1. Introduction
In the 21st century, the rapid acceleration of global industrialization has resulted in a significant increase in greenhouse gas emissions, leading to a series of critical environmental issues, such as global warming, extreme weather events, ecosystem disruption, and rising sea levels, which pose direct threats to human survival and development. To address these challenges, China has put forward the goals of "carbon peak" and "carbon neutrality".[1] In recent years, renewable energy has attracted significant attention due to its green, low-carbon, and sustainable attributes. However, the intermittent [2] and volatile [3] nature of renewable sources like wind and solar power presents substantial challenges to maintaining balance and reliability in the power grid. To tackle these issues, energy storage systems have emerged [4] as a solution to ensure a stable and high-quality power supply. [5] Among various energy storage technologies, electrochemical energy storage stands out due to its flexible configuration, rapid response time, and high level of control, driving the transformation of the global energy system. At present, lithium-ion batteries, sodium-ion batteries, flow batteries, and solid-state batteries have become the focal points of research. Through the optimization of key structural components such as electrode materials and electrolytes, researchers around the world have greatly improved the energy density, cycle life, and safety of these batteries, [6] making battery energy storage systems more economically viable across different sectors. This paper reviews the working principles, technical characteristics, development status, and existing challenges of major battery technologies, and forecasts their future development trends, aiming to offer a multi-dimensional perspective for further research into electrochemical energy storage technologies.
2. Lithium-Ion Battery
2.1. Operating principle
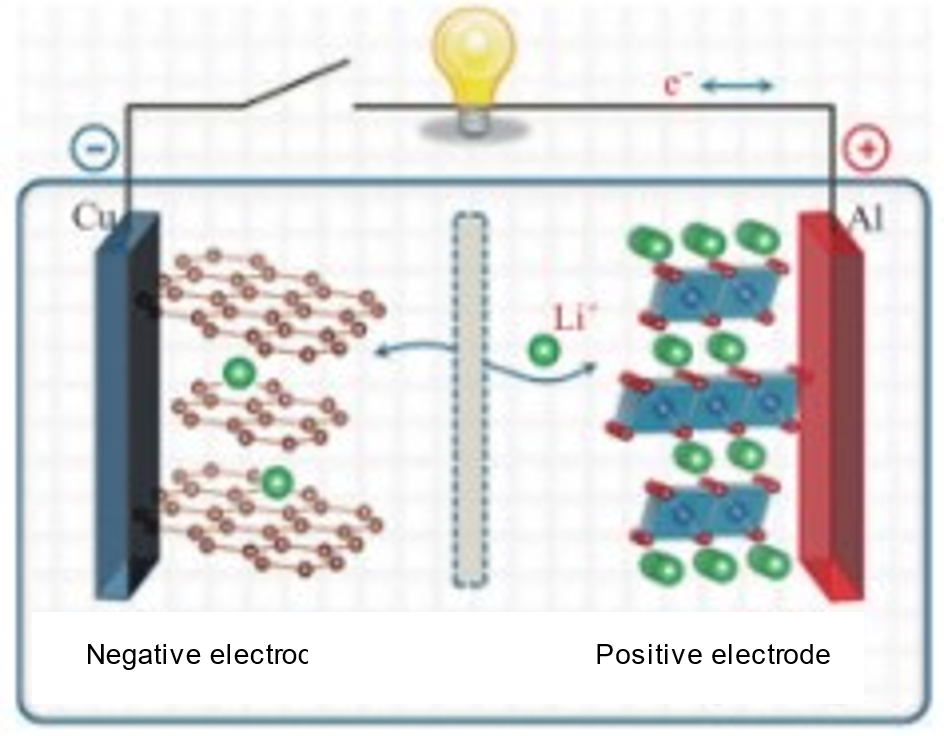
Lithium-ion batteries (LIBs) are currently among the most advanced electrochemical energy storage systems that are mass-produced. These batteries consist of two electrodes—an anode and a cathode—separated by an electrolyte and a separator. [7] The fundamental operating mechanism of LIBs is based on the intercalation and deintercalation of lithium ions between the positive and negative electrodes during charging and discharging. When charging, an external power source applies a voltage across the electrodes, causing lithium ions to be extracted (deintercalation) from the anode material and move through the electrolyte toward the cathode, where they are inserted (intercalation) into the cathode material. Simultaneously, electrons are released from the anode material and flow through an external circuit toward the cathode to maintain charge balance. During discharge, lithium ions are deintercalated from the cathode material, pass through the electrolyte, and are intercalated back into the anode material. Electrons flow in the opposite direction, from the cathode back to the anode via the external circuit, creating a current that powers external devices. [8] Figure 1 illustrates the working mechanism of the widely used lithium cobalt oxide/graphite LIB, and the corresponding electrochemical reaction is as follows:
Positive electrode reaction:LiCoO2 ⇌ Li1-xCoO2 + xLi+ + xe- (1)
Anodic reaction:6C + xLi+ + xe- ⇌ LixC6 (2)
Total reaction:LiCoO2 + 6C ⇌ Li1-xCoO2 + LixC6 (3)
The performance and safety of lithium-ion batteries are heavily dependent on the choice of materials for the cathode, anode, and electrolyte. The materials used for these components play a crucial role in determining key battery characteristics, such as energy density, cycle longevity, safety, and charge/discharge rates. Each material selection can significantly impact how well the battery performs across these metrics.
|
|
Figure 1: The operating principle of lithium-ion batteries. | Figure 2: The lithium storage mechanism of anode materials for lithium-ion batteries. |
The positive electrode plays a crucial role in supplying lithium ions for the electrochemical reaction. When selecting cathode materials, key considerations include achieving high voltage, high capacity, and stability, while also accounting for factors such as electrical conductivity, ionic conductivity, and environmental sustainability. Traditional cathode materials are typically categorized into three structural types: trigonal layered transition metal oxides (e.g., LiCoO₂), spinel-type materials (e.g., LiMn₂O₄), and polyanion-type materials (e.g., LiFePO₄).[9] As the most developed battery energy storage technology, lithium-ion batteries continue to advance. High-nickel ternary materials, such as NCM811 and NCA, are gaining significant attention due to their high energy density. However, they still face challenges related to safety and cycle life. Researchers are working on methods like doping and surface coating to enhance these materials' performance. Lithium iron phosphate (LFP) batteries, known for their safety and cost-effectiveness, are already widely used in grid energy storage and some electric vehicle applications.
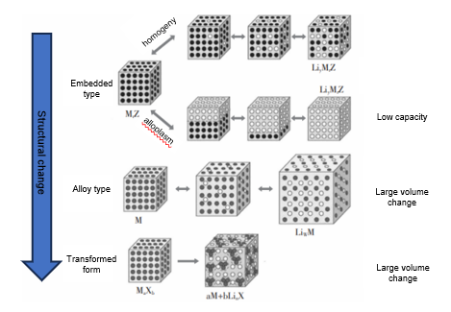
For the negative electrode, low voltage, high capacity, cycling stability, and environmental compatibility are primary concerns. The material must exhibit high lithium intercalation/ deintercalation efficiency, avoid dendrite formation from lithium metal deposition, and ensure the battery's overall safety. As shown in Figure 2, lithium-ion batteries are divided into three types of anode materials based on their lithium storage mechanisms: intercalation-type, alloying-type, and conversion-type. Graphite is the most commonly used anode material due to its low voltage plateau around 0.1V, which allows for a significant voltage difference between the anode and cathode, resulting in relatively high energy density. Recent studies show that silicon-based anode materials have a theoretical capacity of up to 4200 mAh/g—more than ten times that of traditional graphite—making them a major focus of current research. However, the stability of the anode structure is critical to prevent issues like electrode cracking or detachment caused by volume changes during charge/discharge cycles, which would reduce battery lifespan. While graphite exhibits excellent cycling stability, the volume expansion of silicon-based materials during cycling is a challenge that requires the development of composite technologies to mitigate.
