1. Introduction
The interface electronic coupling phenomena usually refer to the interaction between electronic states at the interface of two materials, and this phenomenon could also cause charge transfer and energy transfer at the interface of these materials. However, since human beings are facing serious systemic challenges confronting humanity across critical domains spanning energy security and climate governance, it’s crucial to develop and use clean energy. And the solid-state lithium battery shows great potential as an energy resource. However, the solid-state lithium battery has some deadly defects, like low efficiency and so on. This research’s conclusion would help to solve the problem that the common battery’s efficiency is excessively low and to improve the battery’s energy transportation property. This thesis mainly discusses the interface electronic coupling phenomena of the 2D MXene materials and their heterostructures and the reaction mechanism. And also discuss how this phenomenon works to improve the properties of the battery. This research is conducted by the document analysis method. This research could provide those researchers with a new train of thought to discover a new method to improve the efficiency and properties of the solid-state lithium metal batteries. As for the reference significance for researchers on related subjects in the future, this thesis could be seen as a summary of previous research and conclusions, which can help them easily get to know about the research’s rate of progress and the previous conclusions.
2. The structure and property of the MXene material
The MXene material is a kind of two-dimensional transition metal carbides, nitrides, or carbonitrides derived from MAX-phase ceramics. In microscopic conditions, the MXene materials’ microstructure usually emerges as a layered structure, with each layer possessing atomic-scale thickness. Each layer of MXene is connected by the Van der Waals forces. So the shape of the three-dimensional structure of the MXene material is just featured as a cylinder. Besides the three-dimensional structure, the surface of the MXenes is terminated with chemical functional groups, and these functional groups are crucial in deciding the MXene’s chemical properties like reactivity, hydrophilicity and electrical conductivities. Also, MXene typically exhibits a hexagonal symmetric structure, but some other MXenes may exhibit slight variations in symmetry due to differences in their composition.
3. The way to construct the heterostructure
One fundamental way to construct the heterostructure of the MXene is that MXene can composite with other 2D materials. In this research, MXene is composited with the graphene, which is a flat sheet with the carbon atoms arranged in a honeycomb lattice. In this substance, the great conductivity of the graphene and the high surface area and great electrochemical performance of MXene are combined. In other words, the heterostructure graphene composite with the MXene can be used in the supercapacitors and lithium-ion battery’s electrodes to improve the conductivity and other properties [1-2] In order to improve the property of the battery’s cathode, researchers would use the Van der Waals force to build heterostructures on the surface of different two-dimensional materials. Lately, Son and other researchers have composited a heterostructure of MXene and MoS2, it’s believed that this material possesses great electrical properties, could motivate the adsorption of the lithium ions, and has good circulatory stability [3].
4. The application of the MXene in solid-state lithium metal battery
4.1. The work principle of the solid-state lithium metal battery
The fundamental work principle of the solid-state lithium metal battery is just like the work principle of the ordinary chemical battery, but one different thing between the ordinary one and this solid-state lithium metal battery is that solid-state lithium battery uses a solid-state electrolytem to transfer to electrons (see Figure 1). The underlying logic of the solid-state lithium metal battery is the the insertion and detachment of ions in cathodes and anodes.
However, when the Liquid electrolyte is replaced by the solid electrolyte, the way ions transfer would also change. The ionic conductivity of solid electrolytes is usually lower than that of liquid electrolytes, so the optimized MXene material is very essential to improve the transfer efficiency of the lithium ions.
