1. Introduction
High-entropy alloys (HEAs) are a novel class of alloy materials composed of multiple (typically five or more) metallic elements in equal or near-equal molar ratios. Their unique structure and multi-element composition endow them with exceptional mechanical strength, thermal stability, and corrosion resistance [1, 2]. In recent years, with the expanding application of HEAs in fields such as catalysis, energy storage, and aerospace, corresponding preparation technologies have also continued to develop. Although HEAs exhibit significant advantages in mechanical performance, thermal stability, and corrosion resistance [3], their potential in practical applications remains constrained by certain challenges in the preparation process, such as the risk of phase separation, high equipment costs, and insufficient stability under specific conditions [4, 5]. To overcome these obstacles and achieve broader and more efficient applications, researchers have been continuously exploring the optimization and modification of preparation methods. By developing and applying versatile techniques, such as carbothermal shock, electrochemical synthesis, and spark plasma sintering, scientists aim to enhance the performance and controllability of HEAs, thereby promoting their practical application across various fields [6, 7].
This paper outlines the primary preparation techniques for HEAs and their optimization, while discussing future directions for improvement and potential application prospects. Besides, corresponding characterizations used for HEAs are summarized from the aspect of principle and related examples. We hope this paper would give some guideline for exploring high-performance HEAs.
2. Preparation Method of High-entropy Alloy
The preparation methods for HEAs differ depending on application requirements and alloy composition. At present, common techniques for the preparation of HEAs include carbothermal shock, electrochemical methods, and spark plasma sintering, among others.
2.1. Carbothermal Shock
Carbothermal shock is a technique used for the rapid synthesis of high-entropy alloy (HEA) materials. Its core principle involves subjecting different elements to a brief period of high-temperature heating followed by rapid cooling [8]. This process transitions the atomic arrangement from disorder to an ordered alloy structure, thereby achieving alloy formation and the synthesis of HEAs. Figure 1 illustrates the synthesis scheme for L-CFP/FeCoNiCuZn-X based on the carbothermal shock method. This synthesis technique was first proposed by Professor John B. Goodenough’s team at the University of Texas at Austin. They described a novel approach for the rapid synthesis of HEA nanoparticles using carbothermal shock, which achieves high-temperature conditions within a few milliseconds, enabling effective control over the alloy’s composition and structure [9]. Building upon this, the team led by Ju Li and researchers from Honda Research Institute USA optimized the carbothermal shock synthesis method. They achieved the in-situ synthesis of high-entropy oxide (HEO) catalysts through ultrafast Joule heating and instantaneous quenching, which refined the alloy composition and enhanced the mechanical stability and electrochemical activity of the catalysts. Measurements conducted via cyclic voltammetry (CV) revealed that the electrochemically active specific area (ECSA) of the optimized FeNiCoCrV high-entropy oxide catalyst was approximately an order of magnitude greater than that of the FeNiCo catalyst, increasing from 13.16 mF cm−2 to 111.8 mF cm−2. This significantly improved the catalytic activity and stability of the reaction [10].
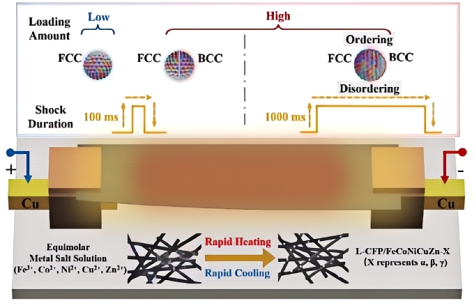
Figure 1: The preparation scheme of L-CFP/FeCoNiCuZn-X based on Carbothermal shock [11]
Recently, the Chuang Xue team at Dalian University of Technology has made significant advancements in the carbothermal shock synthesis method. By precisely controlling the elemental composition and crystal structure of HEAs, the L-CFP/FeCoNiCuZn-γ sample demonstrated an effective absorption bandwidth exceeding 2.16 GHz in the low-frequency range (2–4 GHz) with a thickness of 5.58 mm. This significantly enhances the catalyst's performance in low-frequency electromagnetic wave absorption while imparting additional thermal conductivity, hydrophobicity, and electrocatalytic properties to the material [11]. In summary, this method achieves high-temperature catalyst synthesis within an extremely short time (a few milliseconds). Through adjustments to temperature, duration, and the type of carbon source, the composition and structure of HEAs can be flexibly tuned. The resulting nanoparticles exhibit high specific surface area and activity and are more environmentally friendly compared to traditional methods. However, carbothermal shock synthesis may carry the risk of elemental phase separation, particularly in the synthesis of HEAs. The fast-moving bed pyrolysis (FMBP) strategy addresses this issue by ensuring that mixed metal precursors decompose simultaneously at high temperatures. This promotes the formation of uniform high-entropy alloy nanoparticles rather than phase-separated alloys.