The electrolyte in lithium-ion batteries is essential for the transport of lithium ions between the anode and cathode. Typically, this electrolyte is an organic solution containing lithium salts or a solid electrolyte. Organic liquid electrolytes are commonly used due to their high ionic conductivity, which enables the efficient movement of lithium ions during battery charge and discharge. To ensure the battery’s long-term stability, the electrolyte must possess a wide electrochemical stability window, allowing it to operate within the voltage limits of the electrodes without decomposing. The compatibility of the electrolyte with both the anode and cathode materials is also critical; it must not engage in side reactions that could impair battery performance or raise safety concerns. Solid electrolytes, along with certain liquid electrolytes, offer the advantage of high thermal stability, which helps maintain battery performance at elevated temperatures by preventing decomposition, gas evolution, or thermal runaway. Solid electrolytes, in particular, are non-flammable and non-volatile, making them highly attractive for improving both the safety and lifespan of lithium-ion batteries. As a result, they have garnered considerable interest from researchers. The commercialization of all-solid-state batteries is anticipated within the next decade, with several automotive companies and battery manufacturers already positioning themselves strategically. However, challenges such as low ionic conductivity and high interfacial resistance in solid electrolytes [10] still need to be addressed to facilitate widespread adoption.
2.2. Technical characteristics
Lithium-ion batteries offer key technical features such as high energy density, long cycle life, a low self-discharge rate, and the absence of a memory effect.[11] Compared to conventional nickel-cadmium and nickel-metal-hydride batteries, lithium-ion batteries possess a higher energy density, significantly reducing their size and weight. As a result, they are widely used in portable electronic devices. Moreover, they offer a long cycle life, typically capable of enduring several hundred to thousands of charge-discharge cycles, making them ideal for applications requiring frequent charging and discharging. Guerifi et al.[12] tested batteries using LiFePO4 and graphite as the cathode and anode materials, respectively. Their results showed that the initial discharge capacity of the lithium-ion battery was 150 mAhg⁻¹, and even after 100 cycles, the capacity remained at 128 mAhg⁻¹. Additionally, lithium-ion batteries have a low self-discharge rate of about 1-2% per month, allowing them to retain a substantial charge when not in use for extended periods. Unlike nickel-cadmium batteries, lithium-ion batteries do not exhibit a memory effect, eliminating the need for full discharges before recharging, which adds convenience for users.
Due to their high energy density and long life span, lithium-ion batteries are extensively applied in devices like smartphones, laptops, and tablets, contributing to the convenience of modern life and work. In recent years, with the rapid development of electric vehicles (EVs), lithium-ion batteries have also become the primary energy source for EVs. Companies such as Tesla and BYD have made notable progress in this area, driving technological advancements in lithium-ion battery technology. Additionally, lithium-ion battery energy storage systems play a crucial role in balancing grid loads and improving energy utilization efficiency. Their application is expanding from home energy storage to large-scale grid energy storage. Moreover, because of their high energy density and low maintenance requirements, lithium-ion batteries are also widely used in industrial equipment, such as drones and electric tools. However, despite these advantages, lithium-ion batteries face several challenges. Most commercially available lithium-ion batteries use flammable organic liquids as electrolytes, which can lead to thermal runaway in extreme conditions such as overcharging, short circuits, or high temperatures, posing significant safety risks. As the electric vehicle industry continues to expand rapidly, full electrification of automobiles has become inevitable. Nonetheless, reports of EV fires are not uncommon, primarily due to the use of flammable liquid electrolytes. Furthermore, liquid electrolytes are prone to volatility and may dry out or leak during extended use, which affects the cycling performance and reduces the battery's service life. These issues contradict current market demands, which emphasize improved safety and battery longevity, posing a significant obstacle to the expansion of lithium-ion batteries in the power sector. LiCoO₂, with a relatively high specific capacity (greater than 145 mAhg⁻¹), good cycling stability, and ease of synthesis, remains an irreplaceable cathode material in lithium-ion batteries, particularly for electronic devices like mobile phones and computers. However, LiCoO₂ has a typical α-NaFeO₂-type layered two-dimensional structure, which offers poor resistance to overcharging. Excessive lithium-ion extraction from the structure can cause its collapse, leading to reduced cycling stability.[13] To control the amount of lithium-ion extraction, the voltage of LiCoO₂ is typically limited to between 3.6 and 4.2 V. Nevertheless, this traditional LiCoO₂ system faces difficulties in further increasing energy density, which is necessary for lightweight, portable devices. Efforts to raise the working voltage of LiCoO₂ to increase energy density have shown promising results. For instance, when the charging voltage is increased from 4.2 V to 4.45 V, the specific capacity exceeds 180 mAhg⁻¹. Qian et al.[14] demonstrated that doping LiCoO₂ with La and Al impurities could improve performance, yielding a reversible capacity of 190 mAhg⁻¹ at a high cut-off voltage of 4.5 V and a 96% capacity retention after 50 cycles, confirming good cycling stability.
Moreover, the production and recycling of lithium-ion batteries involve many chemical substances that, if handled improperly, could result in environmental pollution. The proposed battery cascade utilization strategy to tackle electric vehicle battery recycling has encountered challenges such as high costs and incompatibility, making it difficult to sustain. Thus, efficiently recycling and reusing waste lithium-ion batteries remains a significant challenge for researchers. Although lithium-ion batteries boast high energy density, they face a theoretical limit. In November 2017, the world’s first 2000-ton-class new energy pure electric ship began operating in the Pearl River Delta. Its popularity was driven by its zero emissions, low noise, and simple structure. Looking ahead, breakthroughs in technologies like electric ships and electric aircraft will place higher demands on power battery energy density (see Figure 3). Significantly increasing the energy density of lithium-ion batteries, while maintaining the stability of other key parameters, will be a crucial research topic in the coming years.
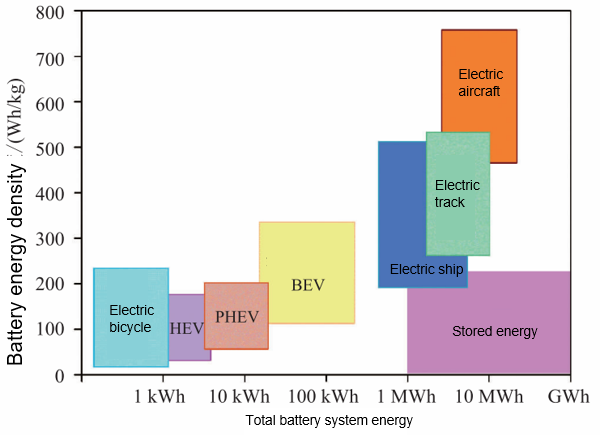
Figure 3: Demands for Energy Density and System Scale of Lithium-Ion Batteries in Various Applications
3. Sodium-ion Battery
3.1. Operating principle
The energy density and system scale requirements of lithium-ion batteries vary significantly across different application scenarios. Sodium-ion batteries have emerged as a promising alternative technology to lithium-ion batteries, garnering attention due to their abundant raw materials and lower costs. Although the current energy density of sodium-ion batteries is lower than that of lithium-ion batteries, they offer potential advantages in cost-sensitive fields with relatively lower energy density requirements, such as large-scale energy storage.
