1. Background and Introduction
Before getting into the topic of retrosynthesis, we have to have a clear understanding of: what exactly is retrosynthesis? Retrosynthesis, in essence, is a method used in chemistry that involves deconstructing a target molecule to determine the starting materials needed for its synthesis. To illustrate, consider the task of constructing a triangle. One might suggest using sticks and glue, or perhaps assembling smaller triangles to form a larger one of equivalent area. There are countless methods to achieve the same outcome. Similarly, in retrosynthesis, chemists approach the problem by working backward from the desired molecule, exploring various pathways to construct it from available starting materials. This backward-thinking approach not only fosters creativity but also aids in the efficient synthesis of complex molecules.


Figure 1&2: Methods of how to construct a triangle
2. Methods of Retrosynthesis
Let us now turn to one of the most critical aspects of retrosynthesis: the methodologies employed in this process. Drawing on the earlier example of constructing a triangle, we approached the task by breaking it down into smaller components. In a similar manner, a fundamental strategy in retrosynthesis is the selective breaking of chemical bonds. The goal is to identify and cleave the appropriate bond to simplify the target molecule into more manageable fragments. Consider, for instance, the mortise and tenon structure in woodworking. The most effective way to separate the two connected pieces is to cut at the joint; attempting to do so elsewhere would prove significantly more challenging. Thus, the careful selection of which bond to break is crucial in retrosynthesis. The question then arises: how do we determine the correct bond to break? The following methods provide some guidance in this regard.
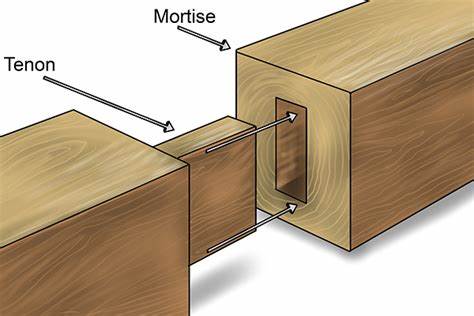
Figure 3: Breaking a mortise and tenon structure from the joint
2.1. Bond-breaking
2.1.1. Classification: Heterolysis and homolysis
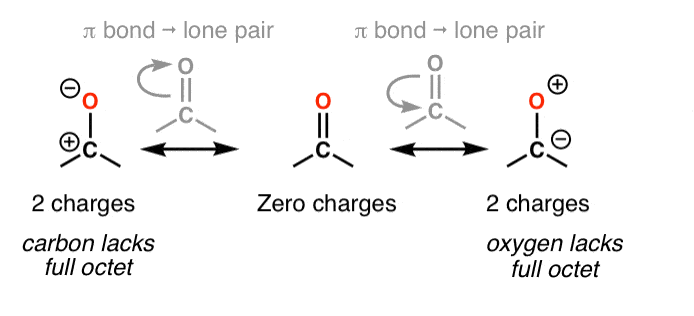
To begin, bond-breaking strategies can be categorized based on the transfer of electrons, namely into heterolysis and homolysis. Heterolysis involves the complete transfer of an electron pair, resulting in the formation of ions, whereas homolysis entails the equal splitting of an electron pair, leading to the creation of two radicals. In the context of retrosynthesis, heterolysis is the more prevalent method, as it produces synthons—molecular fragments that are either anionic or cationic. However, because these charged synthons cannot exist independently in their ionic forms, they must be paired with neutral counterparts in actual total synthesis. This is where the concept of synthetic equivalence comes into play. Identifying the appropriate synthetic equivalents for each type of synthon is a fundamental aspect of organic synthesis. Below are some examples that illustrate this concept.