2.2. Electrochemical reduction
Electrochemical methods provide an efficient, environmentally friendly, and relatively low-cost approach for the synthesis of HEAs. As shown in Figure 2, the core principle of the electrochemical method for HEA preparation is electrodeposition. This process involves the simultaneous reduction of multiple metal ions on a conductive substrate through electrochemical reactions, forming HEA films or nanostructures [12, 13]. In 2017, the Jagadeesh Sure team first proposed the synthesis of HEAs using electrochemical methods. They utilized molten CaCl2 as the electrolyte at 1173 K and applied cathodic polarization to pre-mixed solid metal oxides, achieving the one-step alloying synthesis of equiatomic HEAs such as TiNbTaZr and TiNbTaZrHf [14].
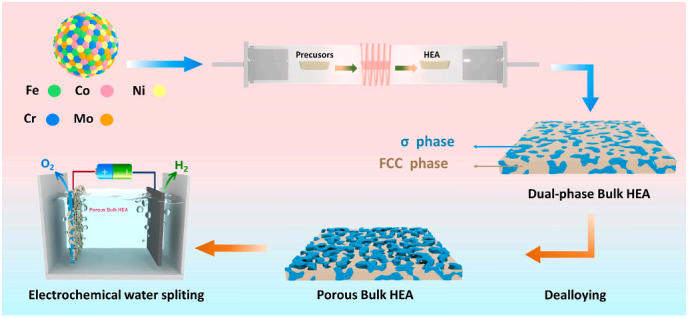
Figure 2: Process outline for the electrochemical dealloying of FeCoNiCrMo-HEA[15]
Subsequently, the Fateme Yoosefan team conducted further research and discovered that the composition, film thickness, crystal size, and structure of HEAs synthesized under different pulse frequencies and duty cycles exhibit varied combinations. For instance, in the preparation of CoCrFeMnNi HEA films using the pulse electrodeposition method, increasing the duty cycle from 50% to 60% resulted in an increase in Cr content from 7.23% to 9.94%. Thus, by adjusting the parameters of pulse electrodeposition, the composition, structure, and properties of HEA films can be effectively controlled [16]. Recently, the Zhongsheng Hua team investigated the effects of different concentrations of nitric acid (HNO3) solutions (ranging from 0.1 M to 1 M), different potentials (0 to 3 V vs. RHE), and different durations (0 to 20 minutes) on the microstructure of HEA surfaces through electrochemical dealloying treatment. This process effectively optimizes the surface microstructure and electrocatalytic performance of HEAs [15].
In summary, electrochemical methods for HEA synthesis offer advantages such as rapid processing, low cost, compositional uniformity, controllable microstructures, and environmental friendliness. These attributes make them suitable for producing materials with excellent catalytic and mechanical properties, particularly in the fields of energy conversion and storage. However, despite these advantages, limitations such as challenges in compositional control and instability in structure and phase composition restrict their broader application in certain areas. Future developments may require the integration of other preparation methods or the improvement of process conditions to overcome these challenges.