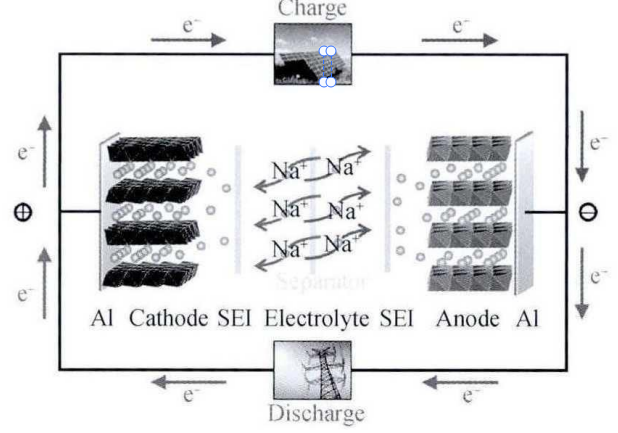
Presently, several companies have commenced small-scale production of sodium-ion batteries, conducting demonstration projects in applications like grid energy storage and electric bicycles. With ongoing technological advancements, it is anticipated that sodium-ion batteries will achieve widespread application within the next 5 to 10 years. The operational principle of sodium-ion batteries is similar to that of lithium-ion batteries, with both technologies relying on the intercalation and deintercalation of ions between the positive and negative electrodes during charging and discharging. As illustrated in Figure 4, during the charging process, an external power supply applies voltage to the battery, driving the deintercalation of sodium ions from the negative electrode material. These sodium ions then migrate through the electrolyte to the positive electrode material for intercalation. Concurrently, the electrons released from the negative electrode flow through the external circuit to the positive electrode, achieving charge equilibrium. During discharging, sodium ions deintercalated from the positive electrode material, migrate back through the electrolyte to the negative electrode, and intercalate into it. Simultaneously, electrons return to the negative electrode through the external circuit, generating a current for external device utilization. Taking the NaMnO₂/hard carbon sodium-ion battery as an example, its electrochemical reaction can be expressed by the following equation:
Positive electrode reaction:NaMnO2 ⇌ Na1-xMnO2 + xNa+ + xe- (4)
Anodic reaction :C + xNa+ + xe- ⇌ NaxC (5)
Total reaction: C + NaMnO2 ⇌ Na1-xMnO2 + NaxC (6)
|
|
Figure 4: The operating principle of sodium-ion batteries | Figure 5: The operating voltages, specific capacities and energy densities of common cathode materials. |
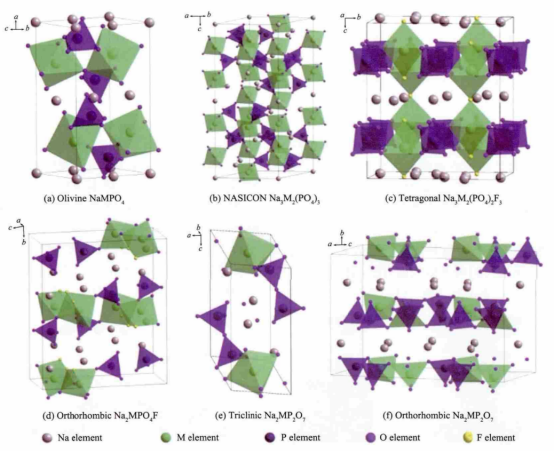
Figure 6: The crystal structures of various polyanionic cathode materials.
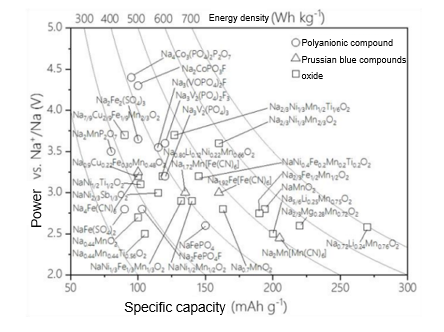
The selection criteria for cathode materials in sodium-ion batteries include a high redox potential, significant specific capacity, a crystal structure that facilitates the insertion and extraction of sodium ions, and good structural stability. Additionally, these materials should demonstrate favorable ionic and electronic conductivity and be environmentally friendly. Current research on cathode materials focuses on layered transition metal oxides (e.g., NaCoO₂, NaMnO₂), polyanionic compounds, and Prussian blue compounds. These materials are capable of providing adequate capacity while maintaining structural stability over multiple cycles. Figure 5 summarizes the fundamental electrochemical parameters of these three common types of cathode materials for sodium-ion batteries. Polyanionic cathode materials, such as olivine-structured NaFePO₄, NASICON-structured Na₃V₂(PO₄)₃, as well as various fluorophosphates and pyrophosphates, typically feature open three-dimensional frameworks. This structural characteristic leads to improved rate and cycling performance, as depicted in Figure 6. However, these materials generally exhibit poor conductivity, necessitating auxiliary measures like surface carbon coating, nanonization, and the design of porous structures to enhance their electrochemical performance. Unfortunately, these enhancements can result in a lower volumetric energy density of the battery. Prussian blue compounds have emerged as an attractive option for cathode materials due to their ease of synthesis and excellent electrochemical performance, making them a focal point of research in sodium-ion batteries. For instance, Cui et al.[15] reported a manganese-based cyanide compound, Na₂Mn[Mn(CN)₆], with a reversible capacity of 209 mA·h/g and an initial discharge insertion specific capacity of 80 mA·h/g. Based on these findings, the inferred true reversible capacity of this cathode is approximately 129 mA·h/g, and it also demonstrates impressive rate performance. Anode materials for sodium-ion batteries prioritize low voltage, high capacity, and cycling stability. Graphite, a common anode material in lithium-ion batteries, has a relatively small interlayer spacing of 0.335 nm, which poses challenges for sodium ion intercalation due to the larger size of sodium ions. Consequently, researchers do not consider graphite an ideal anode material for sodium-ion batteries. Instead, amorphous carbon materials with larger interlayer spacings have garnered significant attention. Among these, hard carbon—with its favorable low-voltage platform and relatively high reversible specific capacity—has become a key focus of research. Hard carbon materials can maintain structural integrity during repeated sodium intercalation and deintercalation processes, effectively avoiding issues like pulverization or shedding due to volume changes. This attribute ensures a long cycling life for the battery. Rong's team [16] used phenolic resin as a precursor and ethanol as a pore-forming agent, and by precisely controlling the microstructure, they achieved a reversible specific capacity of up to 410 mA·h/g for the resulting hard carbon anode.
The performance of sodium-ion batteries is primarily determined by the combined properties of the cathode and anode materials. The electrolyte plays a crucial role, as it must complement these materials while meeting various requirements, including high ionic conductivity, chemical and thermal stability, safety, and environmental friendliness. Typically, organic liquid electrolytes containing sodium salts (such as NaPF₆ and NaClO₄) are employed. Additionally, the electrolyte must possess a wide electrochemical stability window to accommodate the voltage ranges of the cathode and anode materials, thereby preventing electrolyte decomposition and side reactions. Common electrolyte solvents, such as a combination of ethylene carbonate (EC) and dimethyl carbonate (DMC), can provide an appropriate stability window for these applications.
3.2. Technical Characteristics
Sodium-ion batteries offer a series of advantages, with some indicators even surpassing those of lithium-ion batteries, demonstrating significant development potential. Sodium, as an abundant element in the Earth's crust, has far greater resource reserves than lithium and is widely distributed. This abundance leads to lower raw material costs for sodium-ion batteries and reduces reliance on resources from specific regions, effectively guarding against potential regional resource risks and safeguarding the energy strategic security of countries and regions.[17]
Research findings indicate that sodium-ion batteries essentially do not exhibit phenomena such as fires or explosions during safety performance testing and have a lower risk of thermal runaway under high temperatures and overcharging conditions, showcasing superior safety compared to lithium-ion batteries. With the transformation of the global energy structure and the modernization of power systems, large-scale energy storage technology is not only essential for ensuring energy security and promoting sustainable development but also serves as a crucial factor driving innovation and economic growth within the energy industry, occupying a vital position in the modern energy system. Additionally, sodium-ion batteries demonstrate relatively balanced performance across capacity, lifespan, and energy density, revealing substantial potential advantages in the field of large-scale energy storage. They also exhibit superior low-temperature performance, making them suitable for applications in cold regions. As an important complement to lithium-ion batteries, sodium-ion batteries may play an increasingly significant role in the future of energy storage and specific application scenarios. In contexts such as grid peak shaving and the integration of renewable energy, sodium-ion batteries are regarded as potential choices for large-scale energy storage systems. While lithium-ion batteries currently dominate the electric vehicle market, sodium-ion batteries also hold application prospects in electric two-wheelers, low-speed electric vehicles, and short-distance transportation tools. In consumer electronic products with less demanding energy density requirements, sodium-ion batteries may serve as an alternative solution. Furthermore, sodium-ion batteries have broad emerging markets. With technological advancements and further cost reductions, sodium-ion batteries could play a greater role in meeting the energy storage demands of developing countries, assisting these regions in achieving sustainable energy development.
