sodium ions in sodium-ion batteries, while maintaining high conductivity and cycle stability, so that SnO2 electrodes can achieve high efficiency charge and discharge process and extend battery life.
During the charging process, the SnO2 electrode receives sodium ions and is embedded in its structure, forming an Na-Sn alloy or Sn-Na compound. During the charging process, the structure of SnO2 will undergo volume changes, but compared with lithium in lithium-ion batteries, the ion radius of sodium ions is larger, which is more dependent on the better structural stability of SnO2 electrodes when embedding and releasing sodium ions[15]. It is widely believed that the reaction between SnO2 and Na can be described by the following two-step natritization process:
\( Sn{O_{2}}+4{Na^{+}}+4{e^{-}}→Sn+2Na\ \ \ (1) \)
\( Sn+x{Na^{+}}+x{e^{-}}→{Na_{x}}Sn(0≤x≤3.75)\ \ \ (2) \)
2.2.2. Sn-S
Due to the interlayer structure of Sn sulfide, its suitable lattice gap and weak van der Waals forces between layers[16], it can accommodate ions with a larger radius, so sodium ions can be more easily embedded or removed between layers, which undoubtedly contributes to the rapid diffusion of sodium ions. The Sn sulfur electrode improves the reversibility of the battery electrode reaction and its cycle stability.
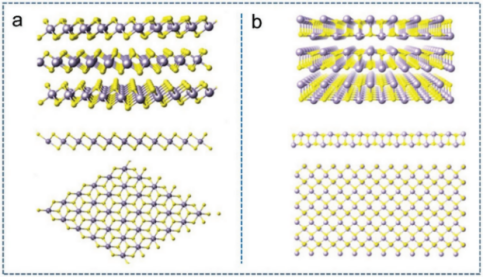
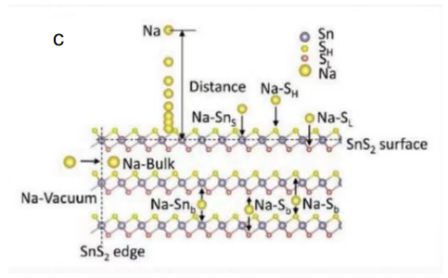
Figure 2: (a) hexagonal cell SnS2, (b) crystal structure of orthorhombic SnS, (c) schematic diagram of Na-SnS2 surface distance and Na surface adsorption site/body insertion site[17].
Its theoretical specific capacity is 1137 mAh g-1. SnS2 has a larger interlayer spacing (0.59 nm), can carry a greater amount of Na+, and has greater tolerance to the expansion of the volume during the cycle of sodium-ion batteries, and has better cycle stability.
2.2.3. Sn-P
At present, the main components of Sn-phosphorus alloy phases are Sn4P3, Sn3P, SnP and SnP3[18]. Sn4P3, as a Sn-phosphorus alloy electrode, shows excellent electrochemical performance, providing a high reversible capacity of 718 mAh g−1, and has very stable cycling performance with almost no capacity attenuation after 100 cycles[19]. The sodium storage reaction in the negative electrode of Sn4P3 is as follows: Sn atom and P atom in Sn4P3 react with Na to generate Na15Sn4 and Na3P, respectively. The main electrode reactions are as follows[20]:
\( S{n_{4}}{P_{3}}+24N{a^{+}}+24{e^{-}}→N{a_{15}}S{n_{4}}+3N{a_{3}}P\ \ \ (3) \)
\( N{a_{15}}S{n_{4}}→24Sn+15N{a^{+}}+15{e^{-}}\ \ \ (4) \)
\( N{a_{3}}P→P+3N{a^{+}}+3{e^{-}}\ \ \ (5) \)
2.3. Other Sn-based electrodes
In addition to the above negative materials, there are many other types of Sn-based negative materials, such as stannate negative (M2SnO4)[1]. Compared with the electrode with only Sn participating in the reaction, the introduction of other cations can make the electrode reaction more diversified, and the M and MO as products can reduce the volume expansion caused by the reaction to Na15Sn4, and improve the cycle stability of the battery. The reaction principle is as follows[1]:
\( {M_{2}}Sn{O_{4}}+8N{a^{+}}+8{e^{-}}→2M+Sn+4N{a_{2}}O\ \ \ (6) \)
\( Sn+xN{a^{+}}+x{e^{-}}→N{a_{x}}Sn(0≤x≤3.75)\ \ \ (7) \)
\( Sn+2N{a_{2}}O→Sn{O_{2}}+4N{a^{+}}+4{e^{-}}\ \ \ (8) \)
\( M+N{a_{2}}O→MO+2N{a^{+}}+2{e^{-}}\ \ \ (9) \)
3. Modification method
When sodium ions are charged into the negative electrode of Sn base, the volume of Sn material will change seriously, which will lead to serious attenuation of effective capacity, serious loss of sodium ions and low coulomb efficiency[4]. In order to overcome the above problems, people have carried out a lot of research in various aspects of battery modification, structural design and size adjustment. At present, the more effective optimization and improvement methods mainly include size adjustment to the nanometer level, composite with carbon materials, alloying with other metals and structural design of electrodes[5–8]. The following will introduce the principles, advantages and latest research progress of several improved methods respectively.
3.1. Nanoscale synthesis
By preparing the Sn-based anode material into nanometer size, the electrode performance can be improved in many aspects: (1) The gap between the nanoparticles can provide reserved buffer space for the volume expansion, thereby reducing the volume expansion at the macro level. (2) Nanostructures can provide more space and structural stability, reducing the micro-cracks or spalling caused by volume changes in the electrode material. (3) The closer distance between nanoparticles shorens the charge diffusion path of ions and electrons, and charges and ions can be transferred between nanoparticles more quickly[5].
