1. Introduction
In 1991, a Japanese professor who called Iijima evaporated graphite electrodes with vacuum arc and found carbon nanotubes, a multilayer tube of carbon with nanometer size, under a high-resolution transmission electron microscope. From that time, carbon nanotubes be known by everyone as a new type of nanomaterial. It has been widely used in composite materials, electronic materials, and energy materials [1] because of its special electrical, mechanical, optical, and thermal properties. Lu Yong et al. have developed carbon nanotube rubber composites which can use in green fuel-saving tires with high-performance, high-strength, and their fuel consumption level has reached the EU labeling law C level or above, and the antistatic performance of the green fuel-saving tires meets the standard. A multinational company called Bayer MaterialScience has integrated carbon nanotube technology effectively and put it into commercial production. Plus, they used new materials extensively in wind energy construction [2]. Showa Corporation of Japan uses the planktonic catalytic method to achieve the meteorological preparation of carbon nanotubes and apply them to the anode material of lithium-ion batteries. In addition, the Nanocyl company from Belgium has applied carbon nanotubes to some fields, such conductive composites and tires. DuPont company from United States industrialized and promoted the technology about prepare carbon nanotube thin film composites from Nanocomp company [3]. All of them were promoted the development of carbon nanotube technology.
Nowadays, have about three common methods for preparing carbon nanotubes, such as arc discharge method, chemical vapor deposition method and laser ablation method. In the midst of these, the standard production method for the preparation of carbon nanotubes is chemical vapor deposition, but this preparation prices is expensive and the economy is poor because of the high cost and low utilization rate of some reactants. However, Laser ablation has the same problem. The arc discharge method is the most useful way to solve the current problem of chemical vapor deposition because of the high product quality and controllable preparation process.
Although the arc discharge method has some advantages, such as high yield, high product quality, controllable preparation process and high degree of graphitization, but in actual production process, it affected by factors of discharge atmosphere, catalyst type and proportion, and temperature. Wu [4] analyzed the surface morphology of carbon nanotubes prepared by He, N2 , and Ar atmospheres. In addition, they found that carbon nanotubes were intertwined with each other and the tube diameter was small by prepared by He atmosphere. And the diameter of carbon nanotubes prepared by N2 atmosphere was different anf has a number of fractures. However, the diameter distribution of carbon nanotubes is uniform when the discharge atmosphere is Ar and the surface was smooth and flat. Qui [5] found that the catalyst addition ratio has very important effects in the tyoe of carbon nanotube product. Zhao found that temperature has different effects on purity and yield of siglewalled carbon nanotubes. Therefore, in order to solve these problems, this passage will explore the influence on arc discharge method to preparation of carbon nanotubes by discharge atmosphere, catalyst type and proportion, and temperature and provide more ideas on actual preparation of carbon nanotubes in the future.
2. Discharge atmosphere
In preparation of carbon nanotubes, discharge atmosphere plays an important character. In the arc discharge method, discharge atmosphere has very important influence on the type of carbon nanotube products. Wang [6,7] use Yunnan anthracite as raw material. When the discharge atmosphere is He, they get cathode product which is "Y" bifurcated carbon nanotubes. The inside diameter ranges from 40 to 50 nm and the outside diameter ranges from 20 to 60 nm. When the discharge atmosphere is Ar, they get product which is copper nanowires filled with carbon nanotubes. Its diameter ranges from 30 to 80 nm, the length to ratio reaches 200-360. The wall thickness of carbon nanotubes is about 20 layers, interlamellar spacing is about 0.34 nm. All of these phenomena are attributed to Ar atmosphere is rapid pyrolysis of anthracite than He atmosphere to produce hydrocarbon molecules or aromatic components.
At the same time, the discharge atmosphere can not only accelerate the production of carbon nanotubes, but also can affect the surface mrophologgy and structure. Wu [4] respectively analyzed the surface mrophologies of carbon nanotubes prepared under He atmosphere, N2 atmosphere and Ar atmosphere. They discover the carbon nanotubes under He atmosphere (Fig. 1a) has a small pipe diameter, about 30 nm. Under the N2 atmosphere, the diameter of carbon nanotubes (Fig. 1b) from 80 to 120 nm, with uneven thickness and multiple fractures. The open bamboo carbon nanotubes (Fig. 1c) were obtained under Ar atmosphere, with a diameter about 500 nm, and the distribution was uniform and smooth. It can be seen from Figure 2, the shape of carbon nanotubes is bamboo, corresponding to its SEM image and the tube wall is thick, about 20 nm. It is attribute to the inner layer has formed a template and the outer layer continues to grow on the template during the growth of carbon nanotubes, so the carbon nanotubes have a thick wall. It can be seen from Fig.2b, the tube wall spacing of carbon nanotubes is about 0.34 nm, it signifies that carbon nanotubes also have a certain degree of graphitization (graphite d002=0.337 nm).