Table 1: Comparison of Key Characteristics of Lithium and Sodium.
Element | Lithium | Sodium |
Relative atomic mass | 6.9 | 23 |
Ionic radius/nm | 0.076 | 0.106 |
Standard electrode potential/V(vs.SHE) | -3.04 | -2.74 |
Specific capacity/(mAh/g) | 3 829 | 1 165 |
The price of carbonates/($/kg) | 5 | 0.15 |
Geological reserves/% | 0.002 | 2.64 |
Sodium and lithium are part of the same main group in the periodic table and share similar physicochemical characteristics (see Table 1). However, sodium ions have a larger ionic radius compared to lithium ions, which notably hampers their mobility within sodium-storage materials. This reduced transport rate adversely impacts the overall electrochemical performance and efficiency of sodium-ion batteries. The increased ionic radius can also cause material fractures during the insertion and extraction of sodium ions in electrode materials, compromising the structural integrity and functionality of the battery. Additionally, sodium has a higher relative atomic mass, and its standard electrode potential is 0.33 V greater than that of lithium. As a result, when utilizing electrode materials of the same structural type in sodium-ion batteries, the potentials and theoretical specific capacities are lower, leading to a reduced energy density for sodium-ion batteries. In light of the rapid development of portable electronic devices and the growing demand for high energy density, sodium-ion batteries currently exhibit disadvantages and face challenges in competing with lithium-ion counterparts. Furthermore, research on sodium-ion batteries began relatively later and has progressed more slowly. At present, the material systems for sodium-ion batteries are less developed compared to those for lithium-ion batteries, and the mass production processes are not fully optimized, particularly concerning the selection of anode materials and electrolyte enhancement. Recent research efforts have focused on understanding sodium-storage mechanisms, refining both positive and negative electrode materials, and optimizing electrolytes. The goal is to develop more efficient synthesis methods and modifications for electrode materials, resulting in higher stability and more compatible electrolytes, thus improving the competitive edge of sodium-ion batteries in terms of energy density and cycle life. Currently, lithium-ion batteries dominate the market with widespread applications, while sodium-ion batteries face considerable challenges for market acceptance in the short term, requiring time for users to build confidence in these emerging technologies.
4. Flow Battery
4.1. Operating principle
Flow batteries represent a high-performance electrochemical energy storage technology that operates based on reversible redox reactions involving active substances in positive and negative electrolyte solutions. Originally proposed by Thaller in 1974, this technology features the separation of positive and negative electrolyte solutions, allowing each to circulate independently for chemical reactions. Key advantages of flow batteries include high capacity, broad applicability, long cycle life, safety, and environmental friendliness. [18-19]
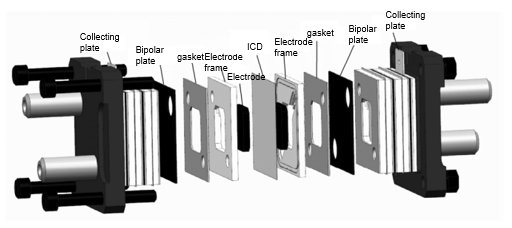
The essential components of a flow battery comprise the stack unit, electrolyte solutions, electrolyte storage and supply unit, and the management and control unit. The system consists of two electrolyte liquids, one for the positive electrode and the other for the negative electrode. These solutions are stored in separate tanks, with the stack formed by multiple single cells placed between them. Figure 8 illustrates the typical structure of a vanadium redox flow battery stack. The stack acts as the heart of the flow battery energy storage system, where the electrolyte is pumped through the battery for chemical reactions. The performance of this stack is critical in determining the overall efficiency of the energy storage system. Additionally, an ion-conductive membrane is typically present within the stack to separate the two electrolyte solutions. This membrane must exhibit high conductivity, a fast ion exchange rate, strong corrosion resistance, and minimal water migration.
|
|
Figure 8: The typical structure of the all-vanadium redox flow battery stack. | Figure 9: Schematic diagram of all-vanadium redox flow battery energy storage system. |
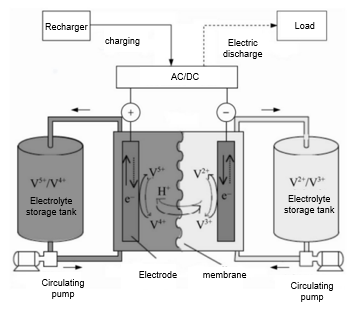
These two electrolyte liquids generally consist of distinct chemical substances that possess different redox properties. For instance, the vanadium redox flow battery (VRFB) is one of the most developed and commonly used liquid flow battery technologies (see Figure 9). In a VRFB, vanadium ion solutions serve as the active substances for both the positive and negative electrodes. The positive electrolyte solution contains V(V) and V(IV) ions, while the negative electrolyte solution consists of V(III) and V(II) ions. During the charging phase, an external power source supplies current to the battery, resulting in the oxidation of VO₂⁺ ions in the positive electrolyte, producing VO₂⁺ ions. The V(IV) ions in the charged positive electrolyte release an electron, which is transferred to the V(III) ions in the negative electrolyte, facilitating a reduction reaction that converts them into V(II) ions. The specific electrode reaction equations are as follows:
Positive electrode reaction:VO2+ + 2H+ + e-⇌ VO2+ + H2O (7)
Anodic reaction:V3+ + e- ⇌ V2+ (8)
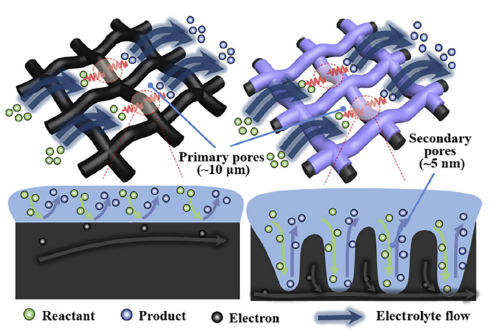
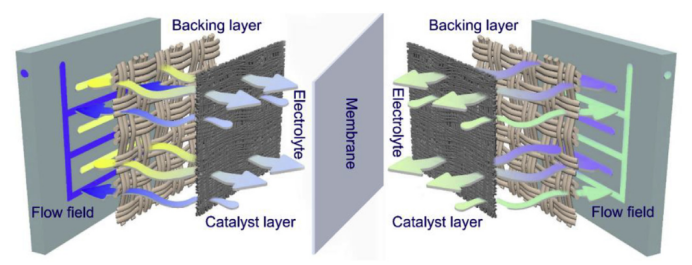
During this process, the vanadium battery stores electrical energy as chemical energy in the sulfuric acid electrolyte containing vanadium ions in various valence states. An external pump generates mechanical force to circulate the electrolyte through the stack, flowing within the closed circuits of different storage tanks and half-cells. When discharging, the electrolyte solution flows parallel to the electrode surface and undergoes the reverse electrochemical reaction. The current is collected and transmitted via the bipolar plate, converting the stored chemical energy into electrical energy for the external circuit. The stack uses a proton exchange membrane to allow H⁺ ions to pass through the electrolyte, completing the electrical circuit. Currently, the most widely used membrane is the perfluoro sulfonic membrane, such as the Nafion membrane, which achieves voltage efficiency exceeding 90%. Typically, vanadium redox flow batteries utilize carbon-based electrodes, including materials like carbon felt, graphite felt, and carbon cloth. [20-21] These materials offer good conductivity, corrosion resistance, and chemical stability. However, they exhibit low electrochemical activity and hydrophilicity, necessitating modifications for better electrolyte transport and electrochemical reactions. For instance, Zhou et al. [22] treated carbon paper with KOH to create secondary micropores with a high specific surface area (5 nm) on the carbon fibers, which have primary macropores (10 μm), as shown in Figure 10. The primary macropores act as transport channels for the electrolyte, while the etched micropores serve as active sites for rapid electrochemical reactions. Measurements indicated that this modified electrode's specific surface area increased 16 times compared to the original, achieving battery energy efficiency over 80% at a current density of 400 mA cm⁻². In another approach, Wu et al. [23] developed a bilayer thin-film gradient electrode comprising a backing layer and a catalyst layer. They used large-pore carbon paper, carbon cloth, and graphite felt as the backing layer, while the catalyst layer consisted of small-pore electro spun fiber felt. Experimental results showed that the bilayer electrode made from carbon cloth and electro spun fiber felt delivered optimal performance, achieving an energy efficiency of 80.2% at a current density of 240 mA cm⁻², with no significant performance degradation after 800 cycles.
