1. Introduction
Fabric softeners, typically formulated with cationic surfactants, emerged in the 1950s as a countermeasure to the abrasions inflicted by synthetic detergents. Now, households use fabric softeners to soften apparel and provide fragrance, deodorization, and bacterial resistance to clothes. Despite their widespread use, the producing of fabric softeners and surfactants is still environmentally hazardous. Furthermore, current products have potential side effects, such as causing skin irritation and reducing the clothes’ water and air permeability. As a result, modern research aims to lower the ecotoxicity of fabric softeners while optimizing their effectiveness. Numerous theories exist regarding the mechanism of these products. However, studies still have not fully confirmed the exact mechanism of cationic surfactants. In terms of engineering eco-friendly alternatives, while the process of producing fabric softeners has been redesigned to minimize the addition of non-biodegradable compounds and several replacements have been proposed (such as esterquats), researchers have not yet found an alternative product that is more eco-friendly and equally effective as traditional products. This paper synthesizes the prevailing theories on the mechanism of fabric softeners, clarifies the environmental issues that fabric softeners imply, and evaluates the efficacy of the use of esterquats, a widely considered alternative for fabric softeners. Through a comprehensive literature review, it aims to provide guidance for future research, emphasizing the need to reduce the environmental footprint of fabric softeners and explore the potential of esterquats as sustainable solutions.
2. Historical Context
The concept of softening agents can be traced back to prehistoric times. During this era, people used fats and oils to condition skins and hides [1]. However, it was not until the 1930s that the application of cationic surfactants as fabric softeners was discovered accidentally during an experiment on the effects of conditioning agents for cotton fibers [2]. In 1955, the first household fabric softeners hit the American market [2]. Subsequently, in 1962, the Japanese market introduced the Kao Softer, the first softener with both fabric softening and antistatic properties [2]. Modern fabric softeners are mostly classified as cationic surfactants. Apart from softening apparel, fabric softeners nowadays also impart fragrance, deodorization, and bacterial resistance [2].
The industrial production process of surfactant components has also undergone significant evolution. A surfactant consists of a hydrophilic head and a hydrophobic tail. One way to produce the hydrophobic chain is to polymerize short-chain olefins, particularly propylene [3]. The trimerization of propylene produces an alpha-olefin, which is used as an alkylate in a Friedel-Crafts reaction, producing an alkyl benzene. Through sulfonation and neutralization, an alkyl-benzene sulfonate is produced at a lower cost than soap bars formed from natural oil and fat [3]. However, the branched alkylate is difficult to biodegrade, so most nations have banned it and replaced it with linear counterparts [3]. Instead, they are introduced into the synthesis process in the form of either alkyl benzenes or alkyl phenols. Ethoxylated alkyl phenols were quite popular in the 1970s - 80s for liquid dishwashing applications. Nevertheless, they have been found to pose toxicity problems and are now being replaced substitutes. While these alcohol substitutes are more environmentally friendly, they do not match the effectiveness of the previous compounds [3]. Over the years, the major challenge of this process has been to find biodegradable, non-petrochemical, and equally effective substitutes.
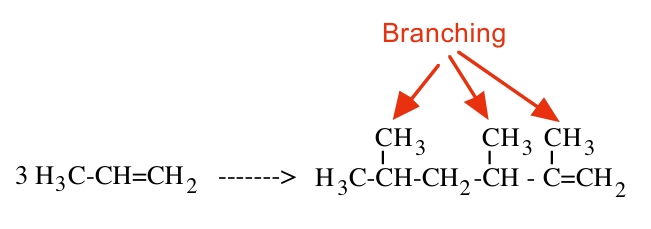
Figure 1: The Trimerization of Propylene [3]
3. Mechanism
3.1. The Softening Effect
A surfactant molecule consists of a hydrophilic head and a hydrophobic tail. In a solution, the molecules self-organize into micelles. In this structure, the hydrophobic chains are oriented inwards, while the hydrophilic heads are exposed to the surrounding polar environment. Surfactants can be categorized into nonionic, anionic, cationic, and amphoteric surfactants depending on the type of charge their hydrophilic heads carry. When employed as fabric softeners, surfactant molecules, typically cationic ones, function to soften the fabric by reducing the friction between fibers. After water wets cotton fibers, the bound water between the cotton fibers forms cross-linkages. This increases the hardness of cotton threads. In general, fabric softeners prevent the formation of this cross-linkage [2]. The negatively charged surfaces of the fibers absorb cationic micelles of the softener molecules through electrostatic interactions [2]. During the drying process, the micelles collapse, forming a monolayer of protective film. The alkyl groups facing the air now have lower surface energy, which lowers the inter-fiber friction. The details of this conventional explanation are explained in Figure 2. Crutzen argues that the hydrophobic long-chain alkyl groups drive the process of absorption [4]. Thus, theoretically, the same mechanism can be applied to other non-charged fabric surfaces. For instance, DTDMAC/quat (ditallowdimethylammonium chloride) exhibits a strong affinity for cellulose. However, it binds to cellulose via London dispersion forces rather than electrostatic binding.