2.3. Spark plasma sintering
Spark plasma sintering (SPS) is a rapid sintering technique widely utilized for fabricating bulk HEAs. SPS combines the advantages of powder metallurgy and rapid sintering, achieving the rapid densification of metal powders through the application of pulsed electric currents and pressure, thereby enabling the preparation of HEAs [17]. The Z.Y. Fu team was the first to employ spark plasma sintering to produce HEA samples with a relative density of 98% and a Vickers hardness of 432 HV. They synthesized high-entropy solid solution alloys using the mechanical alloying (MA) method and subsequently sintered them at 800°C for 10 minutes using SPS [18].
In 2020, the Junfeng Chen team from Fuzhou University in China fabricated equiatomic TiZrNbMoTa HEAs. They prepared a face-centered cubic (FCC) solid solution phase powder using mechanical alloying and subsequently sintered it at various temperatures using spark plasma sintering. It was found that the HEA sintered at 1400°C achieved the highest compressive fracture strength (3759 MPa) and fracture strain (12.1%), with the alloy hardness reaching a maximum of 1181 HV. By adjusting the sintering temperature in SPS, the microstructure and mechanical properties of HEAs can be significantly influenced, thereby optimizing their performance for high-temperature applications[19]. Recently, the Pradip Kumar Verma team discovered that in the process of synthesizing HEAs using a combination of MA and SPS, optimizing the ball milling time effectively enhances the coercivity of the alloys while maintaining an appropriate magnetization intensity. With an increase in ball milling time, the saturation magnetization of CoMoMnNiV HEAs decreased from 42.648 emu/g to 25.977 emu/g, while the coercivity increased from 135.25 Oe to 371.5 Oe [20].
In summary, spark plasma sintering is a rapid and efficient technique for producing dense, high-performance materials in the synthesis of HEAs. By controlling the sintering temperature, pressure, and duration, the microstructure, phase composition, and magnetic properties of the materials can be significantly optimized. However, SPS equipment is complex and expensive, particularly under high-temperature and high-pressure conditions, where specialized equipment incurs high costs. Moreover, SPS is primarily suitable for the preparation of small-sized samples, making it challenging to directly apply this technique to the sintering of large-scale components. As a result, the application of SPS is limited in both academic research and industrial production, especially for large-scale manufacturing.
3. Morphology and elemental characterization techniques
Characterization serves as the foundation for understanding the microstructure, composition, and physical and chemical properties of materials. In cutting-edge research on HEAs, characterization is indispensable for accurately determining the internal structure, crystal morphology, and phase composition of materials, thereby enabling performance optimization. By evaluating whether a material or product meets the desired performance criteria, characterization can promptly identify issues and guide improvements in production processes. Nanostructures are challenging to resolve with traditional optical microscopy. Transmission electron microscopy (TEM) provides a powerful alternative for examining fine features smaller than 100 nanometers and, in some cases, achieving atomic-scale resolution [21].
As shown in Figure 3, which depicts a schematic diagram and photograph of a TEM, the imaging principle of TEM operates as follows: An electron beam is generated by an electron gun and focused through metallic apertures and electromagnetic lenses within the TEM column. The focusing mechanism of electrons is based on their wave-like properties. As negatively charged particles, electrons are deflected by magnetic or electric fields, and only electrons within a narrow energy range can pass through, forming a well-defined electron beam. The electron beam then interacts with a sample mounted on a specimen holder (also referred to as a TEM grid, consisting of a metallic frame and a carbon-based film). The sample thickness is typically less than 100 nm to allow electron transmission. Factors such as the sample's density and composition influence the transmission of the electron beam—for example, porous materials permit more electrons to pass through, while denser materials allow fewer electrons to transmit. Using a condenser lens in TEM, the crystal structure of the sample can be determined from parallel electron beams [22].
After passing through the sample, the electron beam is refocused and magnified by two sets of electromagnetic lenses and projected onto a phosphorescent screen, where the electron image is converted into a visible image. Throughout the imaging system, the direction of electron emission can be adjusted via electromagnetic lenses. The entire TEM imaging process is analogous to photography [23].