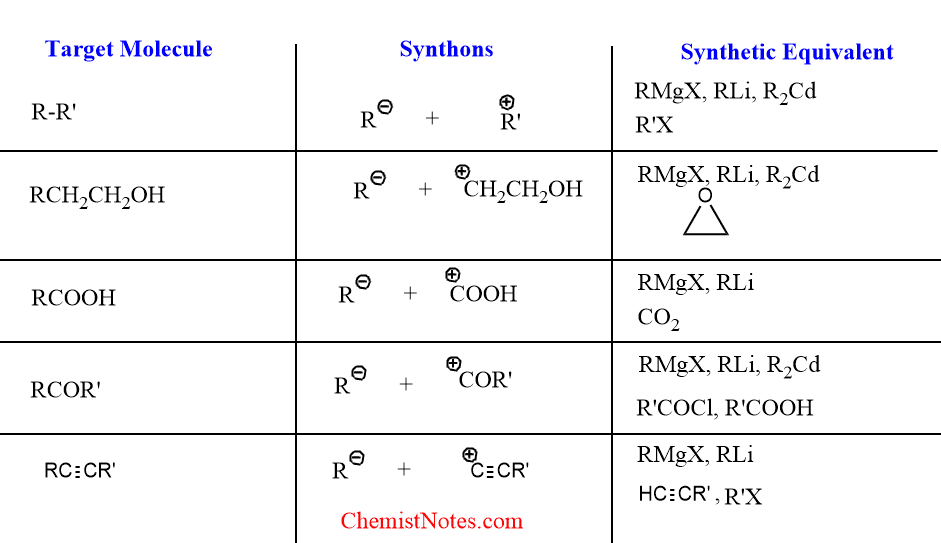
Figure 4: Examples of synthons [1]
2.1.2. One-functional-group disconnection for heterolysis
To further elaborate on heterolysis, it can primarily be divided into two types: one-functional-group disconnection and two-functional-group disconnection (which are also known as one-group-disconnection and two-group-disconnection for abbreviation). In the case of one functional-group disconnection, it is helpful to consider the resonance forms of the molecule. Referring to Figure 6, we observe the ketone and its resonance form, particularly the one on the left, where the oxygen carries a negative charge and the carbon carries a positive charge. In this configuration, the oxygen can be identified as a hydroxyl functional group (with an additional hydrogen). Thus, when breaking bonds, through a process known as Functional Group Interconversion (FGI), which will be discussed later, the ketone can be transformed into a hydroxyl group with a positively charged connected carbon, consistent with the resonance form of the ketone.
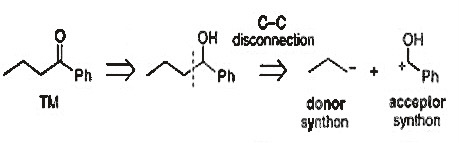
Figure 5: Example of one group disconnection
Figure 6: Resonance form of ketone [2]
2.1.3. Two-functional-group disconnection for heterolysis
For two functional-group disconnection, we can analyze it from the perspective of the consonant pattern. In this scenario, the two functional groups involved are hydroxyl and ketone (Figure 7). Given that oxygen is an electronegative atom, it tends to attract the shared electron pair towards itself, creating a partially positive charge on the carbon to which it is connected. Consequently, this partially positive carbon draws electrons from the adjacent carbon, imparting a partially negative charge to it. This results in a positive-negative-positive pattern, referred to as a consonant pattern, which facilitates the bond-breaking process. It is important to note that bonds corresponding to a dissonant pattern are more difficult to break than those in a consonant pattern.

Figure 7: Example of two-group disconnection [3]
2.2. Other strategies besides bond-breaking
However, bond-breaking alone is not sufficient to address all challenges in retrosynthesis, prompting the introduction of additional strategies.
2.2.1. Fine-tuning
One such strategy is fine-tuning, which involves making small adjustments during the retrosynthesis process. While fine-tuning alters the problem at hand, it does not necessarily simplify it. Within this approach, we have Functional Group Interconversion (FGI), which transforms one functional group into another; Functional Group Addition (FGA), which introduces a new functional group; and Functional Group Removal (FGR), which eliminates a functional group, although it is crucial to understand how this group will be reintroduced during total synthesis.