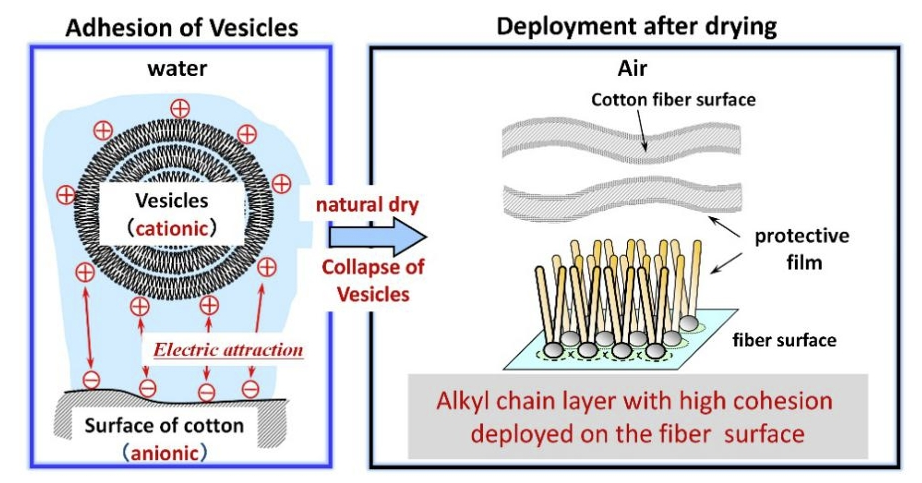
Figure 2: The Conventional Explanation of the Softening Effect [2]
3.2. Other Properties
Apart from softening fabric, fabric softeners also have important properties. Firstly, they serve as effective antistatic agents [1]. This property is likely attributed to the bonding that occurs between the softener molecules and the fabric. It is believed hat the hydrophilic part of the softener forms a layer of moisture on the fabric surface that captures large particles and dissipates them [1]. Secondly, fabric softeners tend to cause yellowing and color change in fabrics [1]. Research indicates that certain organic and silicone - based softeners are prone to inducing yellowing after repeated use [5]. Additionally, leached-out optical brightening agents (OBA) can also interact with these softeners to induce precipitation or spots on the fabric [5]. Finally, the aging of softeners and the fabric itself can also add to the yellowing effect [5]. Finally, fabric softeners can extend fabric life by making the fabric more resistant to abrasion and tearing [1]. However, this area of study remains highly contentious as the research community has yet to reach a unified conclusion. Test results [1] indicate that abrasion resistance is improved because the fibers face less stress when softeners are applied. Furthermore, cationic softeners have been confirmed to improve fiber elongation and tear strength [1]. Tear strength appears to increase with all fibers except rayon, and it has been theorized [6] that the lubricant coating provided by the hydrophobic tails additionally protects the fabric against heat. Nevertheless, the exact effects of fabric softeners on these miscellaneous properties require further research.
4. Strengths & Weaknesses
4.1. Strengths & Additional Features
The strengths of using fabric softeners are evident. They make the fabric feel “softer”, disperse electrostatic molecules, deliver fragrance, and extend garment life. Household softeners are more diverse to meet different customer demands. For instance, modern softeners often coat yarn and fiber with both lubricants and humectants [7]. The lubricants make the fabric smooth and soft, whereas the humectants retain moisture for antistatic purposes. Variants of softeners are also developed to be more compatible with the washing process. For example, dryer sheet softeners are minorly different from other cationic compounds to “ensure compatibility” with the clothes dryer [7]. Dryer sheet softeners impart less lubrication and therefore less softening than rinse cycle softeners; however, they reduce static cling to a greater extent than rinse cycle softeners [7]. In addition, manufacturers have been incorporating new features into products to mitigate side effects. Fabric pilling, also known as surface fuzz, is a phenomenon that makes fabrics look “dull” [7]. Nevertheless, some laundry product brands have added cellulase enzymes, which degrade the formation of microfibrils that detach from the main fiber, to reduce fabric pilling [8]. Apart from cationic softeners, nonionic softeners have also gained much popularity because of their compatibility with different chemicals [9]. Nonionic softeners carry no electrical charge, making them suitable for a wide range of fabrics. They are stable across different temperature and do not cause yellowing of fabrics [10]. These advantages make nonionic softeners ideal for maintaining optically brightened white fabrics [10]. Compared to cationic and silicone softeners, nonionic softeners perform better at retaining water and vapor permeability. This will be discussed later in this paper.