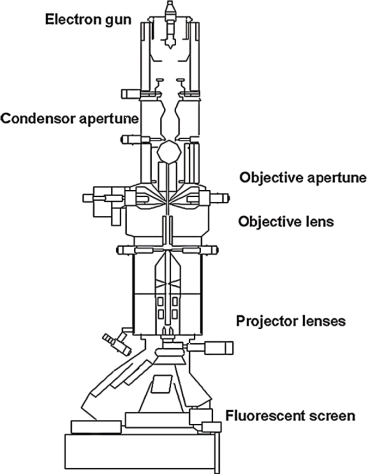
Figure 3: Transmission electron microscopy structure [23]
In addition, transmission electron microscopy (TEM) combined with energy dispersive spectroscopy (EDS) is widely used for characterizing the microstructure and elemental composition of HEAs. Figure 4 illustrates SEM and TEM images of HEA powder. TEM provides high-resolution microstructural images, while EDS analyzes the distribution of different elements within the sample by detecting the energy distribution of X-rays. The Mina Zhang team employed TEM in conjunction with EDS to investigate the elemental distribution and microstructure of AlCoCrCuFeNi HEAs [24]. TEM revealed the alloy’s multiphase structure, while EDS identified the elemental composition of each phase. The study showed enrichment of Al and Ni in specific regions, forming Al-Ni-rich precipitates, whereas Cr, Fe, and Co were uniformly distributed in the matrix. This approach elucidated the nanoscale elemental segregation behavior of the HEA [25, 26].
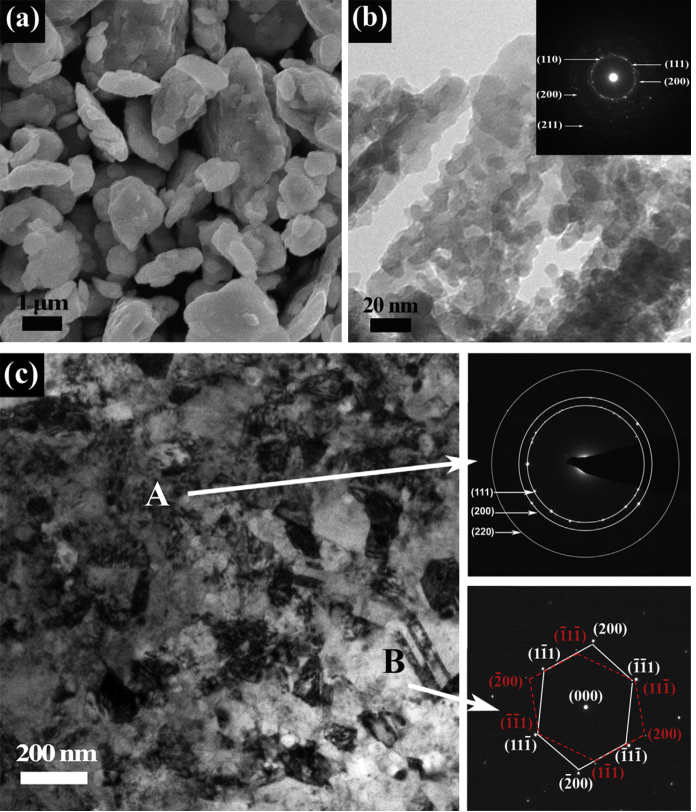
Figure 4: Microstructures of as-preserved CoCrFeNiMnHEAs: (a)-(b) SEM and TEM images of 60 h milled HEA powder, respectively; and (c) TEM bright field image of SPSed CoCrFeNiMn HEA.[27]
4. Conclusion
In conclusion, as an emerging class of materials, high-entropy alloys (HEAs) demonstrate a diversity of preparation methods and advantages across various applications. Techniques such as carbothermal shock synthesis, spark plasma sintering (SPS), and electrochemical synthesis each possess unique characteristics, catering to diverse technical demands and performance requirements. The continuous optimization and combined application of these methods have propelled the development of HEAs in fields such as catalysis, mechanical performance, and electromagnetic properties, providing exceptional material solutions.