Li et al.[21]designed a bimetallic SnS2/NiS2@S-rGO nanocomposite with layered flower-like structure using a simple solvothermal method (Figure. 3). The synergistic effect of NiS2 and SnS2 components enhanced the electrochemical performance. The synthetic SnS2/NiS2@S-rGO composite electrode used as the anode material for sodium ion batteries has an ultra-stable cycle life (380.9 mAh g−1 at 1 A g−1, 4000 cycles; 340.7 mAh g−1at 2 A g−1, 5000 cycles ) and excellent magnification performance (330.4 mAh g−1 at 10.0 A g−1).The sodium-ion full battery (NVP@rGO//SnS2/NiS2@S-rGO) has an excellent magnification performance of 220.9 mAh g−1 at 2.0 A g−1. Different from other schemes, Zhang et al.[22]used hydrothermal method to prepare ultra-fine SnSx nanoparticles with abundant heterostructures on N-rGO nanosheets, which can provide abundant surface active sites and effectively shorten the ion diffusion path. The internal electric field at the heterogeneous interface can significantly promote ion diffusion, accelerate surface reaction kinetics, and promote electron transfer. It has A large discharge capacity of 1273 mAh g-1 at 0.1 A g-1 and an excellent magnification performance of 594 mAh g-1 at 10 A g-1. After 1000 cycles at 1 A g−1, SnSx/N-rGO-30 nanocomposites also showed excellent cycling performance with a discharge capacity of 1450 mAh g−1. SnSx/NrGO-30 nanocomposite, as A negative electrode material for sodium-ion batteries, has A high reversible discharge capacity of 640 mAh g-1 at 0.1 A g-1 and an excellent magnification capacity of 225 mAh g-1 at 10 A g-1 (Figure 3). Zhu et al.[23]designed a novel porous double-carbon shell structure to grow Sn-MOF directly on the GO template by hydrothermal method. SnO2 nanoparticles (SnO2/C/rGO) encapsulated in a double-carbon conductive network were then synthesized by a simple solvothermal method (Figure 3). When assembled in a sodium-ion battery, the SnO2/C/rGO anode exhibits excellent rate performance and cycle stability. Even at high current densities, the specific capacities of SnO2/C/rGO anodes in sodium-ion batteries (0.5 A g−1,100 cycles) are 500.1 mAh g−1 and 206.2 mAh g−1, respectively.
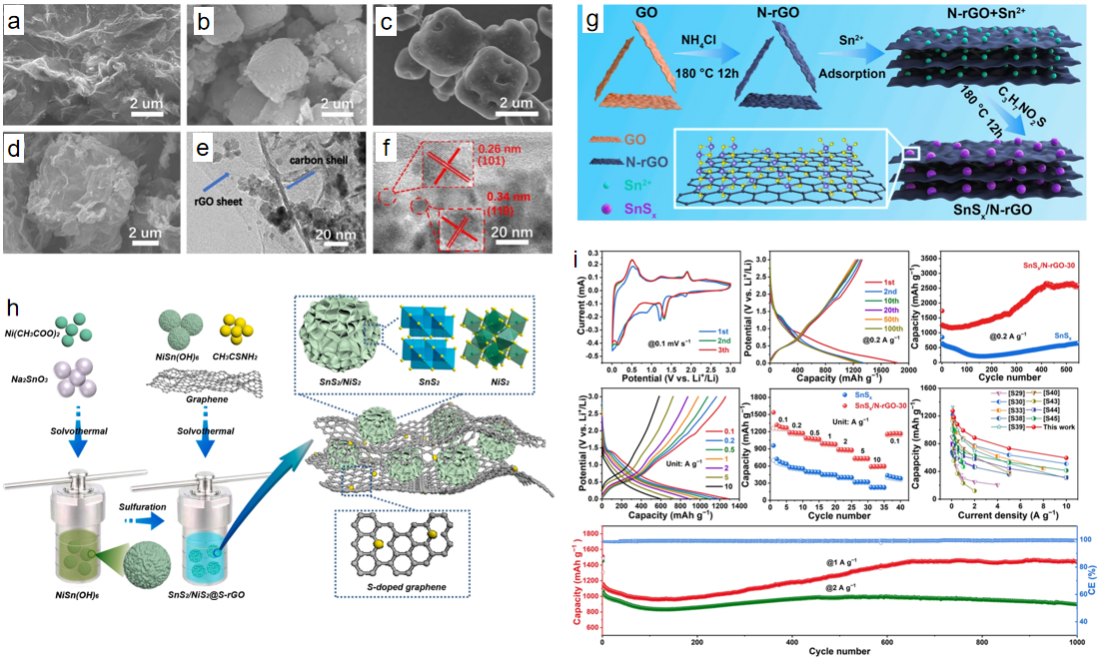
Figure 3: SEM photos of (a) SnO2/rGO, (b) Sn-MOF, (c) SnO2/ c and (d) SnO2/ c /rGO. (e) TEM photos of SnO2/C/rGO. (f) HRTEM photo of SnO2/C/rGO[23]. (g) Schematic diagram of synthesis process of SnSx/N-rGO-30 composite[22]. (h) Preparation process and structure diagram of SnS2/NiS2@S-rGO nanocomposites[21]. (i) Electrochemical properties of SnSx/N-rGO-30 composites[22].