4.2. Weaknesses
4.2.1. Environmental Concerns
The production of surfactants raises environmental concerns. Surfactants in general can be detrimental to cellular functions. On a cellular level, anionic surfactants change the folding of proteins by binding to bioactive macromolecules [11]. This modifies biological functions. Nonionic surfactants increase the permeability of membranes and vesicles, causing cell death and loss of ions/amino acids [11]. Among cationic surfactants, the most prevalent type is quaternary ammonium compounds (QAC). These are composed of hydrophobic hydrocarbon chains connected to a hydrophilic nitrogen-containing head [11]. Hydrophobic interactions play a role in surfactant toxicity. Specifically, QACs disrupt the inner membranes of cells using their alkyl chains [11].
Surfactants are unselective at attacking cellular structures, which compromises organismic health and other industrial processes. Although cationic surfactants are widely utilized for their antibacterial properties, the excessive use and improper disposal of any type of surfactants can severely impair the ecosystem [11]. For example, oil dispersant mixtures containing anionic and nonionic surfactants appear toxic to brown algae (Macrocystis pyrifera) [11]. Dodecylbenzene sodium sulfonate, an anionic surfactant, modifies the behavior of catfish by lowering the level of lipids in its cells [11]. Furthermore, surfactants can disrupt wastewater treatment. Even if most of the cationic surfactants are degradable under aerobic conditions, their biodegradability decreases when more non-methyl alkyl groups are added to the overall molecule [11]. A lower biodegradability implies a faster rate of accumulation of surfactants in sewage and wastewater. There is a concern [11] that high concentrations of surfactants in sewage sludge prevent the microorganisms from breaking down pollutants, calling for regulation on the level of disposal of popular surfactants.
4.2.2. Product Side Effects
Although mainstream brands have optimized their products, the surfactants employed in fabric softeners are still prone to causing side effects, such as skin irritation. Raw surfactants are known to trigger irritant skin reactions [12]. Research findings [12] indicate that when surfactants bind to keratins, which form the outermost layer of the skin, the cell membrane swells. This swelling is a probable sign of skin irritation. Moreover, certain substances within surfactant-based products are cytotoxic. For instance, a 1% solution of benzalkonium chloride (BAC), used for cleaning polyethylene and nylon tubing, alters the permeability of the skin, particularly the water-holding capacity of the stratum corneum [12]. Additionally, BAC has been found to be cytotoxic to human keratinocytes [12]. Most fabric softeners also affect the water and air permeability of the fabric. A “comfortable” fabric should be able to reduce the humidity of the skin by releasing the moisture into the surroundings as the body sweats or stops sweating [9]. The thermal comfort of fabric is dependent on its ability to exchange air and vapor with the external environment. Experiment results [9] show that fabric softeners decrease both air and water permeability, with nonionic surfactants retaining more permeability than cationic and silicone surfactants.
5. Esterquats as a Solution
Most of the proposed solutions focus on finding “greener” alternatives for current fabric softener products that are more biodegradable and less ecotoxic. Multiple studies [13] point out that esterquats can improve the softeners’ biodegradability by replacing dialkyldimethylammonium surfactants. Esterquats are QACs with two fatty acid chains and two weak ester linkages [13]. Common types of esterquats can be classified into choline-type and betaine-type [14]. Under ambient conditions, the ester-amide bonds are abiotically degradable under ambient conditions, which facilitates further biodegrading. As a result, esterquats exhibit favorable hydrolysis resistance and high biodegradability [15]. Furthermore, they are good antibacterial agents and are more prone to being absorbed into the bacterial cell membrane than traditional softeners [14]. Betaine-type esterquats can bind to the fabric surface through esterification, allowing the fabric to exhibit excellent antibacterial properties. Finally, esterquats are diverse chemical structures that can be modified to different needs [14]. Nevertheless, there is also a substantial lack of literature regarding the effects of esterquats on the environment. For instance, researchers [14] doubt whether every type of esterquat is as “less ecotoxic” as claimed. In the hydrolysis process of esterquats, free choline is converted into glycine betaine. During this process, formaldehyde and other toxic compounds may be released into the environment [14]. The toxicity of esterquats on other organisms is unclear, making it hard to arrive at a definitive conclusion [14]. Furthermore, while some esterquats are indeed less toxic than conventional QACs, others, such as DEEDMAC, an esterquat that has replaced DTDMAC for treating cellulose fabrics, are proven to be “harmful to aquatic life with long-lasting effects” [14].
