1. Introduction
In recent years, brain-computer interfaces (BCIs) have garnered global attention as a method of establishing direct communication between the human brain and external devices[1]. Among them, BCIs have played a promoting role in human research on brain neuromodulation. At the same time, it also makes critical contributions in helping or treating cases of paralysis, neurodegeneration and other diseases. A key technology in BCIs is implantable microelectrodes, which are miniature electronic devices that can be inserted into the human body, particularly the brain, to record or stimulate neural activity. These devices are utilized in areas such as brain-computer interfaces and therapeutic intervention. As technology evolves, significant advances have been made in the design and fabrication of implantable microelectrodes, improving performance, biocompatibility, and durability. These microelectrodes typically consist of arrays of thin-film electrodes that can be implanted into specific brain regions to enable precise targeting of neural circuits. Implantable microelectrodes have broad application prospects in neuroengineering research and clinical applications. They can help stimulate the brain and record the electrical activity of neurons in the brain, revealing patterns of brain network activity and mechanisms of neural regulation. These recorded neural signals are decoded and processed to determine the user's intentions, which are then converted into signals for controlling external devices, such as prosthetics. This technology could assist disabled and paralyzed patients in basic rehabilitation training [2].
2. Implantable Microelectrodes
Implantable Microelectrodes are tiny electronic devices that can be inserted into the body, particularly the brain, to record or stimulate neural activity for applications such as BCIs and therapeutic interventions.
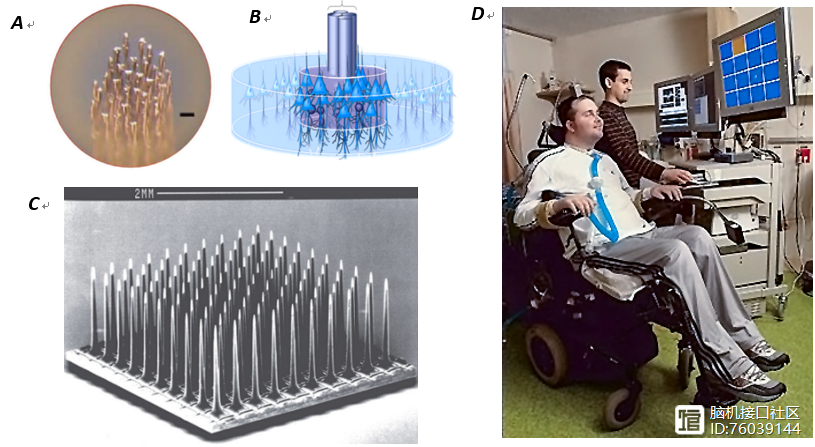
Figure 1. A. the feature of microwire electrodes array; B. the illustration of tetrode in neuraltissue; C. Utah array [3].
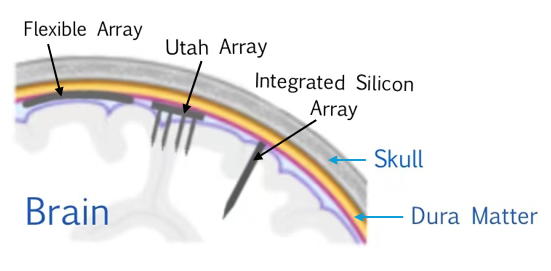
Figure 2. The mentioned types of neural electrode interfaces.
Here Figure 1 and 2 lists three commonly used microelectrodes in BCIs: Microwire electrodes, Utah arrays and Michigan probes. Microwire electrodes are single-channel or multi-channel microelectrodes made of fine metal wires, usually platinum or tungsten. These electrodes are suitable for recording the electrical activity of single or multiple neurons [4]. Utah arrays are high-density electrode arrays consisting of many microelectrodes. These arrays have high spatial resolution, can record the activity of multiple neurons simultaneously, and are widely used in brain-computer interface research and clinical applications. The number of neurons present in the recording significantly influences task performance, with an increase in neuron numbers reducing their sensitivity to stimulation [5]. Michigan probes are silicon-based micro-electrodes typically composed of multiple tiny electrodes that can record neural activity with micron-level resolution. Michigan probes have excellent spatial resolution and can be used to study the connections between neurons and the activity of neural networks. These probes are in high demand, in part because they can be designed with multiple precisely located sites to achieve measurements that are not possible by other means [6].