3.2. Carbon composite structure
The combination of Sn-based materials with various carbon materials is also widely considered to be one of the strategies to improve the performance of sodium-ion batteries, and the main carbon substrates such as amorphous carbon, carbon nanotubes[24] and graphene[25] are widely used at present. Through the composite with carbon materials, especially with high surface area and elasticity of carbon materials such as carbon nanotubes, graphene, etc., can effectively alleviate the volume expansion of Sn-based materials[6].
Graphene is one of the most promising carbon composite materials. Wang[26] et al. constructed SnO2 nanocrystals anchored to nitrogen/sulfur codoped graphene (NSGS) by using a simple LBL assembly technique and an interface linker. This method enables ultra-small, high-content SnO2 nanocrystals to uniformly anchor doped graphene due to coordination effects. The optimized NSGS has A high initial lithium storage capacity of 2123.9 mAh g−1 at 0.1 A g−1 and a high reversible capacity of 480.2 mAh g−1 after 2000 cycles at 2 A g−1. Wu[27] et al. successfully prepared SnP2O7 encapsulated in N and P double-doped graphene (SnP2O7/NPG) using hexachlorocyclotriphosphorus as N and P sources. The resulting SnP2O7/NPG as an anode material for a sodium-ion battery showed excellent Na ion storage performance, with good stability of 86.2 mAh g−1 after 1000 cycles at 2A g−1 and a high rate capacity of 182.3 mAh g−1 at 2A g−1. Two sodium-based batteries were assembled with graphite as positive electrode material and SnP2O7/NPG as negative electrode material respectively, which showed A high dielectric discharge voltage of 2.5 V and a high discharge capacity of 86.6 mAh g−1 at 0.1 A g−1, which has great practical application potential.
3.3. Alloying control
In recent years, metal oxid-based conversion cathode has been considered as a promising candidate to replace commercial graphite due to its abundant natural reserves and excellent electrochemical properties. The reaction mechanism in the conversion anode is either embedding/ejection (MxOy + nLi++ ne- ↔LinMxOy) or conversion reaction (MxOy + 2yLi++ 2ye- ↔yLi2O + xM)[28].By introducing other elements (such as antimony, nickel, etc.) to form alloys with Sn, the mechanical strength and stability of Sn-based materials can be improved, the electron transport path can be optimized, the internal resistance of electrode materials can be reduced, the conductive properties of electrode materials can be improved, the charge transfer rate and the magnicity performance of electrodes can be increased[7].
Zhao[29] et al. used electrospinning technology to synthesize SnSe quantum dots (E-SnSe) and SnSe nanoparticles (B-SnSe), respectively. E-SnSe has a unique three-dimensional (3D) conducting carbon network structure (Figure 4). The E-SnSe electrode provides A larger discharge capacity of 268 mAh g−1 after 750 cycles at 2 A g−1 and a significant cycle durability of more than 1500 cycles even at higher rates of 5 A g−1. The assembled Na3V2(PO4)3@C//E-SnSe full battery showed an output voltage of over 2.0V, A long cycle life of 1500 cycles, a high reversible discharge capacity of about 100 mAh g−1 at 1 A g−1, and a capacity retention rate of up to 86.4% after 35 cycles, with good electrochemical performance. Zhang et al. [30] prepared p-SnO2/rGO/Se microspheres using SnCl2, graphene oxide (GO) and SeO2 as raw materials by a simple single-pot spray pyrolysis method. In the presence of RGO, SeO2 was pyrolyzed to Se, and a garnet-like RGO structure was constructed, which alleviated the large volume change of SnO2 and accelerated the charge transfer. The p-SnO2/rGO/Se sphere provides A high capacity of 506.7 mAh g-1 at a current of 30 mA g-1 and maintains a capacity of 188.9 mAh g-1 at an ultra-high current density of 10 A g−1 (Figure 4).
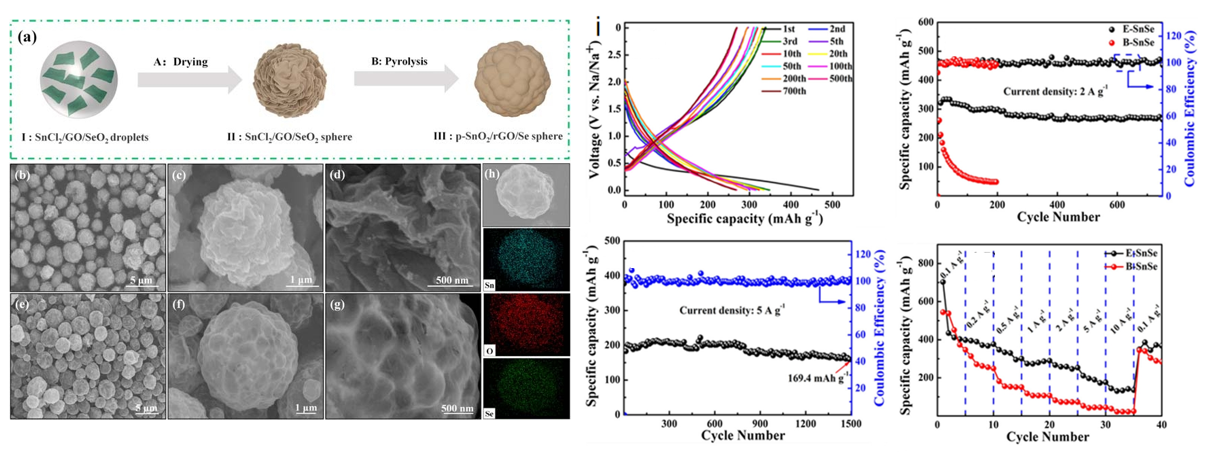
Figure 4: (a) Schematic diagram of the preparation process. SEM images of (b-d) f-SnO2/rGO ball and (e-g) p-SnO2/rGO/Se ball. (h) SEM images of the distribution of Sn, O and Se elements in p-SnO2/rGO/Se spheres with EDS [30]. (i) Electrochemical performance test of E-SnSe[29].