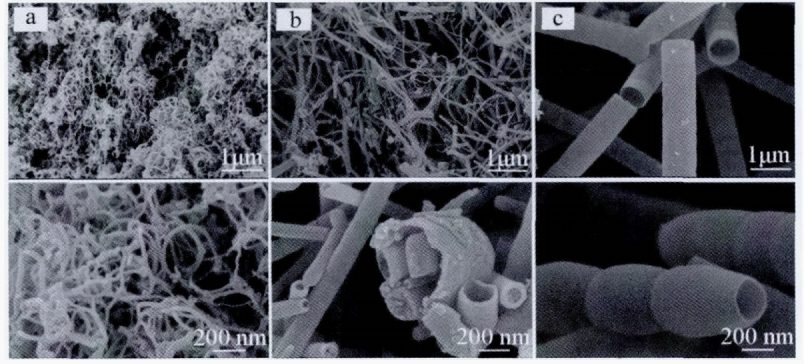
Figure 1: FESEM images of CNTs synthesized under different atmospheres. a: He; b: N2; c: Ar
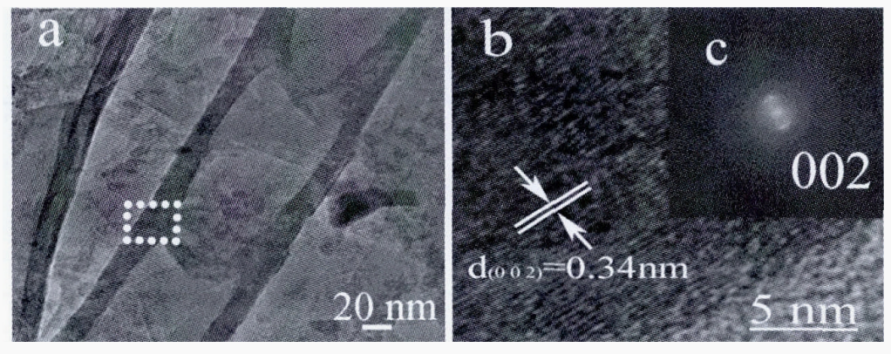
Figure 2: TEM images of CNTs synthesized under Ar atmospheres. a: TEM image of CNTs; b: HRTEM image for the wall of a CNT; c: The corresponding electron diffraction pattern of the CNT in b.
In addition to the type of discharge atmosphere has a certain effect on the forming of carbon nanotubes, the yield of carbon nanotubes is also significantly impacted by the discharge atmosphere's pressure. Qiu [8] use Taixi anthraciteas raw material. They found that yield of carbon nanotubes showed a “volcanic” trend with discharge atmosphere pressure. At 0.055 MPa, the maximum yield was 9.2%, it signifies that when the buffer gas pressure is low, the carbon species generated by anode ionization are easily diffused to areas outstide the arc center region, and the possibility of generating carbon nanotubes is reduced. When the buffer gas pressure is high, the carbon species generated by ionization are concentrated in the center of arc, generate carbon nanotubes and molten carbon materials instead of carbon nanotubes. To sum up, the type and pressure of discharge atmosphere paly very important character in the preparation of carbon nanotubes by arc discharge method, and the pressure of a suitable discharge atmosphere needs to be adjusted.