|
|
Figure 10: Schematic diagram of the dual-scale prous electrode structure. | Figure 11: Schematic diagram of the liquid flow cell with dual-electrode structure. |
Technical characteristics
The flow battery's unique characteristic lies in the independent configurability of its capacity and power. The energy storage capacity is determined by the volume of the electrolyte, while the output power is influenced by the number of stack units, allowing for flexible energy and power design. For example, increasing either the electrolyte volume or the number of stack units can enhance both energy storage capacity and output power. Unlike conventional batteries, which typically rely on solid electrode surfaces for electrochemical reactions and suffer from electrode degradation, flow batteries conduct these reactions within the flowing electrolyte. This design minimizes electrode consumption and significantly extends the battery's cycle life.
In China, established flow battery technologies include vanadium redox flow batteries, iron-chromium flow batteries, and zinc-bromine flow batteries. Notably, these batteries can achieve a 100% depth of charge and discharge without damage, a feat not possible with mainstream lithium-ion batteries, which helps mitigate safety risks like combustion or explosion from overcharging or over-discharging. Flow battery electrolytes are usually aqueous solutions, which are less flammable and corrosive, and the separation of reaction sites and active substance storage further reduces thermal runaway risks, enhancing safety. The flexible design and high safety of flow batteries make them ideal for long-duration and large-capacity energy storage applications. For example, integrating vanadium redox flow batteries with renewable energy systems can ensure stable power supply and facilitate grid services such as peak shaving, frequency modulation, and load curve smoothing. They also show promise for applications in emergency backup power, EV charging stations, communication base stations, and remote area power supply. The Austrian company Cellstrom has developed a 1 kW stack for 10 kW/100 kWh vanadium redox flow battery systems, meeting practical application power requirements from several kilowatts to tens of megawatts, with energy storage needs ranging from several megawatt-hours to hundreds of megawatt-hours. Dalian Rongke Company operates a vanadium redox flow battery energy storage and peak shaving power station with 100 MW/400 MWh, positioning itself at the forefront of the domestic and international flow battery market. Despite their advantages, liquid flow batteries are not ideal for small-scale energy storage due to high initial investment costs, particularly for stacks and electrolytes, posing challenges for market adoption. Managing the electrolyte in liquid flow batteries involves complex aspects like flow control, temperature regulation, and sealing, which complicate system design and maintenance. These batteries are sensitive to temperature, requiring effective thermal management. For instance, vanadium redox flow batteries operate optimally at temperatures between 35 to 40 degrees Celsius. Lower temperatures can lead to crystallization of vanadium ions, reducing capacity, while higher temperatures risk hydrolysis of V(V) ions, compromising electrolyte stability. Compared to traditional lithium-ion batteries, liquid flow batteries generally have lower energy densities. Vanadium redox flow batteries offer energy densities of 25–35 Wh/L, while iron-chromium flow batteries range from 15–25 Wh/L. The zinc-bromine flow battery achieves higher energy densities of 70–80 Wh/L, but the volatility of bromine presents challenges, including self-discharge due to reactions with zinc and environmental pollution from atmospheric diffusion. The corrosive nature of bromine increases the demands on material selection and system sealing. Although liquid flow batteries typically offer longer cycle lives, issues related to stack component efficiency and lifespan—especially concerning electrode material stability and membrane durability—continue to require ongoing optimization.
5. Solid-state Battery
5.1. Operating principle
Solid-state batteries represent an emerging energy storage technology that replaces the liquid electrolytes commonly used in traditional batteries with solid electrolytes. This innovation fundamentally addresses the flammability and leakage issues associated with liquid electrolytes, significantly enhancing battery safety, while achieving higher energy density and longer cycle life. Solid-state batteries have considerable developmental potential and are expected to disrupt existing lithium-ion battery technologies, marking an inevitable trend in the future evolution of battery technologies.
Based on differences in electrolytes, solid-state batteries can be classified into three types: semi-solid, quasi-solid, and all-solid. In semi-solid batteries, the mass percentage of liquid electrolyte is less than 10%; in quasi-solid batteries, it is less than 5%; while all-solid batteries contain no liquid electrolyte. The operating principle of solid-state batteries is similar to that of traditional liquid batteries, storing and releasing electrical energy through electrochemical reactions. For instance, in solid-state lithium batteries, during charging, lithium ions detach from the positive electrode material and migrate through the solid electrolyte to the negative electrode, where they undergo a chemical reaction with the negative electrode material. Concurrently, electrons flow from the positive electrode to the negative electrode through the external circuit, completing the charging process. During discharging, lithium ions deintercalate from the negative electrode, return to the positive electrode through the solid electrolyte, while electrons flow from the negative electrode to the positive electrode through the external circuit, releasing electrical energy. Currently, solid electrolytes attracting significant research attention can be broadly categorized into three groups: inorganic solid electrolytes, polymer solid electrolytes, and composite solid electrolytes. Inorganic solid electrolytes come in various forms, generally classified into oxide electrolytes and sulfide electrolytes. Oxide electrolytes exhibit good thermal stability, air stability, a wide electrochemical window, and high mechanical strength. However, they tend to have lower electrical conductivity, high hardness, and poor toughness, leading to suboptimal interface contact and excessive interfacial impedance. In contrast, sulfide electrolytes have a weaker binding effect on lithium, facilitating lithium ion migration, resulting in higher electrical conductivity compared to equivalent oxides, approaching or even exceeding that of liquid electrolytes. Additionally, sulfides possess a soft texture that makes them easier to process, and interface contact can be enhanced through compression, thereby improving battery performance. Nevertheless, sulfide solid electrolytes exhibit poor electrochemical stability, a narrow electrochemical window, and high sensitivity to moisture, reacting with trace amounts of water in the air to generate toxic, flammable, and explosive hydrogen sulfide gas. Most existing lithium battery manufacturing relies on wet processes, making moisture contact difficult to avoid, which complicates the production process and limits their application in high-energy-density scenarios. Polymer solid electrolytes are formed through the complexation of a polymer matrix and lithium salts. Table 2 lists the physicochemical properties of various solid polymer matrices, with poly (ethylene oxide) (PEO) being the most widely used. Compared to inorganic solid electrolytes, polymer electrolytes are flexible, lightweight, and exhibit good interface compatibility, making them suitable for various flexible devices. However, their ionic conductivity at room temperature is relatively low, requiring heating to higher temperatures for normal charging and discharging. Furthermore, polymer electrolytes have a narrow electrochemical window, making them prone to electrolysis under large potential differences, which limits their overall performance ceiling. Composite solid electrolytes are created by incorporating inorganic particle fillers into polymer solid electrolytes. This combination effectively merges the advantages of both organic and inorganic solid electrolytes, resulting in high ionic conductivity, stability, and good interface contact performance, making them the most likely candidates for large-scale practical applications. [24,25]
Table 2: Common solid polymer matrices exhibit various physical and chemical properties.