6. Conclusion
Modern fabric softeners predominantly rely on cationic surfactants. Beyond softening fabric and countering the effects of using synthetic detergents, most products offer antibacterial properties, reduce electrostatic forces, and impart pleasant scents to clothing. However, both the production and usage of fabric softeners contain potential drawbacks, such as raising environmental concerns and affecting the fabric’s permeability and thermal comfort. The popular solution to address these drawbacks is the usage of esterquats, which are theoretically greener and more efficient than conventional QACs. Generally speaking, esterquats are promising alternatives for developing the next generation of fabric softeners in line with pro - ecological initiatives. However, further research is essential to accurately determine their toxicity, given the significant gap in literature. To improve the softeners’ biodegradability, future research should consider the further investigation and refinement of esterquats’ physiochemical properties. Studies should be conducted to examine the esterquats’ toxicity in relation to common organisms. In addition to all these aspects, the industry must conduct a cost-benefit analysis when scaling up the production of the new-generation fabric softeners. This paper faces three major limitations. Firstly, it only discusses esterquats as the alternative for current fabric softeners. There are many approaches to improving fabric softeners’ biodegradability and effects on fabric. Secondly, this paper does not provide a conclusive literature review on improving the fabric’s air and water permeability. Instead, it merely implies that nonionic surfactants are better at retaining air and water permeability than cationic and silicone surfactants. Finally, this paper does not further its investigation regarding the refinement of the production of the conventional industrial process of producing fabric softener products. The three mentioned limitations all require further research and literature to support and clarify.
References
[1]. A. R. Tickel, “THE EVALUATION OF THREE TYPES OF FABRIC SOFTENER,” 1974.
[2]. T. Igarashi and K. Nakamura, “Mechanism of Softening Effect of Fabric Softener,” Journal of Materials Science Research, vol. 8, no. 1, p. 35, Dec. 2018, doi: 10.5539/jmsr.v8n1p35.
[3]. J.-L. Salager, “Surfactants Types and Uses,” 2002.
[4]. A. M. Crutzen, “Study of the ditallowdimethylammonium chloride interaction with cellulose,” Journal of the American Oil Chemists’ Society, vol. 72, no. 1, pp. 137–143, Jan. 1995, doi: 10.1007/BF02635791.
[5]. D. Naresh, M. Saraf, and D. v Alat, “Effect of pH on Whiteness Index Effect of OBA Conc on Whiteness Index Yellowing of White Fabrics and Garments Yellowing of White Fabrics and Garments,” Aug. 2006. [Online]. Available: www.textilebookstore.com
[6]. W. S. Sollenberger, “Cationic Softeners—Their Secondary Effects on Textile Fabrics,” American Dyestuff Reporter, Jan. 1957.
[7]. A. Chiweshe and P. Crews, “Influence of Household Fabric Softeners and Laundry Enzymes on Pilling and Breaking Strength,” Sep. 2000.
[8]. T. Sooksai, “Production and characterization of cutinase from tropical fungi Production and characterization of cutinase from tropical fungi and application with detergent for stain and fuzz removal from and application with detergent for stain and fuzz removal from spun polyester fabrics spun polyester fabrics,” 2018. [Online]. Available: https://digital.car.chula.ac.th/chulaetd/2171
[9]. U. K. Sahin and S. Cimilli Duru, “EFFECTS OF SOFTENER APPLICATIONS ON AIR AND WATER VAPOR PERMEABILITY OF COTTON KNITTED FABRICS PRODUCED WITH DIFFERENT YARNS,” Apr. 2017.
[10]. K. Nostadt and R. Zyschka, “Softeners in the textile finishing industry,” Jan. 1997.
[11]. T. Ivanković and J. Hrenović, “Surfactants in the environment,” Arhiv za Higijenu Rada i Toksikologiju, vol. 61, no. 1. pp. 95–110, Mar. 01, 2010. doi: 10.2478/10004-1254-61-2010-1943.
[12]. . Effendy and H. I. Maibach, “Surfactants and experimental irritant contact dermatitis,” Contact Dermatitis, vol. 33, no. 4. pp. 217–225, 1995. doi: 10.1111/j.1600-0536.1995.tb00470.x.
[13]. E. K. Oikonomou, N. Christov, G. Cristobal, C. Bourgaux, I. Boucenna, and J.-F. Berret, “Design of biopolymer based eco-friendly fabric softeners,” 2019.