2.2.2. Electrocyclic disconnection
Electrocyclic disconnection is another method, which involves the reverse process of cycloaddition by reorganizing and breaking bonds through the movement of electrons within a ring. An example of this is the reverse process of Diels-Alder reaction [4].
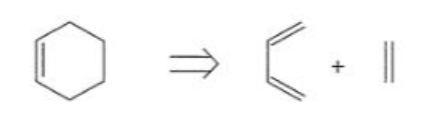
Figure 8: Reverse process of Diels-Alder reaction
2.2.3. Illogical disconnection
Illogical disconnection is a method that paradoxically simplifies the molecule by forming new bonds.
2.2.4. Rearrangement
Rearrangement strategies are employed to further refine the retrosynthetic pathway by rearranging the molecule with certain conditions.
3. Guiding Principles
3.1. Focus on Simplification
The first guiding principle is that we should always focus on simplification of the molecule. Since methods such as fine-tuning can only shift the problem instead of simplifying them, we should focus on simplification by reducing reliance on fine-tuning and taking advantage of symmetry to streamline the process.
3.2. Most Reactive Functional Group Needs to be Cut Off the First
The second guiding principle is that the most reactive functional group should be cut off first, as these groups are typically the last to be introduced in a forward synthesis. To use a house-building analogy, if we consider the carbon-carbon skeleton as the framework of the house, then the bonds to heteroatoms can be likened to the furniture. So frame first, funiture last. And in backwards process, which is retrosynthesis, furniture first, then framework.
3.3. Two Group Disconnection is Usually Better than One Group Disconnection
Last but not the least, the third guiding principle is that two-functional-group disconnections are generally preferred over one functional-group disconnections. Due to the presence of consonant patterns, it is often easier to break two functional groups apart.
4. Real world example
With reference to a real-life example, we can analyze how we can do retrosynthesis with the methods mentioned above.
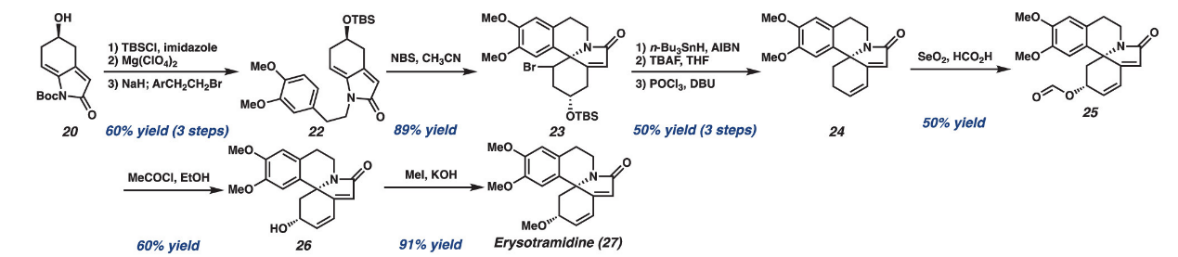
Figure 9: Real-life total synthesis process [5]
Firstly, we can start our retrosynthesis by analyzing which molecule is the target molecule, which is the starting material, and what are the similarities between them to therefore think of a process to retrosynthesize it.

Figure 10: Starting material (left) and target molecule (right)
The figure shown above are the starting material (left) and target molecule (right). The two parts circled are the similarity between the two molecules since them have similar ring structures and consist similar elements.
So our target is to remain this structure in the target molecule, not cutting it, and try to convert it to the ring structure in the starting material.
Since this functional group, MeO, does not appear in the starting material, we have to cut this off. With reference to guiding principle 2, most reactive cut off first. Since MeO is not that reactive for us to directly cut off, we use FGI to convert this ether into hydroxyl and then into CO2, a much more reactive functional group, which is a lot easier to cut off.

Figure 11: Methodology of how to convert MeO into CO2
After removing the functional group, CO2, by FGR, via FGA, we introduce a bromine (third molecule) for the construction of a double bond in the next step (second molecule) and an OTBS (third molecule) to change into hydroxyl functional group (second molecule). So next, we use electrocyclic and bond breaking to from this double bond (second molecule) and break the ring structure in the third molecule. Finally, by breaking the bond in the second molecule and with a FGI, we got the starting material.