3. Catalyst
The actalyst has very important effect on formation of carbon nanotubes. Awasthi [9] use carbon rods made from Indian bituminous coal as raw material, before add catalyst, the produce is multi-walled carbon nanotubes, and the diameter is 8-20 nm and the length is 5-10 μm. When Fe is added as a catalyst, keeping other reaction conditions unchanged, the products underwent a transformation into single-walled carbon nanotubes, measuring an average of 1.7 nm in diameter and 0.2 μm in length. Wuxia use Kuqa coal as raw material and get carbon nanotubes yield of up to 1.53% (relative anode carbon rod mass) without catalysis. When adding 12% wt as a catalyst, the yield increased to 5.22%. When adding Ni and FeS (both added amounts of 6 wt%) as catalysts, the yield increased to 22.64%. It means that the carbon nanotubes were formed more quickly when the catalyst was added., and the catalytic effect of Fe catalyst was better than Ni catalyst. In addition to the type of catalyst, the catatlyst addition ratio has very important effect on the product type of carbon nanotubes. Qiu [10] take coke rod filled with Fe powder and coke powder (mass ratio 2:1) as raw material, the product is single-walled carbon nanotubes and the diameter is 1.24-2.19 nm. When the mass ratio of Fe powder to coke powder is 1:1, the product becomes “bamboo shaped” carbon nanotubes. It is due to the addition ratio of catalyst resulting in the different proportion of metal catalyst vapor and carbon vapor in the discharge process, and the corresponding products are different. In the bimetallic catalyst system, the ratio of catalyst also has an important effect on the purity and yield of carbon nanotubes. Itkis [11] found that different ratios of Ni/Y catalysts correspond to different product purity of carbon nanotubes. In the range of 0-8, with the increase of the ratios of Ni/Y atoms, the purity of carbon nanotubes showed a trend of first increased and then decreased, and reached a maximum value of 67% when the ratio was 4. Under comparable circumstances, Williams [12] created single-walled carbon nanotubes that ranged in diameter from 1.2 to 1.7 nm. Kumar [13] use Zr/Ni bimetal as catalyst and found that within the range of 0.25-3, with the increase of Zr/Ni mass ratio, the yield of purified carbon nanotubes gradually increased from 6 mg to 46 mg. Then, with the Zr/Ni ratio continues to increased (in the range of 3 to 6), the carbon nanotubes yield gradually decreased to 3 mg. It is due to the fact that at the optimal ratio, the bimetallic catalyst system forms a stable liquid eutectic phase system, and the catalyst particle size is small, which promotes the creation of carbon nanotubes with a single wall. When the bimetal ratio is increased or decreased, the eutectic structure changes, leading to the development of bigger catalyst particles, which is not conducive to the formation of single-walled carbon nanotubes. To sum up, the type and proportion of catalyst play a key character in the morphology and yield of carbon nanotubes. Selecting and optimizing catalyst conditions is a sensible approach to enhance the performance of carbon nanotubes.
4. Temperature
Carbon nanotube quality and growth are directly influenced by temperature. A range of temperatures that is appropriate for chemical vapor deposition promotes the formation of carbon nanotubes and the effective breakdown of carbon source gases. In laser evaporation, High temperature laser pulses also stimulate carbon atoms and catalyst particles by providing a certain amount of heat to encourage the synthesis of carbon nanotubes. Carbon nanotubes are manufactured using these two extensively utilized processes because of their respective advantages. Reasonable calcination temperature is conducive to the dispersion of the active substance on the surface of the carrier, in order to increase the pore capacity and specific surface area of the catalyst, so that the carbon nanotubes with high yield, uniform diameter, high graphitization degree, less ash and low resistivity can be prepared. When the calcination temperature is 500 °C, the diameter distribution of carbon nanotubes is more uniform, concentrated in the range of 10-14 nm, and the defect degree is the smallest, only 0.98. At the same time, temperature not only affects the yield and purity, but also directly affects the morphology of carbon nanotubes. If the temperature is very high or very low, the appearance of carbon nanotubes is often uneven and the impurity increases. Yue [14] found that the diameter of carbon nanotubes increased from 75 nm to 104 nm when the temperature was increased in the range of 810-860 °C, and the diameter of carbon nanotubes decreased to 78 nm when the temperature was increased in the range of 860-900 °C. This is due to the fact that at low temperatures, C2H4 cannot be fully decomposed, resulting in low diffusion capacity of generated carbon atoms, and Fe atoms cannot be liquefied and nucleated at low temperatures, resulting in low concentration and poor catalytic effect, resulting in a smaller diameter of carbon nanotubes. At high temperatures, C2H4 can be fully decomposed, and there are enough carbon sources to generate carbon nanotubes, while the catalyst has a high concentration of Fe atoms and a large particle size, so the diameter of carbon nanotubes increases.
However, too high a temperature will also deactivate the catalyst and reduce the diameter of the carbon nanotubes. When Qiu [15] prepared single-wall carbon nanotubes using commercial gas as raw material, it was found that when the calcination temperature was lower than 750 °C, the catalyst concentration would decrease, and only less black deposits would be produced. When the calcination temperature is higher than 1100 °C, the product is a mixture of amorphous carbon and carbon nanotubes. When Seo [16] catalyzed amorphous carbon to crack CH4 to prepare carbon nanotubes, it was found that in the temperature range of 350-600 °C, the generation of carbon nanotubes would gradually increase with the increase of temperature, and eventually become an array. This is explained by the fact that rising temperatures quicken the rate at which carbon atoms diffuse and react. In summary, the primary aspect influencing the development and caliber of carbon nanotubes is temperature, and the morphology and properties of the products under different temperature conditions are significantly different.