3. Application in Neuroregulation
3.1. Deep Brain Stimulation (DBS)
Deep brain stimulation therapy involves using stereotactic technology to accurately locate and implant electrodes in specific brain areas, delivering continuous stimulation pulses to targeted deep brain tissues to achieve therapeutic effects. For patients with severe and chronic refractory depression, deep brain stimulation can significantly and sustainably improve symptoms. Although deep brain stimulation is generally considered to have a low risk, it, like any surgical procedure, carries potential risks of complications. Similarly, brain stimulation itself can also produce side effects. Deep brain stimulation does not cure the disease [7], but it can help reduce symptoms, and in some cases, some conditions may still require medication [8].
3.2. Neural Feedback Control
The specific practice of neurofeedback training involves measuring the brain nerve activity related to a specific function. The measurement results are fed back to the trainee in real time through visual, auditory, or tactile means, often using a reward mechanism [9]. This feedback helps the trainee learn to autonomously adjust and improve the target brain nerve activity. Improving targeted brain activity can lead to the repair or enhancement of corresponding brain functions. Similar to how physical exercise builds muscle for health, neurofeedback training strengthens and optimizes brain function [10].
3.3. Neural Recording and Stimulation
Nerve stimulation involves the application of electrodes directly or indirectly placed in the field of nerve tissue innervation, or around the nerve root, the dorsal horn of the spinal cord. The device used to deliver these stimulation impulses is called a nerve stimulator. Its purpose is to improve the pathological state of patients, clinical symptoms, and even to achieve therapeutic effects [11]. On the basis of neural regulation, this biomedical engineering technology came into being, the concept utilizes implantable or non-implantable technology, the use of electrical stimulation or drug means, to change the activity of the central nervous system, peripheral nervous system or autonomic nervous system. The objective is to alleviate symptoms and enhance patients' quality of life.
4. Application in Medicine
Implantable microelectrodes are utilized in the motor recovery of brain-computer interfaces. Motor recovery involves employing various methods and techniques to restore motor function in patients with impaired mobility. In terms of upper limb control, technologies such as exoskeleton control and fine finger control are commonly used [12,13]. In terms of lower limb motor recovery, the more critical technologies include gait reconstruction and standing control. Gait reconstruction aims to use technologies such as exoskeletons and neuromuscular stimulation systems to help paralyzed patients regain their ability to walk. Standing control focuses on supporting the balance and stability of patients in a standing position through external support devices and neural control technology. At the same time, real-time motion control and feedback systems are integral to the rehabilitation process for paralyzed patients. Real-time motion control can adjust the movement of external devices in real time according to the patient's movement intentions and neural signals, providing more accurate and natural motion control. The feedback system can provide patients with perception and feedback of the movement process, helping them to better adjust their balance.
Implantable microelectrodes provide solutions for the treatment and rehabilitation of a variety of diseases and conditions in clinical applications. The following are three notable clinical applications of implantable microelectrodes.
1) Parkinson's Disease: DBS mentioned in Part 3 is widely used to relieve symptoms and improve quality of life. Compared with simple drug treatment, DBS can produce long-term therapeutic effects on patients by optimizing electrode placement and surgical strategies [14,15].
2) Epilepsy: Implantable microelectrodes can provide another adjunctive treatment option for patients with partial epilepsy. It can help reduce the frequency of epileptic seizures and regulate and control epileptic seizures through responsive cortical stimulation [16].
3) Amputees: In terms of prosthetic control, implantable electrodes can provide more precise and reliable control than surface electrodes [17].
