1. Introduction
Biologically-based hydrogels are three-dimensional polymer networks characterised by their high water retention, biocompatibility, biodegradability, and low toxicity. Derived from natural polymers like alginate, chitosan, gelatin, and collagen, they closely mimic the extracellular matrix. Recent advances in 3D bioprinting, microfabrication, and stimuli-responsive designs have significantly broadened the scope of their potential applications.
Despite some progress, challenges remain in optimizing the mechanical properties, degradation kinetics and long-term stability of hydrogels under physiological conditions. Future research is expected to focus on multimaterial hydrogels, nanocomposite enhancement and bioactive functionalization to further improve their performance in clinical and industrial settings.
2. Preparation methodology
Biological-based hydrogels are prepared using physical, chemical, hybrid, and emerging cross-linking strategies, each offering distinct advantages and limitations that influence their mechanical, chemical, and biological properties for specific applications.
2.1. Physical cross-linking
Physical cross-linking methods rely on non-covalent interactions, offering a biocompatible and simple approach without toxic reagents, but typically result in hydrogels with limited mechanical strength and stability [1]. To overcome these limitations, natural polymer chemically cross-linked hydrogels (NPCCHs) introduce reversible cross-links between chains, improving structural integrity [2]. However, as noted by Yang et al., the removal of unreacted monomers and cross-linkers complicates post-processing.
Moreover, Jiang et al. demonstrated 3D printing of ultra-strong hydrogels by forming crosslinked DPC networks from polyvinyl alcohol (PVA) and chitosan (CS), integrating advanced printing with high-performance hydrogels [3]. In addition, Tang et al. proposed a completely physically crosslinked dual-network (DN) hydrogel using gelatin and poly (N-hydroxyethyl acrylamide), where hydrogen bonds in both networks yield self-healing, adhesive, and tough mechanical properties, though precise gelatin concentration control is crucial [4].
2.2. Chemical cross-linking
Chemical cross-linking (including covalent cross-linking, click chemistry, photocross-linking, and enzymatic cross-linking) introduces covalent bonds into the hydrogel network, resulting in improved stability and mechanical tunability. However, residual cross-linker toxicity and the need for precise reaction control remain challenges [5]. As detailed in recent studies [6], key chemical strategies for cell encapsulation include free-radical photopolymerization, enzyme-mediated and genipin-mediated cross-linking, click reactions, and sol-gel chemistry, each with distinct pros and cons, as discussed by Echalier et al. Additionally, Rebers et al. compared chemical and physical cross-linking in gelatin methacryloyl acetyl (GMA) hydrogels (GM10, GM2A8, GM2), using infrared spectroscopy-photorheology to monitor real-time kinetics. Their results showed that hydrogel stiffness is primarily governed by total crosslink density, regardless of the nature of the cross-links [7].
2.3. Hybrid approaches
Hybrid cross-linking strategies, such as dual cross-linking and interpenetrating polymer networks (IPNs), combine physical and chemical methods to enhance hydrogel stability, biocompatibility, and dynamic responsiveness, though their fabrication remains complex [8]. Zhao et al. developed doubly cross-linked cellulose hydrogels via sequential cross-linking, achieving superior mechanical strength over single-network systems, though cytocompatibility was not addressed [9]. Xu et al. prepared a hybrid hydrogel that demonstrated significant energy dissipation and improved strength through synergistic effects of both cross-linking types, but only its tensile behaviour was tested [10]. Additionally, studies show that viscoelasticity can be tuned by varying the physical-to-chemical cross-link ratio; however, broader experimentation is needed to establish it as a reliable design parameter for biomaterials and tissue engineering [11].
2.4. Emerging techniques
Recent advances in hydrogel technology aim to enhance stimuli-responsiveness, bioprintability, and functionality, with key emerging technologies including 3D bioprinting, stimuli-responsive hydrogels, and self-assembled systems. These approaches are promising for organ-on-a-chip integration but face challenges such as high cost and technical complexity [12]. Li et al. described hydrogel formation from supramolecular assembly to dynamic covalent bonding, highlighting multi-trigger responsiveness for biomedical use, though issues related to degradation toxicity remain underexplored [13]. Zhang et al. introduced photoresponsive and extrusion-printed hydrogels, emphasising print resolution, mechanical strength, and AI-based design tools like finite element analysis to optimise performance [14]. Appel et al. developed shear-thinning, self-healing hydrogels via polymer-nanoparticle self-assembly, suitable for drug delivery, although their study focused solely on shear-induced flow [15].
The preparation methods discussed above are summarised in figure 1 below.

3. Candidate materials
The selection of materials is critical for designing biologically-based hydrogels, as the material properties directly impact their biocompatibility, degradability, mechanical strength, and functional performance. These materials are primarily derived from polysaccharides, proteins, and other natural biomolecules [16]. This section elaborates on the most commonly used materials.
3.1. Polysaccharides
Polysaccharides are naturally occurring carbohydrate polymers with high biocompatibility and versatility in hydrogel applications [17]. There are four main categories of polysaccharides.