Name | structural unit | Tg/℃ | Tm/℃ | Mechanical strength | Redox stability |
Polyoxyethylene (PEO) | \( -(C{H_{2}}C{H_{2}}O{)_{n}}- \) | -64 | 65 | Weak | Stability |
Polyvinylidene fluoride (PVDF) | \( -(C{H_{2}}C{F_{2}}{)_{n}}- \) | -40 | 171 | Strong | instability |
Polyvinylidene fluoride-hexafluoropropylene copolymer (PVDF-HFP) | \( -(C{H_{2}}C{F_{2}}{)_{x}}-(C{F_{2}}-CF(C{F_{3}}){)_{y}}- \) | -90 | 135 | Medium | instability |
Polyacrylonitrile (PAN) | \( -(C{H_{2}}CH(-CN){)_{n}}- \) | 125 | 317 | Strong | instability |
Polymethyl methacrylate (PMMA) | \( -(C{H_{2}}C(-C{H_{3}})-COOC{H_{3}}){)_{n}}- \) | 105 | — | Weak | Stability |
5.2. Technical characteristics
Solid-state batteries utilize solid electrolytes instead of liquid ones, greatly diminishing the risk of thermal runaway while offering enhanced safety and thermal stability. Although the heat resistance varies across different solid electrolyte components, all exhibit significantly higher maximum operating temperatures compared to liquid batteries. Many inorganic solid electrolyte materials are characterized as non-flammable, non-corrosive, non-volatile, and free from leakage problems. While semi-solid and quasi-solid batteries still pose some flammability risks, their reduced liquid content makes them considerably safer than traditional liquid lithium batteries. In liquid batteries, lithium dendrite growth can easily breach the separator, leading to short circuits. In contrast, solid electrolytes possess greater mechanical strength, resulting in slower and more challenging lithium dendrite growth that fundamentally prevents short-circuiting. This characteristic allows for the use of metallic lithium as the anode, thereby enhancing the battery's safety and extending its lifespan. The stability and safety of solid electrolytes contribute to an electrochemical window exceeding 5V, facilitating the expansion of voltage ranges and accommodating high-capacity positive and negative electrodes, including high-voltage positive electrodes and metallic lithium anodes. Consequently, solid-state batteries can achieve energy densities theoretically 2 to 3 times greater than those of liquid lithium batteries. Additionally, the inherent thermal stability of solid materials ensures reliable operation across a wide temperature range, making them suitable for various extreme environmental conditions. Solid-state batteries feature a compact structure, scalable size, and considerable design flexibility. The solid electrolyte integrates the functions of both electrolyte and separator, significantly shortening the distance between the positive and negative electrodes and reducing the overall battery thickness. This design can also streamline packaging and cooling systems, further minimizing battery weight within constrained spaces, making them ideal for applications where volume and weight are critical, such as in wearable and microelectronic devices. Regarded as the next-generation power source for electric vehicles, solid-state batteries have become central to the development strategies of key regions, including China, the United States, the European Union, Japan, and South Korea, establishing a competitive edge in advanced battery technology. Major automotive manufacturers, such as Toyota, BYD, Dongfeng, and CATL, are actively engaging in research and development of solid-state battery technology. A comparison of solid-state battery strategies between China and Japan reveals that Japan benefits from national research institutions and financial support, prioritizing technological development and leading globally in the number of all-solid-state battery patents. Notably, Japan employs a "one-step" approach by integrating all-solid-state batteries directly into vehicles. In contrast, China adopts a "small-step but fast" strategy, initially incorporating semi-solid batteries into vehicles and progressively shifting to all-solid-state batteries. This approach capitalizes on a comprehensive lithium battery industrial chain and the world's largest electric vehicle consumer market to achieve economies of scale. In the consumer electronics sector, the miniaturization and high energy density of solid-state batteries render them exceptionally suited for devices like smartphones, laptops, and wearables, significantly extending device battery life. Furthermore, solid-state batteries hold great promise for various aerospace applications, including unmanned aerial vehicles and satellites, where they can reduce weight and enhance battery longevity, providing a competitive advantage for advanced defense technologies. Additionally, the long cycle life and safety features of solid-state batteries position them as a strong candidate for future large-scale energy storage systems.
|
|
Figure 12: A schematic diagram of solid-state battery interface problems. | Figure 13: The formation of the interfacial solid electrolyte membrane. |
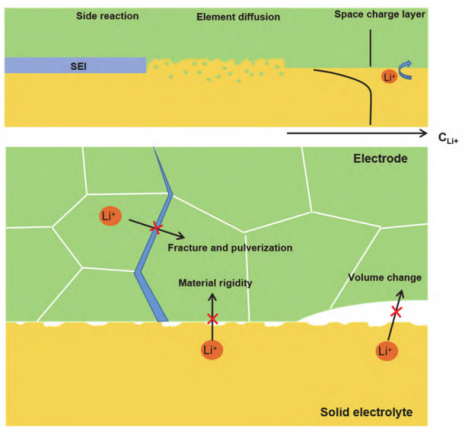
However, the solid electrolyte interface poses a significant challenge that hinders the rapid advancement and widespread application of solid-state batteries. Figure 12 illustrates various interface issues. In traditional lithium batteries, the active material particles are completely immersed in the electrolyte, allowing it to permeate the electrodes freely and establish a favorable ionic conduction network, which ensures effective contact between the electrodes and the electrolyte. Conversely, in solid-state batteries, the contact between the electrodes and the electrolyte is solid-solid. Despite the use of high-pressure pelletizing processes during preparation, the positive and negative electrode materials, along with the electrolyte, do not achieve an optimal close-packed configuration, resulting in voids that can range from 10% to 40%. These voids diminish the interface contact area, leading to increased interface resistance and hindering lithium-ion transport at the interface.
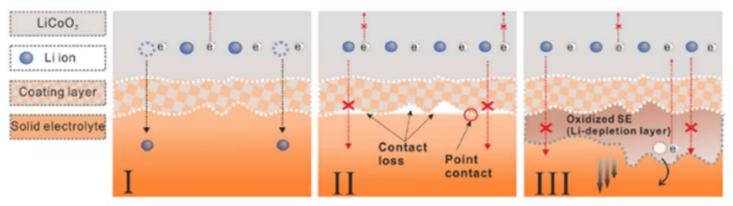
Currently, employing a combination of inorganic-organic solid electrolytes and surface coatings has proven effective in addressing the issue of voids. Zhou et al. [26] developed a solid electrolyte featuring a polymer/oxide/polymer sandwich structure, utilizing the flexibility of the polymer to mitigate the interface contact problems associated with the rigid oxide structure. Han et al. [27] applied atomic layer deposition (ALD) technology to create an ultrathin Al2O3 coating on the oxide solid electrolyte, which reduced interface resistance dramatically from 1710 Ω·cm² to 1 Ω·cm², significantly enhancing interface contact.