Take the currently commercialized battery C/LiCoO2 as an example, the material of the cathode is graphite, while the material of the anode is LiCoO2. The most common electrolyte of this type of battery is LiPF6, and the fundamental electrode reactions are listed as follows [4]:
Cathode: C + xLi + xe- → LixC
Anode: LiCoO2 → Li1-xCoO2 + xLi+ +xe-
Battery: LiCoO2 + C → Li1-xCoO2 + LixC
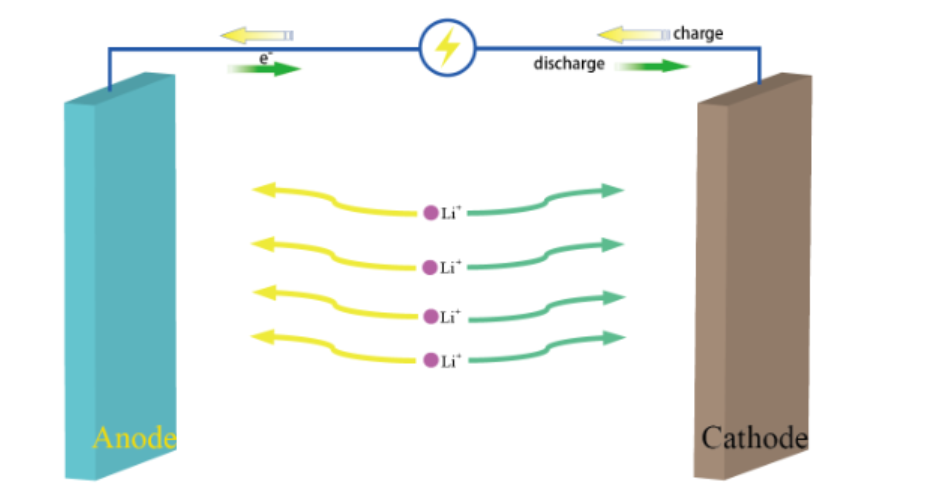
Figure 1: Working principle diagram of the solid lithium battery
4.2. Effect of electronic coupling on electronic properties of MXene interface
The interface electronic coupling effect (will be mentioned as IECE in the following paragraphs) is also a very normal phenomenon that happens between the surface of the MXene materials. To sum up, this phenomena is generated by the interaction of the electrons between two surfaces. This effect means a lot in many physical phenomenons and applications, especially in materials’ electrical properties. When MXene materials connect with other conductive materials, the electrons would transfer from one surface to another, and usually this transportation is dominated by the concentration of electrons. Under normal circumstances, the electrons transport from high concentrations to low concentrations. By this effect, the conductivity of the material would be changed, so it would be possible to improve the conductivity of the heterostructure materials.
When this material is used in the energy storage equipment like battery and capacitor, it would also affect these equipment’s electric chemical properties. For example, at the contact surface, factors like interfacial charge distribution and charge transfer velocity may change the battery’s capacity, cycle stability and charge and discharge rate.
4.3. The coordination mechanism between the effect and the battery
In recent years, a new type of two-dimensional material has been successfully synthesized--transition metal carbides/nitrides (which we also call the MXene materials) [5]. These materials have shown great properties in the storage and release the lithium ions [6-10]. When the topic comes to the coordination mechanism of the electronic coupling effect and the work principle of the solid-state lithium battery, one thing that must be discussed is whether the heterostructure MXene and the battery can work well when they are combined together. Through the research, it’s easy to draw the conclusion that the electronic coupling effect is very important in the function of the solid-state lithium battery. The solid-state lithium battery consists of three parts--the solid-state electrolyte(SSE), the cathode and the anode. Since the work functions of each material are different, the charge would redistribute when they are connected, forming a potential difference. This mechanism, which is called the interfacial charge redistribution, will change the electron concentration at the interface. Meanwhile, the electron coupling effect may lead to the interfacial secondary reaction. For example, the electron-ion synergism may lead to the reconstruction of the interfacial phase, change the diffusion barrier of the interfacial and affect the electrochemical stability. At the nanoscale, the IECE would enhance the local migration of electrons.
4.4. The application of the MXene and the solid-state lithium
There are many choices of material for the anode to choose from, like lithium nickelate and lithium iron phosphate, but the suitable material for the cathode is presenting a situation of scarcity. Although graphene has been commercially used as the cathode material, but is has some fatal flaws. So the scientists changed the research direction to find substitutes for graphene. Based on the previous chapters’ analysis, the MXene material turns out to be a potential material for the cathode and it’s also showing its potential to the consumers. So the most common use of the solid-state lithium and the MXene cathode is to apply it in transportation vehicles. Due to the high energy density and the clean energy emissions, the solid-state lithium battery has been seen as a superior alternative to fossil fuels such as oil and so on. Researchers have pointed out that some core material of the solid-state lithium battery’s mass production has already been achieved and technological breakthroughs have been made. And Solid-state batteries for drones are already available [11]. Under this tendency, it’s easy for us to believe that solid-state lithium batteries will be widely used in more fields in the future.
5. Conclusion
This thesis mainly discusses the interface electronic coupling phenomena of the 2D MXene materials and their heterostructures, and how these can be used to improve the properties of solid-state lithium metal batteries. In the article, the synergistic mechanism between the material and battery is meticulously analyzed. Through the research, it’s easy to draw the conclusion that the interface electronic coupling effect is a phenomenon that exists between the interface of two materials in the electron state. Moreover, the efficiency of the battery could be enhanced when this phenomenon is used in the battery structure. This thesis didn’t use the experimental method and data analysis method to calculate the efficiency improvement. Since the clean energy has been a main tendency now, so it could be easily forecast that the future research direction will be the the further improvement of the battery property, the lighten of the battery weight and the enlarging of the battery volume, so the solid-state lithium batter could be used in the vehicles or other aspects like industrial productions.