5. Advanced Hardware Enhancing Application of BCIs
5.1. Integration of Sensing and Actuation
The integration of sensing and actuation functionalities within a single chip contribute to closed loop system and wireless wearable technology. To begin with, Figure 3 closed-loop systems enhance the precision of neural interfaces by providing real-time neural feedback to increase co-adaptive capabilities of BCIs. This adaptation is crucial for optimizing therapeutic outcomes in applications such as motor rehabilitation and diagnosis and control of pathological brain activity. Moreover, wireless wearable technology benefits from this integration by enabling timely interaction between the implantable device and external systems, allowing for continuous monitoring neural signals and provide adaptive interventions over extended periods [18,19]. This integration allows users to move more freely and better manage conditions such as epilepsy or Parkinson’s disease, facilitating the transition of therapeutic technologies from controlled laboratory settings to real-life applications for patients.
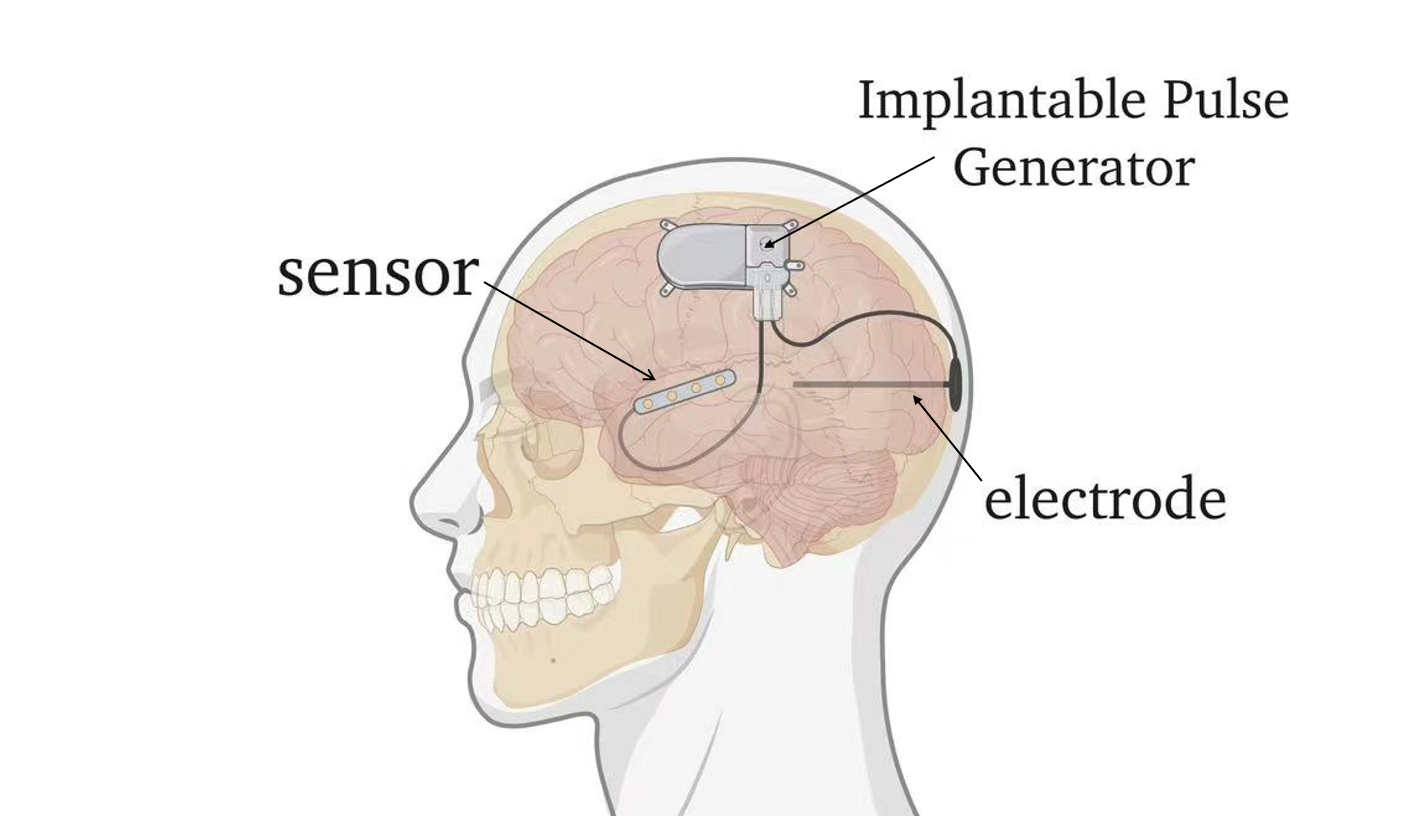
Figure 3. Closed-loop deep brain and cortical stimulation systems.