Despite significant progress, HEAs still face challenges in large-scale industrial applications, including the risk of phase separation during synthesis, high equipment costs, and stability issues under specific conditions. Future research should focus on improving the controllability and environmental sustainability of synthesis processes, while leveraging advanced characterization techniques to gain a deeper understanding of the relationship between microstructure and performance. Ongoing exploration and technological innovation will further promote the widespread application of HEAs in areas such as energy, aerospace, and electronic devices, driving advancements in materials science and industrial technology.
References
[1]. He, Y., et al., (2024), Chemical dealloying derived nanoporous FeCoNiCuTi high-entropy bifunctional electrocatalysts for highly efficient overall water splitting under alkaline conditions. Chemical Engineering Journal, 492.
[2]. Loffler, T., et al., (2021), What Makes High-Entropy Alloys Exceptional Electrocatalysts? Angew Chem Int Ed Engl, 60(52): p. 26894-26903.
[3]. Chen, J., et al., (2024), Sulfurization-induced lattice disordering in high-entropy catalyst for promoted bifunctional electro-oxidation behavior. Chemical Engineering Journal, 489.
[4]. Wang, W., et al., (2024) Flocculent-structured high-entropy-alloy anode electrocatalyst for direct ethylene glycol fuel cells. Applied Catalysis A: General, 681.
[5]. Fan-ying, M., et al., (2023) Research progress on catalytic properties of high-entropy alloys. TRANSACTIONS OF MATERIALS AND HEAT TREATMENT, 44(6): p. 1-8.
[6]. Lu, M., et al., (2023) Ultrafast carbothermal shock strategy enabled highly graphitic po‐rous carbon supports for fuel cells. ScienceDirect, 52(1): p. 228-238.
[7]. Kumar Katiyar, N., et al., (2021) A perspective on the catalysis using the high entropy alloys. Nano Energy, 88.
[8]. Cha, J.H., et al., (2023) Flash-Thermal Shock Synthesis of High-Entropy Alloys Toward High-Performance Water Splitting. Adv Mater,35(46): p. e2305222.
[9]. Yao, Y., et al., (2018) Carbothermal shock synthesis of high-entropy-alloy nanoparticles. RESEARCH, 359: p. 1489-1494.
[10]. Abdelhafiz, A., et al., (2022) Carbothermal Shock Synthesis of High Entropy Oxide Catalysts: Dynamic Structural and Chemical Reconstruction Boosting the Catalytic Activity and Stability toward Oxygen Evolution Reaction. Advanced Energy Materials, 12(35).
[11]. Wang, H., et al., (2024) Low-Frequency Evolution Mechanism of Customized HEAs-Based Electromagnetic Response Modes Manipulated by Carbothermal Shock. Small, 20(31): p. e2309773.
[12]. Abid, T., et al., (2024) Design and development of porous CoCrFeNiMn high entropy alloy (Cantor alloy) with outstanding electrochemical properties. Journal of Alloys and Compounds, 970.
[13]. Runtong, Z., et al., (2023) Recent Progress of High-Entropy Alloys for Electrocatalysis. FOUNDRY TECHNOLOGY, 44(9): p. 1-17.
[14]. Sure, J., D.S.M. Vishnu, and C. Schwandt, (2017) Direct electrochemical synthesis of high-entropy alloys from metal oxides. Applied Materials Today, 9: p. 111-121.
[15]. Xu, Y., et al., (2024) Rapid synthesis of an OER catalytic surface on dual-phase high-entropy alloys via a controllable single-phase corrosion approach. International Journal of Hydrogen Energy, 61: p. 1187-1198.
[16]. Yoosefan, F., et al., (2019) Synthesis of CoCrFeMnNi High Entropy Alloy Thin Films by Pulse Electrodeposition: Part 1: Effect of Pulse Electrodeposition Parameters. Metals and Materials International, 26(8): p. 1262-1269.