References
[1]. Geim.A.K and Grigorieva. I.V. Van der Waals heterostructures[J]. Nature,2013,499,419-425
[2]. Novoselov.K.S., Mishchenko.A, Carvalho. A., et al. 2D materials and Van der Waals heterostructures[J]. Science,2016,353,aac9439
[3]. Wang X., Li H., Lin S., et al. 2D/2D 1T-MoS2/Ti3C2 MXene Heterostructure with ExcellentSupercapacitor Performance [J]. Adv. Funct. Mater. 2020, 0190302-0190312.
[4]. Peng Zhao, Theoretical study on the application of MXene-based two-dimensional heterostructures as anode materials for Li-ion batteries,[D]. College of Chemistry and Chemical Engineering, Liaocheng University, 2022, 1-69
[5]. Naguib. M., Mochalin. V. N., Barsoum. M. W., et al. 25th anniversary article: MXenes: a newfamily of two-dimensional materials[J]. Adv Mater, 2014, 26, 992-1005.
[6]. Anasori. B., Lukatskaya. M. R. and Gogotsi. Y. 2D metal carbides and nitrides (MXenes) forenergy storage[J]. Nat. Rev. Mater., 2017, 2, 16098-16114.
[7]. Pang J., Mendes. R. G., Bachmatiuk. A., et al. Applications of 2D MXenes in energy conversionand storage systems[J]. Chem Soc Rev, 2019, 48, 72-133.
[8]. Xie Y., Naguib. M., Mochalin. V. N., et al. Role of surface structure on Li-ion energy storagecapacity of two-dimensional transition-metal carbides[J]. J Am Chem Soc, 2014, 136, 6385-6394.
[9]. Zhan C., Sun W., Xie Y., et al. Computational Discovery and Design of MXenes for EnergyApplications: Status, Successes, and Opportunities[J]. ACS Appl. Mater. Interfaces, 2019, 11, 24885-24905.
[10]. Zhan C., Sun W., Xie Y., et al. Computational Discovery and Design of MXenes for EnergyApplications: Status, Successes, and Opportunities[J]. ACS Appl. Mater. Interfaces, 2019, 11, 24885-24905.
[11]. Haomin Dong, Junlei Wang, Minghua Xin, Research on Toyota solid state battery technology based on patent analysis [J]. Car Technology, 2021(8): 30-35
Cite this article
Zheng,R. (2025). Interfacial Electronic Coupling of 2D MXene Heterostructures: Cross-Domain Mechanistic Insights for Solid-State Lithium Metal Batteries. Applied and Computational Engineering,148,39-43.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Mechatronics and Smart Systems
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Geim.A.K and Grigorieva. I.V. Van der Waals heterostructures[J]. Nature,2013,499,419-425
[2]. Novoselov.K.S., Mishchenko.A, Carvalho. A., et al. 2D materials and Van der Waals heterostructures[J]. Science,2016,353,aac9439
[3]. Wang X., Li H., Lin S., et al. 2D/2D 1T-MoS2/Ti3C2 MXene Heterostructure with ExcellentSupercapacitor Performance [J]. Adv. Funct. Mater. 2020, 0190302-0190312.
[4]. Peng Zhao, Theoretical study on the application of MXene-based two-dimensional heterostructures as anode materials for Li-ion batteries,[D]. College of Chemistry and Chemical Engineering, Liaocheng University, 2022, 1-69
[5]. Naguib. M., Mochalin. V. N., Barsoum. M. W., et al. 25th anniversary article: MXenes: a newfamily of two-dimensional materials[J]. Adv Mater, 2014, 26, 992-1005.
[6]. Anasori. B., Lukatskaya. M. R. and Gogotsi. Y. 2D metal carbides and nitrides (MXenes) forenergy storage[J]. Nat. Rev. Mater., 2017, 2, 16098-16114.
[7]. Pang J., Mendes. R. G., Bachmatiuk. A., et al. Applications of 2D MXenes in energy conversionand storage systems[J]. Chem Soc Rev, 2019, 48, 72-133.
[8]. Xie Y., Naguib. M., Mochalin. V. N., et al. Role of surface structure on Li-ion energy storagecapacity of two-dimensional transition-metal carbides[J]. J Am Chem Soc, 2014, 136, 6385-6394.
[9]. Zhan C., Sun W., Xie Y., et al. Computational Discovery and Design of MXenes for EnergyApplications: Status, Successes, and Opportunities[J]. ACS Appl. Mater. Interfaces, 2019, 11, 24885-24905.
[10]. Zhan C., Sun W., Xie Y., et al. Computational Discovery and Design of MXenes for EnergyApplications: Status, Successes, and Opportunities[J]. ACS Appl. Mater. Interfaces, 2019, 11, 24885-24905.
[11]. Haomin Dong, Junlei Wang, Minghua Xin, Research on Toyota solid state battery technology based on patent analysis [J]. Car Technology, 2021(8): 30-35