During the cyclic charge-discharge process, most battery electrode materials experience considerable volume changes. The rigid structure of solid electrolytes makes them more susceptible to these volume fluctuations and limits their ability to accommodate the electrodes' deformation, resulting in accumulated internal stress within the battery. If this stress is not effectively relieved, it can lead to cracking and pulverization of the electrode materials, as well as deterioration of the interface contact with the electrolyte, which ultimately reduces battery capacity and cycle life. Figure 13 depicts the potential difference that typically exists between most electrode materials and the electrolyte. When they make contact, a spontaneous redox reaction occurs, creating a solid electrolyte interphase (SEI) at the interface. In many cases, the SEI functions as a mixed electron/ion conductor, which can cause electron leakage and promote ongoing interface reactions, adversely affecting the battery's cycling performance. To effectively suppress these interface reactions, researchers often employ strategies such as coating the electrodes and modifying the electrolyte surface to create an artificial intermediate layer between the electrodes and the electrolyte. For instance, Xie et al. [29] tested LiNbO3-coated LiNi₀.₇Co₀.₁Mn₀.₂O₂ (NCM712) cathode materials. Their results indicated that the interface impedance of the original electrode increased significantly after two charging cycles, whereas the impedance remained largely unchanged after the addition of the LiNbO3 protective layer, confirming that the coating effectively mitigates interfacial side reactions between the Li₅.₅PS₄.₅Cl₁.₅ electrolyte and the NCM721 cathode. Similarly, Sun et al. [30] investigated the Li₁.₃Al₀.₃Ti₁.₇(PO₄)₃ (LATP) electrolyte in conjunction with a LiCoO₂ electrode, utilizing ALD technology to create an artificial Li₃PO₄ interface layer on the LATP surface. Their findings revealed that when the electrolyte was unmodified, significant diffusion of O from LATP into the LiCoO₂ electrode was evident in the O 1s spectrum. However, after applying the Li₃PO₄ coating, the VE-HAXPS spectrum prominently displayed the lattice oxygen characteristics of LiCoO₂, while the proportion of O from Li₃PO₄ and LATP was notably reduced. This indicates that modifying the LATP surface with Li₃PO₄ effectively prevents the mutual diffusion of elements between LATP and LiCoO₂. Additionally, the differing electrochemical potentials of the electrode materials and solid electrolytes lead to the redistribution of lithium ions at the interface, forming a space charge layer where lithium ions accumulate or are depleted. This creates an additional energy barrier for subsequent lithium-ion diffusion, significantly restricting the rate of lithium-ion transport at the interface.
6. Conclusion
With the transformation of the global energy structure and the rapid progress of renewable energy, battery energy storage technology is experiencing unprecedented opportunities for development. Its importance is particularly evident in sectors such as power system innovation, transportation electrification, and large-scale energy storage. Currently, lithium-ion battery technology is the most advanced and widely adopted, having long held a dominant position in the medium and small-scale energy storage markets. However, in the realm of large-scale energy storage, lithium-ion batteries face limitations in terms of performance, cost, and safety, and have yet to achieve a critical technological breakthrough.
At present, all-vanadium redox flow batteries are being demonstrated at the hundred-megawatt scale, with verified technical feasibility, positioning them as potential leaders in the domain of long-duration and large-capacity energy storage. In the future, battery energy storage technology is expected to continue advancing towards higher safety standards, longer lifespans, reduced costs, and improved environmental sustainability. Solid-state batteries are anticipated to emerge as the next generation of energy storage technology, and addressing interface challenges effectively could pave the way for large-scale commercial applications.
The energy storage market is likely to display a trend of diversification, with various battery technologies developing in harmony to provide optimal solutions for different applications. Moreover, establishing a management system compatible with battery technologies, coupled with the use of big data analytics and artificial intelligence algorithms to optimize battery usage strategies, could extend battery lifespans and enhance system efficiency. As battery usage continues to rise, creating a comprehensive battery recycling and reuse system will become an urgent challenge for researchers to address.
References
[1]. Wu H, Wang J, Gong Y, et al. Development status and application prospect analysis of energy storage technology[J]. J. Electr. Power, 2021, 36(5): 434-443.
[2]. Yun T, Fang Y U E, Kaimo G U O, et al. International development trend analysis of next-generation electrochemical energy storage technology[J]. Energy Storage Science and Technology, 2022, 11(1): 89.
[3]. Sun Y S, Yang M, Shi C L, et al. Analysis of application status and development trend of energy storage[J]. High Voltage Engineering, 2020, 46(01): 80-89.
[4]. Xie X, Ma N, Liu W, et al. Functions of energy storage in renewable energy dominated power systems: review and prospect[J]. Proceedings of the CSEE, 2023, 43(1): 158-169.
[5]. Yixue L ,Qing H ,Xingping S , et al.Energy storage in China: Development progress and business model[J].Journal of Energy Storage,2023,72(PA):
[6]. Sheng Z H U, Yiting P, Yulin M I N. Research progress on materials and technologies for electrochemical energy storage[J]. Chemical Industry and Engineering Progress, 2021, 40(9): 4837-4852.
[7]. Detka K, Górecki K. Selected Technologies of Electrochemical Energy Storage—A Review[J]. Energies, 2023, 16(13): 5034.
[8]. Han X, ZHANG C H K, Wu H, et al. Working mechanism and key materials of the lithium ion batteries[J]. Metallic Functional Materials, 2021, 28(2): 37-58.
[9]. Hu Z, Sheng J, Chen J, et al. Enhanced Li ion conductivity in Ge-doped Li 0.33 La 0.56 TiO 3 perovskite solid electrolytes for all-solid-state Li-ion batteries[J]. New Journal of Chemistry, 2018, 42(11): 9074-9079.
[10]. Abbas Q, Mirzaeian M, Hunt M R C, et al. Current state and future prospects for electrochemical energy storage and conversion systems[J]. Energies, 2020, 13(21): 5847.
[11]. Guerfi A, Duchesne S, Kobayashi Y, et al. LiFePO4 and graphite electrodes with ionic liquids based on bis (fluorosulfonyl) imide (FSI)− for Li-ion batteries[J]. Journal of Power Sources, 2008, 175(2): 866-873.
[12]. Liu C, Neale Z G, Cao G. Understanding electrochemical potentials of cathode materials in rechargeable batteries[J]. Materials Today, 2016, 19(2): 109-123.
[13]. Qian J, Liu L, Yang J, et al. Electrochemical surface passivation of LiCoO2 particles at ultrahigh voltage and its applications in lithium-based batteries[J]. Nature communications, 2018, 9(1): 4918.
[14]. Hyun-Wook L ,Y R W ,Mauro P , et al.Manganese hexacyanomanganate open framework as a high-capacity positive electrode material for sodium-ion batteries.[J].Nature communications,2014,5(1):5280.
[15]. Xiaohui R, Yaxiang L U, Xingguo Q I, et al. Na-ion batteries: From fundamental research to engineering exploration[J]. Energy Storage Science and Technology, 2020, 9(2): 515.
[16]. Hu Y S, Lu Y. 2019 Nobel prize for the Li-ion batteries and new opportunities and challenges in Na-ion batteries[J]. ACS Energy Letters, 2019, 4(11): 2689-2690.
[17]. Lv Y, Han C, Zhu Y, et al. Recent advances in metals and metal oxides as catalysts for vanadium redox flow battery: Properties, structures, and perspectives[J]. Journal of Materials Science & Technology, 2021, 75: 96-109.
[18]. Sánchez-Díez E, Ventosa E, Guarnieri M, et al. Redox flow batteries: Status and perspective towards sustainable stationary energy storage[J]. Journal of Power Sources, 2021, 481: 228804.
[19]. Wang Z, Ren J, Sun J, et al. Characterizations and selections of electrodes with optimal performance for large-scale vanadium redox flow batteries through lab-scale experiments[J]. Journal of Power Sources, 2022, 549: 232094.
[20]. Xu Z, Jing M, Liu J, et al. Advanced dual-gradient carbon nanofibers/graphite felt composite electrode for the next-generation vanadium flow battery[J]. Journal of Materials Science & Technology, 2023, 136: 32-42.
[21]. Zhou X L, Zeng Y K, Zhu X B, et al. A high-performance dual-scale porous electrode for vanadium redox flow batteries[J]. Journal of Power Sources, 2016, 325: 329-336.
[22]. Wu Q, Lv Y, Lin L, et al. An improved thin-film electrode for vanadium redox flow batteries enabled by a dual layered structure[J]. Journal of Power Sources, 2019, 410: 152-161.
[23]. Aluko A, Knight A. A review on vanadium redox flow battery storage systems for large-scale power systems application[J]. IEEE Access, 2023, 11: 13773-13793.
[24]. Hu L, Ren Y, Wang C, et al. Fusion Bonding Technique for Solvent‐free Fabrication of All‐solid‐state Battery with Ultra‐thin Sulfide Electrolyte[J]. Advanced Materials, 2024: 2401909.
[25]. Wang Y T, Ju J W, Dong S M, et al. Facile design of sulfide-based all solid-state lithium metal battery: In situ polymerization within self-supported porous argyrodite skeleton [J]. Advanced Functional Materials, 2021, 31(28): 2101523.