[14]. M. Wysocki, W. Stachowiak, M. Smolibowski, A. Olejniczak, M. Niemczak, and J. L. Shamshina, “Rethinking the Esterquats: Synthesis, Stability, Ecotoxicity and Applications of Esterquats Incorporating Analogs of Betaine or Choline as the Cation in Their Structure,” International Journal of Molecular Sciences, vol. 25, no. 11. Multidisciplinary Digital Publishing Institute (MDPI), Jun. 01, 2024. doi: 10.3390/ijms25115761.
[15]. S. Mishra and V. K. Tyagi, “Biodegradable Ester-Amide Fabric Softeners,” Jan. 2006. [Online]. Available: http://jos.jstage.jst.go.jp/en/
Cite this article
Yin,Z. (2025). The Chemistry Behind Softeners: A Literature Review on Surfactants. Applied and Computational Engineering,143,25-30.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Functional Materials and Civil Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. A. R. Tickel, “THE EVALUATION OF THREE TYPES OF FABRIC SOFTENER,” 1974.
[2]. T. Igarashi and K. Nakamura, “Mechanism of Softening Effect of Fabric Softener,” Journal of Materials Science Research, vol. 8, no. 1, p. 35, Dec. 2018, doi: 10.5539/jmsr.v8n1p35.
[3]. J.-L. Salager, “Surfactants Types and Uses,” 2002.
[4]. A. M. Crutzen, “Study of the ditallowdimethylammonium chloride interaction with cellulose,” Journal of the American Oil Chemists’ Society, vol. 72, no. 1, pp. 137–143, Jan. 1995, doi: 10.1007/BF02635791.
[5]. D. Naresh, M. Saraf, and D. v Alat, “Effect of pH on Whiteness Index Effect of OBA Conc on Whiteness Index Yellowing of White Fabrics and Garments Yellowing of White Fabrics and Garments,” Aug. 2006. [Online]. Available: www.textilebookstore.com
[6]. W. S. Sollenberger, “Cationic Softeners—Their Secondary Effects on Textile Fabrics,” American Dyestuff Reporter, Jan. 1957.
[7]. A. Chiweshe and P. Crews, “Influence of Household Fabric Softeners and Laundry Enzymes on Pilling and Breaking Strength,” Sep. 2000.
[8]. T. Sooksai, “Production and characterization of cutinase from tropical fungi Production and characterization of cutinase from tropical fungi and application with detergent for stain and fuzz removal from and application with detergent for stain and fuzz removal from spun polyester fabrics spun polyester fabrics,” 2018. [Online]. Available: https://digital.car.chula.ac.th/chulaetd/2171
[9]. U. K. Sahin and S. Cimilli Duru, “EFFECTS OF SOFTENER APPLICATIONS ON AIR AND WATER VAPOR PERMEABILITY OF COTTON KNITTED FABRICS PRODUCED WITH DIFFERENT YARNS,” Apr. 2017.
[10]. K. Nostadt and R. Zyschka, “Softeners in the textile finishing industry,” Jan. 1997.
[11]. T. Ivanković and J. Hrenović, “Surfactants in the environment,” Arhiv za Higijenu Rada i Toksikologiju, vol. 61, no. 1. pp. 95–110, Mar. 01, 2010. doi: 10.2478/10004-1254-61-2010-1943.
[12]. . Effendy and H. I. Maibach, “Surfactants and experimental irritant contact dermatitis,” Contact Dermatitis, vol. 33, no. 4. pp. 217–225, 1995. doi: 10.1111/j.1600-0536.1995.tb00470.x.
[13]. E. K. Oikonomou, N. Christov, G. Cristobal, C. Bourgaux, I. Boucenna, and J.-F. Berret, “Design of biopolymer based eco-friendly fabric softeners,” 2019.
[14]. M. Wysocki, W. Stachowiak, M. Smolibowski, A. Olejniczak, M. Niemczak, and J. L. Shamshina, “Rethinking the Esterquats: Synthesis, Stability, Ecotoxicity and Applications of Esterquats Incorporating Analogs of Betaine or Choline as the Cation in Their Structure,” International Journal of Molecular Sciences, vol. 25, no. 11. Multidisciplinary Digital Publishing Institute (MDPI), Jun. 01, 2024. doi: 10.3390/ijms25115761.
[15]. S. Mishra and V. K. Tyagi, “Biodegradable Ester-Amide Fabric Softeners,” Jan. 2006. [Online]. Available: http://jos.jstage.jst.go.jp/en/