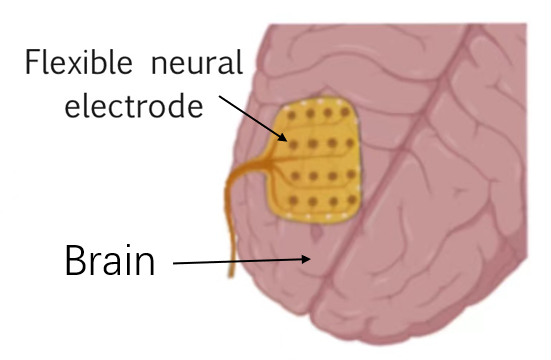
Figure 4. Schematic diagram of a flexible neural electrode.
5.2. Advanced Microfabrication Methods
The development of advanced fabrication techniques results in high-density electrode arrays that offer improved spatial resolution, crucial for detailed and accurate neural activity mapping. Figure 4 illustrates the general principle of a flexible neural electrode. Not only that, advanced lithography techniques and processes such as electron beam lithography are used to create complex patterns on a flexible substrate for the manufacture of flexible neural implants [20]. These implants often feature thin, flexible substrates that can wrap around or conform to the brain's surface, minimizing mechanical stress and improving integration with the tissue. What’s more, smaller devices consume less power, which produces less heat, so in turn reduces tissue damage. Energy-efficient designs also help in prolonging the operational life of the implants without frequent recharges or replacements. Techniques such as electron-beam lithography and deep ultraviolet (DUV) lithography allow for the fabrication of features down to a few nanometers, thus enhancing the specificity of neural recordings. Nanoimprinting, on the other hand, complements lithography by providing a cost-effective and scalable approach to replicate nanoscale patterns over large areas. Furthermore, smaller devices are generally more biocompatible and reduce tissue damage, which helps in maintaining performance of the microelectrode and reducing chronic immune response. The miniaturization technology makes the implantable microelectrode have better performance, Better Biocompatibility, and makes it possible to produce High-density electrode arrays.
5.3. High-Density Electrode Array
The High-Density Electrode Array can make more accurate, multi-region measurement, improve the SNR, to obtain a higher resolution signal. which is very important for the ability to accurately sense and actuate to neural activity [21]. First, the proximity of electrodes to neural tissue reduces the distance over which signals must travel, thereby improving the SNR. Higher SNR making it easier to distinguish between signals from nearby neurons or different brain regions. Furthermore, high-density arrays capture subtle differences in neural activity patterns. This precision is vital for applications requiring accurate targeting of specific brain areas, such as decoding motor intentions for prosthetic control. The ability to processing signals precisely enhances the functionality of neuroprosthetic devices and improve patient experience. Moreover, high-density electrode arrays enable researchers to record signals simultaneously from multiple brain regions. This capability allows for a comprehensive capture of neural dynamics across the brain, helping researchers study how brain signals integrated and how complex cognitive processes occur. By achieving higher electrode density, these arrays can capture precise representation of neural signals, paving the way for more effective brain-computer interface applications and advancing our understanding of complex neural networks.