3.4. Structure Optimization
For Sn-based materials, the electrode properties can also be improved by designing electrode structures, which mainly include porous structures[31], layered structures[32], nanostructures[9] and composite structures[33]. Reasonable structural design can adapt to the volume change of Sn, thereby reducing material damage and structural fatigue, improving its cycle stability and prolonging its service life[8].
Construction of heterogeneous structures is considered to be one of the most effective measures to promote electron transfer and improve surface reaction dynamics by using induced internal construction in an electric field. Tian et al.[34] successfully prepared a carbon coated heterostructure SnO2/SnSe2 NPs as a sodium storage anode material (Figure 5). The material uses the heterogeneous structure to obtain the internal charge transfer driving force and promote the diffusion of sodium ions during charge and discharge. Thanks to its structural characteristics, the specific capacity of C/SnO2/SnSe2@C electrode is 422.3 mAh g-1 at 0.1 A g−1 current density. After 150 cycles, the capacity of C/SnO2/SnSe2@C electrode is still 422.3 mAh g-1. Much higher than the 276.5mAh g-1 at the C/SnO2@C electrode and 252.8mAh g-1 at the C/SnSe2@C electrode.
Cao et al. [35] obtained defect-rich SnSSe/C layered heterostructures by substituting Se anion for S anion through a one-step solvothermal method. The SnSSe crystal structure generates abundant crystal defects and uniformly dispersed S and Se atoms, which effectively controls the nucleation process of Na2S, Na2Se and SnO nanoparticles and the formation of quantum dot distribution intermediates. The SnSSe/C electrode has a high specific capacity of 314.5 mAh g−1 at an ultra-high magnification rate of 20 A g−1, and a very long cycle life with an average capacity decay rate of only 0.002% after 15,000 cycles at 20 A g−1 (Figure 5).
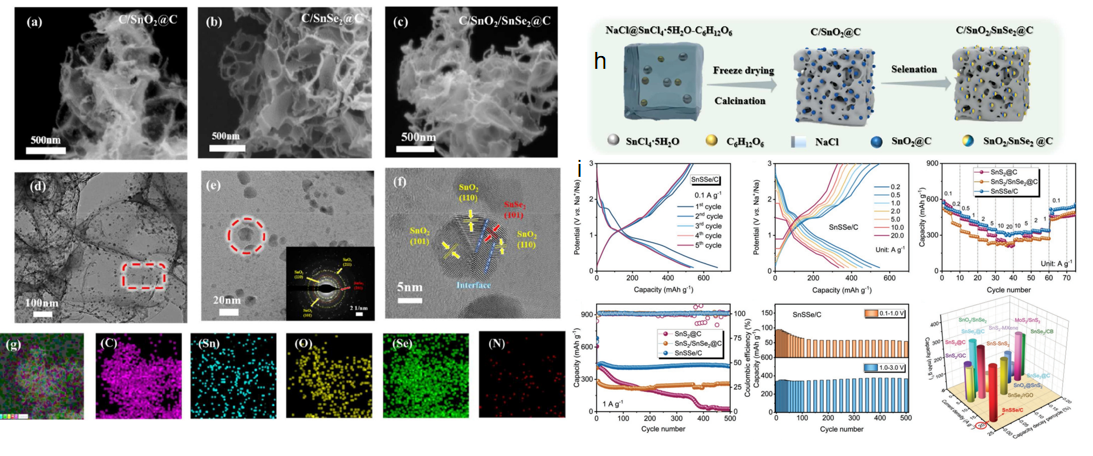
Figure 5: (a~c) SEM images of samples. (d, e) Transmission electron microscopy and selected region electron diffraction of the C/SnO2/SnSe2@C sample (illustration), (f) high-resolution TEM, (g) Energy dispersive X-ray spectral color map. (h) Schematic diagram of synthesis strategy in C/SnO2/SnSe2@C[34]. (i) Na storage performance of SnSSe/C electrodes [37].
4. Conclusion and Prospect
In summary, we summarized the electrode reaction principle and advantages and disadvantages of various Sn-based materials as negative electrode in sodium ion batteries, including Sn metal, Sn sulfide, Sn oxide and Sn phosphating. The SN-based electrodes are mainly alloy electrodes and conversion electrodes.
In order to solve the above problems and improve the performance of Sn-based electrodes, we have developed many methods. (1) nanometer size control. (2) Carbon material composite. (3) Alloying control. (4) Structural design. Sn-based material is a promising electrode material with high theoretical capacity and various electrode types. In the future development, it can be expected that the development direction of Sn-based material electrodes will mainly focus on the following aspects:(1) Material modification and compounding: Improving the properties of Sn-based materials, enhancing electrical conductivity and stability, and reducing volume expansion.(2) Structural optimization: Developing structural designs with better mechanical stability.(3) High-performance battery applications: focusing on the application of Sn-based electrodes in lithium-ion batteries and sodium-ion batteries.
In conclusion, it is believed that the review will help overcome the limitations of existing technologies, promote the development of Sn-based material electrodes in the field of energy storage and conversion.
References
[1]. Y.-K. Duan, Z.-W. Li, S.-C. Zhang, T. Su, Z.-H. Zhang, A.-J. Jiao, Z.-H. Fu, Stannate-Based Materials as Anodes in Lithium-Ion and Sodium-Ion Batteries: A Review, Molecules 28 (2023). https://doi.org/10.3390/molecules28135037.