[17]. Zhou, Y.J., et al., (2007) Solid solution alloys of AlCoCrFeNiTix with excellent room-temperature mechanical properties. APPLIED PHYSICS LETTERS, 90(181904): p. 1-3.
[18]. Zhang, K.B., et al., (2010) Characterization of nanocrystalline CoCrFeNiTiAl high-entropy solid solution processed by mechanical alloying. Journal of Alloys and Compounds, 495(1): p. 33-38.
[19]. Zhu, C., et al., (2020) Microstructure and mechanical properties of the TiZrNbMoTa refractory high-entropy alloy produced by mechanical alloying and spark plasma sintering. International Journal of Refractory Metals and Hard Materials, 93.
[20]. Verma, P.K., A. Singh, and A. Kumar, (2024) Microstructure evolution and magnetic characteristics of a novel high entropy alloy produced by mechanical alloying and spark plasma sintering. Intermetallics, 175.
[21]. Zhang, Y., et al., (2014) Microstructures and properties of high-entropy alloys. Progress in Materials Science, 61: p. 1-93.
[22]. Inkson, B.J., (2016) Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) for materials characterization, in Materials Characterization Using Nondestructive Evaluation (NDE) Methods. p. 17-43.
[23]. Tang, C.Y. and Z. Yang, (2017) Transmission Electron Microscopy (TEM), in Membrane Characterization. p. 145-159.
[24]. Zhang, M., X. Zhou, and J. Li, (2017) Microstructure and Mechanical Properties of a Refractory CoCrMoNbTi High-Entropy Alloy. Journal of Materials Engineering and Performance, 26(8): p. 3657-3665.
[25]. Hodoroaba, V.-D., (2020) Energy-dispersive X-ray spectroscopy (EDS), in Characterization of Nanoparticles. p. 397-417.
[26]. Mishra, R.K., A.K. Zachariah, and S. Thomas, (2017) Energy-Dispersive X-ray Spectroscopy Techniques for Nanomaterial, in Microscopy Methods in Nanomaterials Characterization. p. 383-405.
[27]. Ji, W., et al., (2015) Alloying behavior and novel properties of CoCrFeNiMn high-entropy alloy fabricated by mechanical alloying and spark plasma sintering. Intermetallics, 56: p. 24-27.
Cite this article
Fu,Y. (2025). Preparation and Characterization of High-entropy Alloys for Electrocatalysis. Applied and Computational Engineering,129,123-130.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 5th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. He, Y., et al., (2024), Chemical dealloying derived nanoporous FeCoNiCuTi high-entropy bifunctional electrocatalysts for highly efficient overall water splitting under alkaline conditions. Chemical Engineering Journal, 492.
[2]. Loffler, T., et al., (2021), What Makes High-Entropy Alloys Exceptional Electrocatalysts? Angew Chem Int Ed Engl, 60(52): p. 26894-26903.
[3]. Chen, J., et al., (2024), Sulfurization-induced lattice disordering in high-entropy catalyst for promoted bifunctional electro-oxidation behavior. Chemical Engineering Journal, 489.
[4]. Wang, W., et al., (2024) Flocculent-structured high-entropy-alloy anode electrocatalyst for direct ethylene glycol fuel cells. Applied Catalysis A: General, 681.
[5]. Fan-ying, M., et al., (2023) Research progress on catalytic properties of high-entropy alloys. TRANSACTIONS OF MATERIALS AND HEAT TREATMENT, 44(6): p. 1-8.
[6]. Lu, M., et al., (2023) Ultrafast carbothermal shock strategy enabled highly graphitic po‐rous carbon supports for fuel cells. ScienceDirect, 52(1): p. 228-238.
[7]. Kumar Katiyar, N., et al., (2021) A perspective on the catalysis using the high entropy alloys. Nano Energy, 88.
[8]. Cha, J.H., et al., (2023) Flash-Thermal Shock Synthesis of High-Entropy Alloys Toward High-Performance Water Splitting. Adv Mater,35(46): p. e2305222.
[9]. Yao, Y., et al., (2018) Carbothermal shock synthesis of high-entropy-alloy nanoparticles. RESEARCH, 359: p. 1489-1494.