6. Technological challenges and outlook
Future challenges including ensuring the long-term stability of implanted microelectrodes while maintaining high-quality signal recording. The implantation of foreign materials can trigger inflammatory reactions, leading to scar tissue formation around electrodes and degradation of signal quality. Changes in electrode performance can lead to diminished reducing effectiveness of BCIs for users, impacting their ability to control devices or receive feedback. Thus, research is focused on developing biocompatible materials that minimize immune response and maintain the long-term functionality of implanted devices. Usually, implanted microelectrodes only stay in the body for 1-2 years [22]. Because implantation of foreign materials can trigger inflammatory reactions, leading to scar tissue formation around electrodes and degradation of signal quality. Today's implanted microelectrodes, with better biocompatibility, can stay in the body for five years or more. But they have their limitations [23]. Such as the limited spatial coverage of the Utah Array means that it can only capture neural signals from a confined area of the brain. What’s more, flexible neural implants often suffer from lower electrode density and increased susceptibility to absorption compared to their rigid counterparts. For future advancements, it remains crucial to develop chronic implantable neural interface systems, that offer high spatio-temporal resolution and provide extensive brain coverage.
7. Conclusion
Implantable microelectrodes represent a transformative advancement in brain-computer interfaces (BCIs), offering substantial benefits for neuroregulation, motor recovery, and clinical applications. The integration of sensing and actuation, along with advancements in wireless communication and biocompatible materials, enhances the functionality and versatility of these systems. Despite significant progress, challenges remain in data processing, immune response management, and long-term stability. Future developments must focus on addressing these challenges through innovative electrode designs and improved materials to maximize the potential of implantable microelectrodes in both research and clinical settings, ultimately advancing patient care and neurotechnological applications.
Acknowledgments
This work was financially supported by the Applied Research Project of Public Welfare Technology of Zhejiang Province (2017xxxxxxx).
References
[1]. M. F. Mridha, S. C. Das, M. M. Kabir, A. A. Lima, M. R. Islam, and Y. Watanobe, “Brain-computer interface: advancement and challenges,” Sep. 01, 2021, MDPI. doi: 10.3390/s21175746.
[2]. T. Stieglitz, M. Schuetter, and K. P. Koch, “Implantable biomedical microsystems for neural prostheses,” IEEE Engineering in Medicine and Biology Magazine, vol. 24, no. 5, pp. 58–65, Sep. 2005, doi: 10.1109/MEMB.2005.1511501.
[3]. B. Zhang, C. Deng, C. Cai, and X. Li, “In Vivo Neural Interfaces—From Small- to Large-Scale Recording,” Frontiers in Nanotechnology, vol. 4, Jun. 2022, doi: 10.3389/fnano.2022.885411.
[4]. R. J. Vetter, J. C. Williams, J. F. Hetke, E. A. Nunamaker, and D. R. Kipke, “Chronic Neural Recording Using Silicon-Substrate Microelectrode Arrays Implanted in Cerebral Cortex,” IEEE Trans Biomed Eng, vol. 51, no. 6, pp. 896–904, Jun. 2004, doi: 10.1109/TBME.2004.826680.
[5]. E. M. Maynard, C. T. Nordhausen, and R. A. Normann, “The Utah Intracortical Electrode Array: A recording structure for potential brain-computer interfaces,” Electroencephalogr Clin Neurophysiol, vol. 102, no. 3, pp. 228–239, Mar. 1997, doi: 10.1016/S0013-4694(96)95176-0.
[6]. K. D. Wise, D. J. Anderson, J. F. Hetke, D. R. Kipke, and K. Najafi, “Wireless implantable microsystems: High-density electronic interfaces to the nervous system,” in Proceedings of the IEEE, Institute of Electrical and Electronics Engineers Inc., 2004, pp. 76–97. doi: 10.1109/JPROC.2003.820544.