5. Conclusion
In this paper, the essential elements for the arc discharge method of carbon nanotube synthesis were thoroughly examined. It was found that the discharge atmosphere, the type and proportion of catalyst and temperature had significant effects on the morphology, yield and quality of carbon nanotubes. In the future, with the development of new catalysts and the advancement of green processes, there are numerous applications for carbon nanotubes in the domains of energy, electronics, and composite materials. and will contribute to the global sustainable development and innovation of new material technology.
References
[1]. Volder, M., Tawfick, S., & Baughman, R. (2013). Carbon nanotubes: present and future commercial applications. Science, 339(6119), 535-539. https://doi.org/10.1149/ma2016-01/33/1631.
[2]. Bozhang, Q. (2010). Application progress of carbon nanotubes. New Chemical Materials, 38(2), 16-18+35. https://doi.org/10.3969/j.issn.1006-3536.2010.02.004.
[3]. Qiang, Z., Jiaqi, H., & Mengqiang, Z. (2013). Macro preparation and industrialization of carbon nanotubes. Science in China: Chemistry, 43(6), 641-666. https://doi.org/10.1360/032013-71.
[4]. Xia, W., Luxiang, W., & Lang, L. (2013). Preparation of coal-based carbon nanotubes in Xinjiang. Journal of Inorganic Chemistry, 29(9), 1842-1848. https://doi.org/10.3969/j.issn.1001-4861.2013.00.292.
[5]. Qiu J., Li Y., & Wang, Y. (2003). High-purity single-wall carbon nanotubes synthesized from coal by arc discharge. Carbon, 41(11), 2170-2173. https://doi.org/10.1016/s0008-6223(03)00242-2.
[6]. Wang, Z., Zhao, Z., & Qiu, J. (2006). Synthesis of branched carbon nanotubes from coal. Carbon, 44(7), 1321-1324. https://doi.org/10.1016/j.carbon.2005.12.030.
[7]. Wang, Z., Zhao, Z., & Qiu, J. (2006). In situ synthesis of super-long Cu nanowires inside carbon nanotubes with coal as carbon source. Carbon, 44(9), 1845-1847. https://doi.org/10.1016/j.carbon.2006.04.001.
[8]. Yong, L., Pengzhan, Y., & Huixian, Su. (2004). Preparation methods of carbon nanotubes. Coal Mine Machinery, 8(8), 7-8. https://doi.org/10.3969/j.issn.1003-0794.2004.08.004.
[9]. Shetao, Z. (2013). Feasibility of industrialization of carbon nanotubes at kiloton scale. Guangzhou Chemical Industry, 41(24), 135-138. https://doi.org/10.3969/j.issn.1001-9677.2013.24.050.
[10]. Dongyuan, W. (2010). The industrial development potential of carbon nanotubes is becoming increasingly prominent. Advanced Materials Industry, 1(6), 28-30. https://doi.org/10.3969/j.issn.1008-892X.2010.06.006.
[11]. Itkis, M., Perea, D., & Niyogi, S. (2004). Optimization of the Ni-Y catalyst composition in bulk electric arc synthesis of single-walled carbon nanotubes by use of near-infrared spectroscopy. Journal of Physical Chemistry B, 108(34), 12770-12775. https://doi.org/10.1021/jp0487307.
[12]. Williams, K., Tachibana, M., & Allen, J. (1999). Single-wall carbon nanotubes from coal. Chemical Physics Letters, 310(1), 31-37. https://doi.org/10.1016/s0009-2614(99)00725-3.
[13]. Kumar, R., Singh, R., & Ghosh, A. (2013). Synthesis of coal-derived single-walled carbon nanotube from coal by varying the ratio of Zr/Ni as bimetallic catalyst. Journal of Nanoparticle Research, 15(1), 1406-1416. https://doi.org/10.1007/s11051-012-1406-3.
[14]. Yuchen, Y., Ren, Wei., & Dejun, Li. (2011). Effect of reaction temperature on carbon nanotubes. Vacuum, 48(6), 15-17. https://doi.org/10.13385/j.cnki.vacuum.2011.06.009.
[15]. Qiu, J., An, Y., & Zhao, Z. (2004). Catalytic synthesis of single-walled carbon nanotubes from coal gas by chemical vapor deposition method. Fuel Processing Technology, 85(8), 913-920. https://doi.org/10.1016/j.fuproc.2003.11.033.