[10]. Abdelhafiz, A., et al., (2022) Carbothermal Shock Synthesis of High Entropy Oxide Catalysts: Dynamic Structural and Chemical Reconstruction Boosting the Catalytic Activity and Stability toward Oxygen Evolution Reaction. Advanced Energy Materials, 12(35).
[11]. Wang, H., et al., (2024) Low-Frequency Evolution Mechanism of Customized HEAs-Based Electromagnetic Response Modes Manipulated by Carbothermal Shock. Small, 20(31): p. e2309773.
[12]. Abid, T., et al., (2024) Design and development of porous CoCrFeNiMn high entropy alloy (Cantor alloy) with outstanding electrochemical properties. Journal of Alloys and Compounds, 970.
[13]. Runtong, Z., et al., (2023) Recent Progress of High-Entropy Alloys for Electrocatalysis. FOUNDRY TECHNOLOGY, 44(9): p. 1-17.
[14]. Sure, J., D.S.M. Vishnu, and C. Schwandt, (2017) Direct electrochemical synthesis of high-entropy alloys from metal oxides. Applied Materials Today, 9: p. 111-121.
[15]. Xu, Y., et al., (2024) Rapid synthesis of an OER catalytic surface on dual-phase high-entropy alloys via a controllable single-phase corrosion approach. International Journal of Hydrogen Energy, 61: p. 1187-1198.
[16]. Yoosefan, F., et al., (2019) Synthesis of CoCrFeMnNi High Entropy Alloy Thin Films by Pulse Electrodeposition: Part 1: Effect of Pulse Electrodeposition Parameters. Metals and Materials International, 26(8): p. 1262-1269.
[17]. Zhou, Y.J., et al., (2007) Solid solution alloys of AlCoCrFeNiTix with excellent room-temperature mechanical properties. APPLIED PHYSICS LETTERS, 90(181904): p. 1-3.
[18]. Zhang, K.B., et al., (2010) Characterization of nanocrystalline CoCrFeNiTiAl high-entropy solid solution processed by mechanical alloying. Journal of Alloys and Compounds, 495(1): p. 33-38.
[19]. Zhu, C., et al., (2020) Microstructure and mechanical properties of the TiZrNbMoTa refractory high-entropy alloy produced by mechanical alloying and spark plasma sintering. International Journal of Refractory Metals and Hard Materials, 93.
[20]. Verma, P.K., A. Singh, and A. Kumar, (2024) Microstructure evolution and magnetic characteristics of a novel high entropy alloy produced by mechanical alloying and spark plasma sintering. Intermetallics, 175.
[21]. Zhang, Y., et al., (2014) Microstructures and properties of high-entropy alloys. Progress in Materials Science, 61: p. 1-93.
[22]. Inkson, B.J., (2016) Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) for materials characterization, in Materials Characterization Using Nondestructive Evaluation (NDE) Methods. p. 17-43.
[23]. Tang, C.Y. and Z. Yang, (2017) Transmission Electron Microscopy (TEM), in Membrane Characterization. p. 145-159.
[24]. Zhang, M., X. Zhou, and J. Li, (2017) Microstructure and Mechanical Properties of a Refractory CoCrMoNbTi High-Entropy Alloy. Journal of Materials Engineering and Performance, 26(8): p. 3657-3665.
[25]. Hodoroaba, V.-D., (2020) Energy-dispersive X-ray spectroscopy (EDS), in Characterization of Nanoparticles. p. 397-417.
[26]. Mishra, R.K., A.K. Zachariah, and S. Thomas, (2017) Energy-Dispersive X-ray Spectroscopy Techniques for Nanomaterial, in Microscopy Methods in Nanomaterials Characterization. p. 383-405.
[27]. Ji, W., et al., (2015) Alloying behavior and novel properties of CoCrFeNiMn high-entropy alloy fabricated by mechanical alloying and spark plasma sintering. Intermetallics, 56: p. 24-27.