[7]. T. J. Blanche, M. A. Spacek, J. F. Hetke, and N. V. Swindale, “Polytrodes: High-Density Silicon Electrode Arrays for Large-Scale Multiunit Recording,” J Neurophysiol, vol. 93, no. 5, pp. 2987–3000, May 2005, doi: 10.1152/jn.01023.2004.
[8]. E. B. Montgomery, “Deep brain stimulation for hyperkinetic disorders,” Neurosurg Focus, vol. 17, no. 1, pp. 1–8, Jul. 2004, doi: 10.3171/foc.2004.17.1.1.
[9]. M. Ninaus et al., “Neural substrates of cognitive control under the belief of getting neurofeedback training,” Front Hum Neurosci, vol. 7, 2013, doi: 10.3389/fnhum.2013.00914.
[10]. S. Halder et al., “Neural mechanisms of brain–computer interface control,” Neuroimage, vol. 55, no. 4, pp. 1779–1790, Apr. 2011, doi: 10.1016/j.neuroimage.2011.01.021.
[11]. M. Han, P. S. Manoonkitiwongsa, C. X. Wang, and D. B. McCreery, “In Vivo Validation of Custom-Designed Silicon-Based Microelectrode Arrays for Long-Term Neural Recording and Stimulation,” IEEE Trans Biomed Eng, vol. 59, no. 2, pp. 346–354, Feb. 2012, doi: 10.1109/TBME.2011.2172440.
[12]. A. B. Schwartz, X. T. Cui, D. J. J. Weber, and D. W. Moran, “Brain-Controlled Interfaces: Movement Restoration with Neural Prosthetics,” Oct. 05, 2006. doi: 10.1016/j.neuron.2006.09.019.
[13]. H.-L. Teulings, J. L. Contreras-Vidal, G. E. Stelmach, and C. H. Adler, “Parkinsonism Reduces Coordination of Fingers, Wrist, and Arm in Fine Motor Control,” Exp Neurol, vol. 146, no. 1, pp. 159–170, Jul. 1997, doi: 10.1006/exnr.1997.6507.
[14]. A. Williams et al., “Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson’s disease (PD SURG trial): a randomised, open-label trial,” Lancet Neurol, vol. 9, no. 6, pp. 581–591, Jun. 2010, doi: 10.1016/S1474-4422(10)70093-4.
[15]. M. S. Okun, “Deep-Brain Stimulation for Parkinson’s Disease,” New England Journal of Medicine, vol. 367, no. 16, pp. 1529–1538, Oct. 2012, doi: 10.1056/NEJMct1208070.
[16]. M. J. Morrell, “Responsive cortical stimulation for the treatment of medically intractable partial epilepsy,” Neurology, vol. 77, no. 13, pp. 1295–1304, Sep. 2011, doi: 10.1212/WNL.0b013e3182302056.
[17]. M. Ortiz-Catalan, B. Håkansson, and R. Brånemark, “An osseointegrated human-machine gateway for long-term sensory feedback and motor control of artificial limbs,” Sci Transl Med, vol. 6, no. 257, Oct. 2014, doi: 10.1126/scitranslmed.3008933.
[18]. D. A. Borton, M. Yin, J. Aceros, and A. Nurmikko, “An implantable wireless neural interface for recording cortical circuit dynamics in moving primates,” J Neural Eng, vol. 10, no. 2, p. 026010, Apr. 2013, doi: 10.1088/1741-2560/10/2/026010.
[19]. S. Ha et al., “Silicon-Integrated High-Density Electrocortical Interfaces,” Proceedings of the IEEE, vol. 105, no. 1, pp. 11–33, Jan. 2017, doi: 10.1109/JPROC.2016.2587690.
[20]. Y. Cho, S. Park, J. Lee, and K. J. Yu, “Emerging Materials and Technologies with Applications in Flexible Neural Implants: A Comprehensive Review of Current Issues with Neural Devices,” Advanced Materials, vol. 33, no. 47, Nov. 2021, doi: 10.1002/adma.202005786.