[16]. Seo, J., Choi, W., & Kim, H.. (2011). Growth of metal-free carbon nanotubes on glass substrate with an amorphous carbon catalyst layer. Journal of nanoscience and nanotechnology, 11(12), 11032-11036. https://doi.org/10.1166/jnn.2011.4022.
Cite this article
Sun,W. (2025). Influence of Different Factors on Preparation of Carbon Nanotubes by Arc Discharge Method. Applied and Computational Engineering,129,71-76.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 5th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Volder, M., Tawfick, S., & Baughman, R. (2013). Carbon nanotubes: present and future commercial applications. Science, 339(6119), 535-539. https://doi.org/10.1149/ma2016-01/33/1631.
[2]. Bozhang, Q. (2010). Application progress of carbon nanotubes. New Chemical Materials, 38(2), 16-18+35. https://doi.org/10.3969/j.issn.1006-3536.2010.02.004.
[3]. Qiang, Z., Jiaqi, H., & Mengqiang, Z. (2013). Macro preparation and industrialization of carbon nanotubes. Science in China: Chemistry, 43(6), 641-666. https://doi.org/10.1360/032013-71.
[4]. Xia, W., Luxiang, W., & Lang, L. (2013). Preparation of coal-based carbon nanotubes in Xinjiang. Journal of Inorganic Chemistry, 29(9), 1842-1848. https://doi.org/10.3969/j.issn.1001-4861.2013.00.292.
[5]. Qiu J., Li Y., & Wang, Y. (2003). High-purity single-wall carbon nanotubes synthesized from coal by arc discharge. Carbon, 41(11), 2170-2173. https://doi.org/10.1016/s0008-6223(03)00242-2.
[6]. Wang, Z., Zhao, Z., & Qiu, J. (2006). Synthesis of branched carbon nanotubes from coal. Carbon, 44(7), 1321-1324. https://doi.org/10.1016/j.carbon.2005.12.030.
[7]. Wang, Z., Zhao, Z., & Qiu, J. (2006). In situ synthesis of super-long Cu nanowires inside carbon nanotubes with coal as carbon source. Carbon, 44(9), 1845-1847. https://doi.org/10.1016/j.carbon.2006.04.001.
[8]. Yong, L., Pengzhan, Y., & Huixian, Su. (2004). Preparation methods of carbon nanotubes. Coal Mine Machinery, 8(8), 7-8. https://doi.org/10.3969/j.issn.1003-0794.2004.08.004.
[9]. Shetao, Z. (2013). Feasibility of industrialization of carbon nanotubes at kiloton scale. Guangzhou Chemical Industry, 41(24), 135-138. https://doi.org/10.3969/j.issn.1001-9677.2013.24.050.
[10]. Dongyuan, W. (2010). The industrial development potential of carbon nanotubes is becoming increasingly prominent. Advanced Materials Industry, 1(6), 28-30. https://doi.org/10.3969/j.issn.1008-892X.2010.06.006.
[11]. Itkis, M., Perea, D., & Niyogi, S. (2004). Optimization of the Ni-Y catalyst composition in bulk electric arc synthesis of single-walled carbon nanotubes by use of near-infrared spectroscopy. Journal of Physical Chemistry B, 108(34), 12770-12775. https://doi.org/10.1021/jp0487307.
[12]. Williams, K., Tachibana, M., & Allen, J. (1999). Single-wall carbon nanotubes from coal. Chemical Physics Letters, 310(1), 31-37. https://doi.org/10.1016/s0009-2614(99)00725-3.
[13]. Kumar, R., Singh, R., & Ghosh, A. (2013). Synthesis of coal-derived single-walled carbon nanotube from coal by varying the ratio of Zr/Ni as bimetallic catalyst. Journal of Nanoparticle Research, 15(1), 1406-1416. https://doi.org/10.1007/s11051-012-1406-3.
[14]. Yuchen, Y., Ren, Wei., & Dejun, Li. (2011). Effect of reaction temperature on carbon nanotubes. Vacuum, 48(6), 15-17. https://doi.org/10.13385/j.cnki.vacuum.2011.06.009.
[15]. Qiu, J., An, Y., & Zhao, Z. (2004). Catalytic synthesis of single-walled carbon nanotubes from coal gas by chemical vapor deposition method. Fuel Processing Technology, 85(8), 913-920. https://doi.org/10.1016/j.fuproc.2003.11.033.
[16]. Seo, J., Choi, W., & Kim, H.. (2011). Growth of metal-free carbon nanotubes on glass substrate with an amorphous carbon catalyst layer. Journal of nanoscience and nanotechnology, 11(12), 11032-11036. https://doi.org/10.1166/jnn.2011.4022.