[2]. J. Sun, H.-W. Lee, M. Pasta, H. Yuan, G. Zheng, Y. Sun, Y. Li, Y. Cui, A phosphorene-graphene hybrid material as a high-capacity anode for sodium-ion batteries, Nat. Nanotechnol. 10 (2015) 980–985. https://doi.org/10.1038/nnano.2015.194.
[3]. Y. Wang, W. Zhu, A. Guerfi, C. Kim, K. Zaghib, Roles of ti in electrode materials for sodium-ion batteries, Front. Energy Res. 7 (2019). https://doi.org/10.3389/fenrg.2019.00028.
[4]. P. Gandharapu, A. Das, R. Tripathi, V. Srihari, H.K. Poswal, A. Mukhopadhyay, Facile and Scalable Development of High-Performance Carbon-Free Tin-Based Anodes for Sodium-Ion Batteries, ACS Appl. Mater. Interfaces 15 (2023) 37504–37516. https://doi.org/10.1021/acsami.3c07305.
[5]. H. Ying, W.-Q. Han, Metallic Sn-Based Anode Materials: Application in High-Performance Lithium-Ion and Sodium-Ion Batteries, Adv. Sci. 4 (2017). https://doi.org/10.1002/advs.201700298.
[6]. M.Z. Ansari, S.A. Ansari, S.-H. Kim, Fundamentals and recent progress of Sn-based electrode materials for supercapacitors: A comprehensive review, J. Energy Storage 53 (2022). https://doi.org/10.1016/j.est.2022.105187.
[7]. M. Zhao, Q. Zhao, J. Qiu, H. Xue, H. Pang, Tin-based nanomaterials for electrochemical energy storage, RSC Adv. 6 (2016) 95449–95468. https://doi.org/10.1039/c6ra19877e.
[8]. L. Liu, F. Xie, J. Lyu, T. Zhao, T. Li, B.G. Choi, Tin-based anode materials with well-designed architectures for next-generation lithium-ion batteries, J. Power Sources 321 (2016) 11–35. https://doi.org/10.1016/j.jpowsour.2016.04.105.
[9]. Y. Zhao, X. Li, B. Yan, D. Li, S. Lawes, X. Sun, Significant impact of 2D graphene nanosheets on large volume change tin-based anodes in lithium-ion batteries: A review, J. Power Sources 274 (2015) 869–884. https://doi.org/10.1016/j.jpowsour.2014.10.008.
[10]. M. Lao, Y. Zhang, W. Luo, Q. Yan, W. Sun, S.X. Dou, Alloy-Based Anode Materials toward Advanced Sodium-Ion Batteries, Adv. Mater. 29 (2017). https://doi.org/10.1002/adma.201700622.
[11]. J.M. Stratford, M. Mayo, P.K. Allan, O. Pecher, O.J. Borkiewicz, K.M. Wiaderek, K.W. Chapman, C.J. Pickard, A.J. Morris, C.P. Grey, Investigating Sodium Storage Mechanisms in Tin Anodes: A Combined Pair Distribution Function Analysis, Density Functional Theory, and Solid-State NMR Approach, J. Am. Chem. Soc. 139 (2017) 7273–7286. https://doi.org/10.1021/jacs.7b01398.
[12]. H. Mou, W. Xiao, C. Miao, R. Li, L. Yu, Tin and Tin Compound Materials as Anodes in Lithium-Ion and Sodium-Ion Batteries: A Review, Front. Chem. 8 (2020). https://doi.org/10.3389/fchem.2020.00141.
[13]. J.-M. Liang, L.-J. Zhang, D.-G. XiLi, J. Kang, Research progress on tin-based anode materials for sodium ion batteries, Rare Met. 39 (2020) 1005–1018. https://doi.org/10.1007/s12598-020-01453-x.
[14]. X. Zhu, M. Sun, J. Ni, L. Li, Materials based on group IVA elements for alloying-type sodium storage, Sci. China Chem. 61 (2018) 1494–1502. https://doi.org/10.1007/s11426-018-9347-9.
[15]. J.-K. Meng, W.-W. Wang, Q.-C. Wang, M.-H. Cao, Z.-W. Fu, X.-J. Wu, Y.-N. Zhou, Graphene supported ultrafine tin oxide nanoparticles enable conversion reaction dominated mechanism for sodium-ion batteries, Electrochimica Acta 303 (2019) 32–39. https://doi.org/10.1016/j.electacta.2019.02.072.
[16]. J. Ye, Z. Chen, Q. Liu, C. Xu, Tin sulphide nanoflowers anchored on three-dimensional porous graphene networks as high-performance anode for sodium-ion batteries, J. Colloid Interface Sci. 516 (2018) 1–8. https://doi.org/10.1016/j.jcis.2018.01.045.
[17]. Y. Shan, Y. Li, H. Pang, Applications of Tin Sulfide-Based Materials in Lithium-Ion Batteries and Sodium-Ion Batteries, Adv. Funct. Mater. 30 (2020). https://doi.org/10.1002/adfm.202001298.
[18]. Y.-U. Kim, S.-I. Lee, C.K. Lee, H.-J. Sohn, Enhancement of capacity and cycle-life of Sn4 + δp 3 (0 ≤ δ ≤ 1) anode for lithium secondary batteries, J. Power Sources 141 (2005) 163–166. https://doi.org/10.1016/j.jpowsour.2004.09.007.
[19]. W. Liu, H. Zhi, X. Yu, Recent progress in phosphorus based anode materials for lithium/sodium ion batteries, Energy Storage Mater. 16 (2019) 290–322. https://doi.org/10.1016/j.ensm.2018.05.020.