[21]. J. J. Jun et al., “Fully integrated silicon probes for high-density recording of neural activity,” Nature, vol. 551, no. 7679, pp. 232–236, Nov. 2017, doi: 10.1038/nature24636.
[22]. F. M. Petrini et al., “Six‐Month Assessment of a Hand Prosthesis with Intraneural Tactile Feedback,” Ann Neurol, vol. 85, no. 1, pp. 137–154, Jan. 2019, doi: 10.1002/ana.25384.
[23]. Y. Wang, X. Yang, X. Zhang, Y. Wang, and W. Pei, “Implantable intracortical microelectrodes: reviewing the present with a focus on the future,” Microsyst Nanoeng, vol. 9, no. 1, p. 7, Jan. 2023, doi: 10.1038/s41378-022-00451-6.
Cite this article
Wu,F.;Li,R.;Yue,X. (2025). Applications of Implantable Microelectrodes in Brain-Computer Interfaces. Applied and Computational Engineering,131,98-104.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 2nd International Conference on Machine Learning and Automation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. M. F. Mridha, S. C. Das, M. M. Kabir, A. A. Lima, M. R. Islam, and Y. Watanobe, “Brain-computer interface: advancement and challenges,” Sep. 01, 2021, MDPI. doi: 10.3390/s21175746.
[2]. T. Stieglitz, M. Schuetter, and K. P. Koch, “Implantable biomedical microsystems for neural prostheses,” IEEE Engineering in Medicine and Biology Magazine, vol. 24, no. 5, pp. 58–65, Sep. 2005, doi: 10.1109/MEMB.2005.1511501.
[3]. B. Zhang, C. Deng, C. Cai, and X. Li, “In Vivo Neural Interfaces—From Small- to Large-Scale Recording,” Frontiers in Nanotechnology, vol. 4, Jun. 2022, doi: 10.3389/fnano.2022.885411.
[4]. R. J. Vetter, J. C. Williams, J. F. Hetke, E. A. Nunamaker, and D. R. Kipke, “Chronic Neural Recording Using Silicon-Substrate Microelectrode Arrays Implanted in Cerebral Cortex,” IEEE Trans Biomed Eng, vol. 51, no. 6, pp. 896–904, Jun. 2004, doi: 10.1109/TBME.2004.826680.
[5]. E. M. Maynard, C. T. Nordhausen, and R. A. Normann, “The Utah Intracortical Electrode Array: A recording structure for potential brain-computer interfaces,” Electroencephalogr Clin Neurophysiol, vol. 102, no. 3, pp. 228–239, Mar. 1997, doi: 10.1016/S0013-4694(96)95176-0.
[6]. K. D. Wise, D. J. Anderson, J. F. Hetke, D. R. Kipke, and K. Najafi, “Wireless implantable microsystems: High-density electronic interfaces to the nervous system,” in Proceedings of the IEEE, Institute of Electrical and Electronics Engineers Inc., 2004, pp. 76–97. doi: 10.1109/JPROC.2003.820544.
[7]. T. J. Blanche, M. A. Spacek, J. F. Hetke, and N. V. Swindale, “Polytrodes: High-Density Silicon Electrode Arrays for Large-Scale Multiunit Recording,” J Neurophysiol, vol. 93, no. 5, pp. 2987–3000, May 2005, doi: 10.1152/jn.01023.2004.
[8]. E. B. Montgomery, “Deep brain stimulation for hyperkinetic disorders,” Neurosurg Focus, vol. 17, no. 1, pp. 1–8, Jul. 2004, doi: 10.3171/foc.2004.17.1.1.
[9]. M. Ninaus et al., “Neural substrates of cognitive control under the belief of getting neurofeedback training,” Front Hum Neurosci, vol. 7, 2013, doi: 10.3389/fnhum.2013.00914.