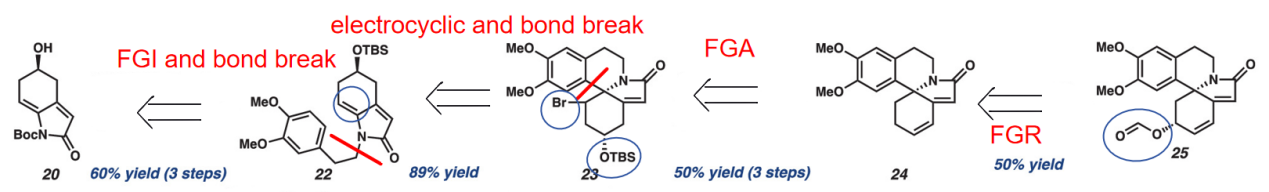
Figure 12: Methodology after removing MeO into the starting material
References
[1]. ChemistRn. "Synthon and synthetic equivalent: Definition, easy examples." ChemistNotes, July 10, 2022 https://chemistnotes.com/organic/synthon-and-synthetic-equivalent/
[2]. James Ashenhurst. "In Summary: Evaluating Resonance Structures." Master Organic Chemistry, September 23, 2024 https://www.masterorganicchemistry.com/2011/12/22/in-summary-resonance/
[3]. Dr. Biswajit Panda. "Retrosynthesis." March 20, 2022 https://www.citycollegekolkata.org/documents/online_course_materials/Retrosyntesis_Biswajit_Pandal.pdf
[4]. Diels, O.; Alder, K.; Stein, G.; Pries, P.; Winckler, H. (1929). "Synthesen in der hydroaromatischen Reihe, VI. Mitteilung, Kurt Alder und Gerhard Stein: Über partiell hydrierte Naphtho- und Anthrachinone mit Wasserstoff in γ- bzw. δ-Stellung. (Mitbearbeitet von Paul Pries und Hans Winckler)". Chem. Ber. 62 (8): 2337.
[5]. Kim AN, Ngamnithiporn A, Du E, Stoltz BM. Recent Advances in the Total Synthesis of the Tetrahydroisoquinoline Alkaloids (2002-2020). Chem Rev. 2023 Aug 9;123(15):9447-9496.
Cite this article
Shi,Y. (2025). Basics of Retrosynthesis Analysis. Applied and Computational Engineering,136,85-91.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 5th International Conference on Materials Chemistry and Environmental Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. ChemistRn. "Synthon and synthetic equivalent: Definition, easy examples." ChemistNotes, July 10, 2022 https://chemistnotes.com/organic/synthon-and-synthetic-equivalent/
[2]. James Ashenhurst. "In Summary: Evaluating Resonance Structures." Master Organic Chemistry, September 23, 2024 https://www.masterorganicchemistry.com/2011/12/22/in-summary-resonance/
[3]. Dr. Biswajit Panda. "Retrosynthesis." March 20, 2022 https://www.citycollegekolkata.org/documents/online_course_materials/Retrosyntesis_Biswajit_Pandal.pdf
[4]. Diels, O.; Alder, K.; Stein, G.; Pries, P.; Winckler, H. (1929). "Synthesen in der hydroaromatischen Reihe, VI. Mitteilung, Kurt Alder und Gerhard Stein: Über partiell hydrierte Naphtho- und Anthrachinone mit Wasserstoff in γ- bzw. δ-Stellung. (Mitbearbeitet von Paul Pries und Hans Winckler)". Chem. Ber. 62 (8): 2337.
[5]. Kim AN, Ngamnithiporn A, Du E, Stoltz BM. Recent Advances in the Total Synthesis of the Tetrahydroisoquinoline Alkaloids (2002-2020). Chem Rev. 2023 Aug 9;123(15):9447-9496.