[20]. J. Qian, Y. Xiong, Y. Cao, X. Ai, H. Yang, Synergistic Na-storage reactions in Sn4P3 as a high-capacity, cycle-stable anode of Na-ion batteries, Nano Lett. 14 (2014) 1865–1869. https://doi.org/10.1021/nl404637q.
[21]. H. Li, Y. He, Y. Dai, Y. Ren, T. Gao, G. Zhou, Bimetallic SnS2/NiS2@S-rGO nanocomposite with hierarchical flower-like architecture for superior high rate and ultra-stable half/full sodium-ion batteries, Chem. Eng. J. 427 (2022). https://doi.org/10.1016/j.cej.2021.131784.
[22]. Y. Zhang, Q. Wang, K. Zhu, K. Ye, G. Wang, D. Cao, J. Yan, Built-in electric field induced interfacial effect enables ultrasmall SnSx nanoparticles with high-rate lithium/sodium storage, Chem. Eng. J. 446 (2022). https://doi.org/10.1016/j.cej.2022.137286.
[23]. S. Zhu, A. Huang, Q. Wang, Y. Xu, MOF derived double-carbon layers boosted the lithium/sodium storage performance of SnO2nanoparticles, Nanotechnology 32 (2021). https://doi.org/10.1088/1361-6528/abf87b.
[24]. Y. Zou, Y. Wang, Sn@CNT nanostructures rooted in graphene with high and fast Li-storage capacities, ACS Nano 5 (2011) 8108–8114. https://doi.org/10.1021/nn2027159.
[25]. M. Ma, J. Yan, C. Yu, S. Guo, Insight into the Formation/Decomposition of Solid Electrolyte Interphase Films and Effects on the Electrochemical Properties of Sn/Graphene Anodes, J. Phys. Chem. C 122 (2018) 25211–25218. https://doi.org/10.1021/acs.jpcc.8b08715.
[26]. H.-G. Wang, Q. Wu, Y. Wang, X. Wang, L. Wu, S. Song, H. Zhang, Molecular Engineering of Monodisperse SnO2 Nanocrystals Anchored on Doped Graphene with High-Performance Lithium/Sodium-Storage Properties in Half/Full Cells, Adv. Energy Mater. 9 (2019). https://doi.org/10.1002/aenm.201802993.
[27]. Q. Wu, J. Wang, H.-G. Wang, Z. Si, C. Li, J. Bai, Doped graphene encapsulated SnP2O7 with enhanced conversion reactions from polyanions as a versatile anode material for sodium dual-ion battery, Electrochimica Acta 369 (2021). https://doi.org/10.1016/j.electacta.2020.137657.
[28]. M. Zheng, H. Tang, L. Li, Q. Hu, L. Zhang, H. Xue, H. Pang, Hierarchically Nanostructured Transition Metal Oxides for Lithium-Ion Batteries, Adv. Sci. 5 (2018). https://doi.org/10.1002/advs.201700592.
[29]. W. Zhao, X. Ma, Y. Li, G. Wang, X. Long, Achieving ultrastable cyclability and pseudocapacitive sodium storage in SnSe quantum-dots sheathed in nitrogen doped carbon nanofibers, Appl. Surf. Sci. 504 (2020). https://doi.org/10.1016/j.apsusc.2019.144455.
[30]. P. Zhang, B. Cao, R.A. Soomro, N. Sun, B. Xu, Se-decorated SnO2/rGO composite spheres and their sodium storage performances, Chin. Chem. Lett. 32 (2021) 282–285. https://doi.org/10.1016/j.cclet.2020.10.006.
[31]. X. Jia, H. Li, B. Huang, J. Yang, Y. Li, Monodisperse yolk-shell SnO2/C submicron spheres fabricated through a facile process as an anode material for lithium-ion batteries, Adv. Powder Technol. 34 (2023). https://doi.org/10.1016/j.apt.2023.104056.
[32]. J. Deng, H. Ji, C. Yan, J. Zhang, W. Si, S. Baunack, S. Oswald, Y. Mei, O.G. Schmidt, Naturally rolled-up C/Si/C trilayer nanomembranes as stable anodes for lithium-ion batteries with remarkable cycling performance, Angew. Chem. - Int. Ed. 52 (2013) 2326–2330. https://doi.org/10.1002/anie.201208357.
[33]. D. Ma, Y. Li, P. Zhang, Z. Lin, Oxygen Vacancy Engineering in Tin(IV) Oxide Based Anode Materials toward Advanced Sodium-Ion Batteries, ChemSusChem 11 (2018) 3693–3703. https://doi.org/10.1002/cssc.201801694.
[34]. S. Tian, X. Cheng, H. Li, M. Wang, X. Wang, A heterogeneous structured C/SnO2/SnSe2@C hybrid: exploring the superior rate performance in sodium ion batteries, Mater. Today Chem. 30 (2023). https://doi.org/10.1016/j.mtchem.2023.101524.
[35]. L. Cao, S. Fang, B. Xu, B. Zhang, C. Wang, Z. Xiao, G. Zou, H. Hou, X. Ou, X. Ji, Enabling Reversible Reaction by Uniform Distribution of Heterogeneous Intermediates on Defect-Rich SnSSe/C Layered Heterostructure for Ultralong-Cycling Sodium Storage, Small 18 (2022). https://doi.org/10.1002/smll.202202134.
Cite this article
Li,T. (2025). Research Progress of Sn-based Anode Materials for SIBs. Applied and Computational Engineering,123,1-9.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 5th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Y.-K. Duan, Z.-W. Li, S.-C. Zhang, T. Su, Z.-H. Zhang, A.-J. Jiao, Z.-H. Fu, Stannate-Based Materials as Anodes in Lithium-Ion and Sodium-Ion Batteries: A Review, Molecules 28 (2023). https://doi.org/10.3390/molecules28135037.