[26]. Weidong Z ,Shaofei W ,Yutao L , et al.Plating a Dendrite-Free Lithium Anode with a Polymer/Ceramic/Polymer Sandwich Electrolyte.[J].Journal of the American Chemical Society,2016,138(30):9385-8.
[27]. Xiaogang H ,Yunhui G ,Kelvin K F , et al.Negating interfacial impedance in garnet-based solid-state Li metal batteries.[J].Nature materials,2017,16(5):572-579.
[28]. Liu S, Liu W, Ba D, et al. Filler‐integrated composite polymer electrolyte for solid‐state lithium batteries[J]. Advanced Materials, 2023, 35(2): 2110423.
[29]. Peng L, Ren H, Zhang J, et al. LiNbO3-coated LiNi0. 7Co0. 1Mn0. 2O2 and chlorine-rich argyrodite enabling high-performance solid-state batteries under different temperatures[J]. Energy Storage Materials, 2021, 43: 53-61.
[30]. Liu Y, Sun Q, Liu J, et al. Variable-energy hard X-ray photoemission spectroscopy: a nondestructive tool to analyze the cathode–solid-state electrolyte interface[J]. ACS applied materials & interfaces, 2019, 12(2): 2293-2298.
Cite this article
Lei,Y. (2025). Research Progress and Prospect of Main Battery Energy Storage Technology. Applied and Computational Engineering,123,72-87.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 5th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Wu H, Wang J, Gong Y, et al. Development status and application prospect analysis of energy storage technology[J]. J. Electr. Power, 2021, 36(5): 434-443.
[2]. Yun T, Fang Y U E, Kaimo G U O, et al. International development trend analysis of next-generation electrochemical energy storage technology[J]. Energy Storage Science and Technology, 2022, 11(1): 89.
[3]. Sun Y S, Yang M, Shi C L, et al. Analysis of application status and development trend of energy storage[J]. High Voltage Engineering, 2020, 46(01): 80-89.
[4]. Xie X, Ma N, Liu W, et al. Functions of energy storage in renewable energy dominated power systems: review and prospect[J]. Proceedings of the CSEE, 2023, 43(1): 158-169.
[5]. Yixue L ,Qing H ,Xingping S , et al.Energy storage in China: Development progress and business model[J].Journal of Energy Storage,2023,72(PA):
[6]. Sheng Z H U, Yiting P, Yulin M I N. Research progress on materials and technologies for electrochemical energy storage[J]. Chemical Industry and Engineering Progress, 2021, 40(9): 4837-4852.
[7]. Detka K, Górecki K. Selected Technologies of Electrochemical Energy Storage—A Review[J]. Energies, 2023, 16(13): 5034.
[8]. Han X, ZHANG C H K, Wu H, et al. Working mechanism and key materials of the lithium ion batteries[J]. Metallic Functional Materials, 2021, 28(2): 37-58.
[9]. Hu Z, Sheng J, Chen J, et al. Enhanced Li ion conductivity in Ge-doped Li 0.33 La 0.56 TiO 3 perovskite solid electrolytes for all-solid-state Li-ion batteries[J]. New Journal of Chemistry, 2018, 42(11): 9074-9079.
[10]. Abbas Q, Mirzaeian M, Hunt M R C, et al. Current state and future prospects for electrochemical energy storage and conversion systems[J]. Energies, 2020, 13(21): 5847.
[11]. Guerfi A, Duchesne S, Kobayashi Y, et al. LiFePO4 and graphite electrodes with ionic liquids based on bis (fluorosulfonyl) imide (FSI)− for Li-ion batteries[J]. Journal of Power Sources, 2008, 175(2): 866-873.
[12]. Liu C, Neale Z G, Cao G. Understanding electrochemical potentials of cathode materials in rechargeable batteries[J]. Materials Today, 2016, 19(2): 109-123.
[13]. Qian J, Liu L, Yang J, et al. Electrochemical surface passivation of LiCoO2 particles at ultrahigh voltage and its applications in lithium-based batteries[J]. Nature communications, 2018, 9(1): 4918.
[14]. Hyun-Wook L ,Y R W ,Mauro P , et al.Manganese hexacyanomanganate open framework as a high-capacity positive electrode material for sodium-ion batteries.[J].Nature communications,2014,5(1):5280.
[15]. Xiaohui R, Yaxiang L U, Xingguo Q I, et al. Na-ion batteries: From fundamental research to engineering exploration[J]. Energy Storage Science and Technology, 2020, 9(2): 515.
[16]. Hu Y S, Lu Y. 2019 Nobel prize for the Li-ion batteries and new opportunities and challenges in Na-ion batteries[J]. ACS Energy Letters, 2019, 4(11): 2689-2690.
[17]. Lv Y, Han C, Zhu Y, et al. Recent advances in metals and metal oxides as catalysts for vanadium redox flow battery: Properties, structures, and perspectives[J]. Journal of Materials Science & Technology, 2021, 75: 96-109.
[18]. Sánchez-Díez E, Ventosa E, Guarnieri M, et al. Redox flow batteries: Status and perspective towards sustainable stationary energy storage[J]. Journal of Power Sources, 2021, 481: 228804.
[19]. Wang Z, Ren J, Sun J, et al. Characterizations and selections of electrodes with optimal performance for large-scale vanadium redox flow batteries through lab-scale experiments[J]. Journal of Power Sources, 2022, 549: 232094.
[20]. Xu Z, Jing M, Liu J, et al. Advanced dual-gradient carbon nanofibers/graphite felt composite electrode for the next-generation vanadium flow battery[J]. Journal of Materials Science & Technology, 2023, 136: 32-42.
[21]. Zhou X L, Zeng Y K, Zhu X B, et al. A high-performance dual-scale porous electrode for vanadium redox flow batteries[J]. Journal of Power Sources, 2016, 325: 329-336.
[22]. Wu Q, Lv Y, Lin L, et al. An improved thin-film electrode for vanadium redox flow batteries enabled by a dual layered structure[J]. Journal of Power Sources, 2019, 410: 152-161.
[23]. Aluko A, Knight A. A review on vanadium redox flow battery storage systems for large-scale power systems application[J]. IEEE Access, 2023, 11: 13773-13793.
[24]. Hu L, Ren Y, Wang C, et al. Fusion Bonding Technique for Solvent‐free Fabrication of All‐solid‐state Battery with Ultra‐thin Sulfide Electrolyte[J]. Advanced Materials, 2024: 2401909.
[25]. Wang Y T, Ju J W, Dong S M, et al. Facile design of sulfide-based all solid-state lithium metal battery: In situ polymerization within self-supported porous argyrodite skeleton [J]. Advanced Functional Materials, 2021, 31(28): 2101523.
[26]. Weidong Z ,Shaofei W ,Yutao L , et al.Plating a Dendrite-Free Lithium Anode with a Polymer/Ceramic/Polymer Sandwich Electrolyte.[J].Journal of the American Chemical Society,2016,138(30):9385-8.
[27]. Xiaogang H ,Yunhui G ,Kelvin K F , et al.Negating interfacial impedance in garnet-based solid-state Li metal batteries.[J].Nature materials,2017,16(5):572-579.
[28]. Liu S, Liu W, Ba D, et al. Filler‐integrated composite polymer electrolyte for solid‐state lithium batteries[J]. Advanced Materials, 2023, 35(2): 2110423.
[29]. Peng L, Ren H, Zhang J, et al. LiNbO3-coated LiNi0. 7Co0. 1Mn0. 2O2 and chlorine-rich argyrodite enabling high-performance solid-state batteries under different temperatures[J]. Energy Storage Materials, 2021, 43: 53-61.
[30]. Liu Y, Sun Q, Liu J, et al. Variable-energy hard X-ray photoemission spectroscopy: a nondestructive tool to analyze the cathode–solid-state electrolyte interface[J]. ACS applied materials & interfaces, 2019, 12(2): 2293-2298.