[10]. S. Halder et al., “Neural mechanisms of brain–computer interface control,” Neuroimage, vol. 55, no. 4, pp. 1779–1790, Apr. 2011, doi: 10.1016/j.neuroimage.2011.01.021.
[11]. M. Han, P. S. Manoonkitiwongsa, C. X. Wang, and D. B. McCreery, “In Vivo Validation of Custom-Designed Silicon-Based Microelectrode Arrays for Long-Term Neural Recording and Stimulation,” IEEE Trans Biomed Eng, vol. 59, no. 2, pp. 346–354, Feb. 2012, doi: 10.1109/TBME.2011.2172440.
[12]. A. B. Schwartz, X. T. Cui, D. J. J. Weber, and D. W. Moran, “Brain-Controlled Interfaces: Movement Restoration with Neural Prosthetics,” Oct. 05, 2006. doi: 10.1016/j.neuron.2006.09.019.
[13]. H.-L. Teulings, J. L. Contreras-Vidal, G. E. Stelmach, and C. H. Adler, “Parkinsonism Reduces Coordination of Fingers, Wrist, and Arm in Fine Motor Control,” Exp Neurol, vol. 146, no. 1, pp. 159–170, Jul. 1997, doi: 10.1006/exnr.1997.6507.
[14]. A. Williams et al., “Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson’s disease (PD SURG trial): a randomised, open-label trial,” Lancet Neurol, vol. 9, no. 6, pp. 581–591, Jun. 2010, doi: 10.1016/S1474-4422(10)70093-4.
[15]. M. S. Okun, “Deep-Brain Stimulation for Parkinson’s Disease,” New England Journal of Medicine, vol. 367, no. 16, pp. 1529–1538, Oct. 2012, doi: 10.1056/NEJMct1208070.
[16]. M. J. Morrell, “Responsive cortical stimulation for the treatment of medically intractable partial epilepsy,” Neurology, vol. 77, no. 13, pp. 1295–1304, Sep. 2011, doi: 10.1212/WNL.0b013e3182302056.
[17]. M. Ortiz-Catalan, B. Håkansson, and R. Brånemark, “An osseointegrated human-machine gateway for long-term sensory feedback and motor control of artificial limbs,” Sci Transl Med, vol. 6, no. 257, Oct. 2014, doi: 10.1126/scitranslmed.3008933.
[18]. D. A. Borton, M. Yin, J. Aceros, and A. Nurmikko, “An implantable wireless neural interface for recording cortical circuit dynamics in moving primates,” J Neural Eng, vol. 10, no. 2, p. 026010, Apr. 2013, doi: 10.1088/1741-2560/10/2/026010.
[19]. S. Ha et al., “Silicon-Integrated High-Density Electrocortical Interfaces,” Proceedings of the IEEE, vol. 105, no. 1, pp. 11–33, Jan. 2017, doi: 10.1109/JPROC.2016.2587690.
[20]. Y. Cho, S. Park, J. Lee, and K. J. Yu, “Emerging Materials and Technologies with Applications in Flexible Neural Implants: A Comprehensive Review of Current Issues with Neural Devices,” Advanced Materials, vol. 33, no. 47, Nov. 2021, doi: 10.1002/adma.202005786.
[21]. J. J. Jun et al., “Fully integrated silicon probes for high-density recording of neural activity,” Nature, vol. 551, no. 7679, pp. 232–236, Nov. 2017, doi: 10.1038/nature24636.
[22]. F. M. Petrini et al., “Six‐Month Assessment of a Hand Prosthesis with Intraneural Tactile Feedback,” Ann Neurol, vol. 85, no. 1, pp. 137–154, Jan. 2019, doi: 10.1002/ana.25384.
[23]. Y. Wang, X. Yang, X. Zhang, Y. Wang, and W. Pei, “Implantable intracortical microelectrodes: reviewing the present with a focus on the future,” Microsyst Nanoeng, vol. 9, no. 1, p. 7, Jan. 2023, doi: 10.1038/s41378-022-00451-6.