[2]. J. Sun, H.-W. Lee, M. Pasta, H. Yuan, G. Zheng, Y. Sun, Y. Li, Y. Cui, A phosphorene-graphene hybrid material as a high-capacity anode for sodium-ion batteries, Nat. Nanotechnol. 10 (2015) 980–985. https://doi.org/10.1038/nnano.2015.194.
[3]. Y. Wang, W. Zhu, A. Guerfi, C. Kim, K. Zaghib, Roles of ti in electrode materials for sodium-ion batteries, Front. Energy Res. 7 (2019). https://doi.org/10.3389/fenrg.2019.00028.
[4]. P. Gandharapu, A. Das, R. Tripathi, V. Srihari, H.K. Poswal, A. Mukhopadhyay, Facile and Scalable Development of High-Performance Carbon-Free Tin-Based Anodes for Sodium-Ion Batteries, ACS Appl. Mater. Interfaces 15 (2023) 37504–37516. https://doi.org/10.1021/acsami.3c07305.
[5]. H. Ying, W.-Q. Han, Metallic Sn-Based Anode Materials: Application in High-Performance Lithium-Ion and Sodium-Ion Batteries, Adv. Sci. 4 (2017). https://doi.org/10.1002/advs.201700298.
[6]. M.Z. Ansari, S.A. Ansari, S.-H. Kim, Fundamentals and recent progress of Sn-based electrode materials for supercapacitors: A comprehensive review, J. Energy Storage 53 (2022). https://doi.org/10.1016/j.est.2022.105187.
[7]. M. Zhao, Q. Zhao, J. Qiu, H. Xue, H. Pang, Tin-based nanomaterials for electrochemical energy storage, RSC Adv. 6 (2016) 95449–95468. https://doi.org/10.1039/c6ra19877e.
[8]. L. Liu, F. Xie, J. Lyu, T. Zhao, T. Li, B.G. Choi, Tin-based anode materials with well-designed architectures for next-generation lithium-ion batteries, J. Power Sources 321 (2016) 11–35. https://doi.org/10.1016/j.jpowsour.2016.04.105.
[9]. Y. Zhao, X. Li, B. Yan, D. Li, S. Lawes, X. Sun, Significant impact of 2D graphene nanosheets on large volume change tin-based anodes in lithium-ion batteries: A review, J. Power Sources 274 (2015) 869–884. https://doi.org/10.1016/j.jpowsour.2014.10.008.
[10]. M. Lao, Y. Zhang, W. Luo, Q. Yan, W. Sun, S.X. Dou, Alloy-Based Anode Materials toward Advanced Sodium-Ion Batteries, Adv. Mater. 29 (2017). https://doi.org/10.1002/adma.201700622.
[11]. J.M. Stratford, M. Mayo, P.K. Allan, O. Pecher, O.J. Borkiewicz, K.M. Wiaderek, K.W. Chapman, C.J. Pickard, A.J. Morris, C.P. Grey, Investigating Sodium Storage Mechanisms in Tin Anodes: A Combined Pair Distribution Function Analysis, Density Functional Theory, and Solid-State NMR Approach, J. Am. Chem. Soc. 139 (2017) 7273–7286. https://doi.org/10.1021/jacs.7b01398.
[12]. H. Mou, W. Xiao, C. Miao, R. Li, L. Yu, Tin and Tin Compound Materials as Anodes in Lithium-Ion and Sodium-Ion Batteries: A Review, Front. Chem. 8 (2020). https://doi.org/10.3389/fchem.2020.00141.
[13]. J.-M. Liang, L.-J. Zhang, D.-G. XiLi, J. Kang, Research progress on tin-based anode materials for sodium ion batteries, Rare Met. 39 (2020) 1005–1018. https://doi.org/10.1007/s12598-020-01453-x.
[14]. X. Zhu, M. Sun, J. Ni, L. Li, Materials based on group IVA elements for alloying-type sodium storage, Sci. China Chem. 61 (2018) 1494–1502. https://doi.org/10.1007/s11426-018-9347-9.
[15]. J.-K. Meng, W.-W. Wang, Q.-C. Wang, M.-H. Cao, Z.-W. Fu, X.-J. Wu, Y.-N. Zhou, Graphene supported ultrafine tin oxide nanoparticles enable conversion reaction dominated mechanism for sodium-ion batteries, Electrochimica Acta 303 (2019) 32–39. https://doi.org/10.1016/j.electacta.2019.02.072.
[16]. J. Ye, Z. Chen, Q. Liu, C. Xu, Tin sulphide nanoflowers anchored on three-dimensional porous graphene networks as high-performance anode for sodium-ion batteries, J. Colloid Interface Sci. 516 (2018) 1–8. https://doi.org/10.1016/j.jcis.2018.01.045.
[17]. Y. Shan, Y. Li, H. Pang, Applications of Tin Sulfide-Based Materials in Lithium-Ion Batteries and Sodium-Ion Batteries, Adv. Funct. Mater. 30 (2020). https://doi.org/10.1002/adfm.202001298.
[18]. Y.-U. Kim, S.-I. Lee, C.K. Lee, H.-J. Sohn, Enhancement of capacity and cycle-life of Sn4 + δp 3 (0 ≤ δ ≤ 1) anode for lithium secondary batteries, J. Power Sources 141 (2005) 163–166. https://doi.org/10.1016/j.jpowsour.2004.09.007.
[19]. W. Liu, H. Zhi, X. Yu, Recent progress in phosphorus based anode materials for lithium/sodium ion batteries, Energy Storage Mater. 16 (2019) 290–322. https://doi.org/10.1016/j.ensm.2018.05.020.
[20]. J. Qian, Y. Xiong, Y. Cao, X. Ai, H. Yang, Synergistic Na-storage reactions in Sn4P3 as a high-capacity, cycle-stable anode of Na-ion batteries, Nano Lett. 14 (2014) 1865–1869. https://doi.org/10.1021/nl404637q.
[21]. H. Li, Y. He, Y. Dai, Y. Ren, T. Gao, G. Zhou, Bimetallic SnS2/NiS2@S-rGO nanocomposite with hierarchical flower-like architecture for superior high rate and ultra-stable half/full sodium-ion batteries, Chem. Eng. J. 427 (2022). https://doi.org/10.1016/j.cej.2021.131784.
[22]. Y. Zhang, Q. Wang, K. Zhu, K. Ye, G. Wang, D. Cao, J. Yan, Built-in electric field induced interfacial effect enables ultrasmall SnSx nanoparticles with high-rate lithium/sodium storage, Chem. Eng. J. 446 (2022). https://doi.org/10.1016/j.cej.2022.137286.
[23]. S. Zhu, A. Huang, Q. Wang, Y. Xu, MOF derived double-carbon layers boosted the lithium/sodium storage performance of SnO2nanoparticles, Nanotechnology 32 (2021). https://doi.org/10.1088/1361-6528/abf87b.
[24]. Y. Zou, Y. Wang, Sn@CNT nanostructures rooted in graphene with high and fast Li-storage capacities, ACS Nano 5 (2011) 8108–8114. https://doi.org/10.1021/nn2027159.
[25]. M. Ma, J. Yan, C. Yu, S. Guo, Insight into the Formation/Decomposition of Solid Electrolyte Interphase Films and Effects on the Electrochemical Properties of Sn/Graphene Anodes, J. Phys. Chem. C 122 (2018) 25211–25218. https://doi.org/10.1021/acs.jpcc.8b08715.
[26]. H.-G. Wang, Q. Wu, Y. Wang, X. Wang, L. Wu, S. Song, H. Zhang, Molecular Engineering of Monodisperse SnO2 Nanocrystals Anchored on Doped Graphene with High-Performance Lithium/Sodium-Storage Properties in Half/Full Cells, Adv. Energy Mater. 9 (2019). https://doi.org/10.1002/aenm.201802993.
[27]. Q. Wu, J. Wang, H.-G. Wang, Z. Si, C. Li, J. Bai, Doped graphene encapsulated SnP2O7 with enhanced conversion reactions from polyanions as a versatile anode material for sodium dual-ion battery, Electrochimica Acta 369 (2021). https://doi.org/10.1016/j.electacta.2020.137657.
[28]. M. Zheng, H. Tang, L. Li, Q. Hu, L. Zhang, H. Xue, H. Pang, Hierarchically Nanostructured Transition Metal Oxides for Lithium-Ion Batteries, Adv. Sci. 5 (2018). https://doi.org/10.1002/advs.201700592.
[29]. W. Zhao, X. Ma, Y. Li, G. Wang, X. Long, Achieving ultrastable cyclability and pseudocapacitive sodium storage in SnSe quantum-dots sheathed in nitrogen doped carbon nanofibers, Appl. Surf. Sci. 504 (2020). https://doi.org/10.1016/j.apsusc.2019.144455.
[30]. P. Zhang, B. Cao, R.A. Soomro, N. Sun, B. Xu, Se-decorated SnO2/rGO composite spheres and their sodium storage performances, Chin. Chem. Lett. 32 (2021) 282–285. https://doi.org/10.1016/j.cclet.2020.10.006.
[31]. X. Jia, H. Li, B. Huang, J. Yang, Y. Li, Monodisperse yolk-shell SnO2/C submicron spheres fabricated through a facile process as an anode material for lithium-ion batteries, Adv. Powder Technol. 34 (2023). https://doi.org/10.1016/j.apt.2023.104056.
[32]. J. Deng, H. Ji, C. Yan, J. Zhang, W. Si, S. Baunack, S. Oswald, Y. Mei, O.G. Schmidt, Naturally rolled-up C/Si/C trilayer nanomembranes as stable anodes for lithium-ion batteries with remarkable cycling performance, Angew. Chem. - Int. Ed. 52 (2013) 2326–2330. https://doi.org/10.1002/anie.201208357.
[33]. D. Ma, Y. Li, P. Zhang, Z. Lin, Oxygen Vacancy Engineering in Tin(IV) Oxide Based Anode Materials toward Advanced Sodium-Ion Batteries, ChemSusChem 11 (2018) 3693–3703. https://doi.org/10.1002/cssc.201801694.
[34]. S. Tian, X. Cheng, H. Li, M. Wang, X. Wang, A heterogeneous structured C/SnO2/SnSe2@C hybrid: exploring the superior rate performance in sodium ion batteries, Mater. Today Chem. 30 (2023). https://doi.org/10.1016/j.mtchem.2023.101524.
[35]. L. Cao, S. Fang, B. Xu, B. Zhang, C. Wang, Z. Xiao, G. Zou, H. Hou, X. Ou, X. Ji, Enabling Reversible Reaction by Uniform Distribution of Heterogeneous Intermediates on Defect-Rich SnSSe/C Layered Heterostructure for Ultralong-Cycling Sodium Storage, Small 18 (2022). https://doi.org/10.1002/smll.202202134.