3.1.1. Alginate
Usually derived from brown algae, alginate is a naturally occurring anionic polymer that forms hydrogels by ionic cross-linking with divalent cations (e.g. Ca2+). It is biodegradable and non-toxic, but mechanically weak under physiological conditions. Alginate’s properties are widely applied in cell encapsulation and drug delivery, and have great potential for development in the biomedical field [18]. The swelling, degradation, mechanical stiffness, cell attachment and binding or release of bioactive molecules in sodium alginate hydrogels were studied by Augest et al. Their work demonstrated that alginate hydrogel is a versatile and adaptable biomaterial that can be used as a support matrix and cell delivery vehicle for tissue engineering. However, specific targeted delivery mechanisms remain underdeveloped [19].
According to research [20], alginate hydrogels offer several advantages as injectable materials, including ease of incorporation of therapeutic agents under mild conditions, minimally invasive local delivery and high profile. However, alginate hydrogels still play a rather passive role in acid reflux and weight control. Thus, alginate hydrogels still need considerable development to become an ideal material for tissue regeneration. Furthermore, Thakur et al. investigated the function of sodium alginate hydrogel in the removal of various water pollutants containing toxic dyes and heavy metal ions, due to its remarkable physicochemical properties such as hydrophilicity, swelling and modifiability [21].
3.1.2. Chitosan
Derived from chitin extracted from the shells of crustaceans, chitosan is obtained by chemically or biologically deacetylating chitin or by combining the two. Chitosan is widely used in wound healing and antimicrobial coatings due to its antimicrobial properties. However, its preparation requires an acidic environment [22].
Fu et al. introduced the stimulus response properties, biomimetic functions and high mechanical strength properties of chitosan hydrogels, focusing on the relationship between the structural features of chitosan hydrogels and their corresponding functions [23]. According to research [24], chitosan (CS), the second most abundant natural polysaccharide after cellulose, can be broken down into harmless products (aminosaccharides) in the human body. Additionally, chitosan offers advantages such as biocompatibility, non-toxicity and biodegradability, making chitosan hydrogel an ideal material for antifungal activity.
Additionally, the application of different types of chitosan hydrogels in biosensors and in smart food packaging systems was investigated by Yang et al. On the one hand, chitosan hydrogels can be used as biosensors due to their ability to respond to external stimuli, either through their swelling behaviour or by embedding bioactive substances capable of interacting with input elements. On the other hand, their strong antimicrobial and antioxidant properties, along with their favourable biodegradation characteristics, make chitosan hydrogels highly promising for smart food packaging systems [25].
3.1.3. Hyaluronic acid (HA)
Hyaluronic acid is a naturally existing unbranched anionic polymer, with good water retention, biocompatibility and bioactivity. Easily chemically modified for controlled degradation, it is widely used for wound healing, antimicrobial coatings and tissue scaffolds. For example, chitosan hydrogels combined with nanosilver are used in antimicrobial wound dressings. However, its rapid degradation remains a key drawback [26]. Luo et al. [27], leveraged hyaluronic acid’s ability to retain water, its biocompatibility and other abilities, and developed various crosslinked hyaluronic acid hydrogels. This customised strategy for the preparation of hyaluronic acid hydrogels has great potential for biomedical applications. However, further optimisation is required to meet complex and specific biomedical needs. In addition, studies have investigated multiple methods of preparing hyaluronic acid hydrogels and assessed the most prominent biomedical applications of each [28]. Nevertheless, most hyaluronic acid hydrogel formulations produced thus far have been developed under incompatible physiological conditions.
3.2. Proteins
Proteins are highly valued for their capability to mimic the extracellular matrix and promote cell adhesion and growth. Due to their biocompatibility, biodegradability, abundant functional groups, and tunable mechanical properties, proteins are considered ideal materials for hydrogels [29].
3.2.1. Collagen
As an essential component of animal connective tissue, collagen is a crucial structural protein in the formation of the extracellular matrix. It is highly biocompatible and promotes cell adhesion and differentiation. Collagen is widely used for skin substitutes, bone regeneration and drug delivery. For example, collagen hydrogels serve as scaffolds for heart tissue engineering. However, collagen’s relatively poor mechanical strength remains a limitation [30].
Yang et al. investigated the effects of gelation conditions, cross-linking agents and additional polymers on the preparation of collagen-based hydrogels. Their study explored the sol-gel phase transition mechanism of collagen-based hydrogels and provided insights into designing and customising hydrogels for specific applications. However, the precise gelation and modification mechanisms require further study [31]. Furthermore, the critical isolation and purification steps of collagen have been studied [32], providing insights into designing and customising collagen-based hydrogels in ophthalmic. Nonetheless, the long-term safety of intraocular hydrogels remains an ongoing challenge.
3.2.2. Gelatin
Gelatin is denatured collagen obtained by hydrolysis. It has a thermoreversible gel, good biocompatibility and is easy to chemically modify. It is used in 3D bioprinting, wound healing and drug delivery. Examples include gelatin methacrylate (GelMA) hydrogels for vascularised tissue engineering. However, thermal instability remains a drawback [33]. According to research [34], gelatin, as a highly renewable, sustainable and versatile hydrogel synthetic polymer, shows promising performance in wastewater remediation by removing pollutants. Additionally, as gelatin-based hydrogels are readily biodegradable, they can be used sustainably in the environment, fulfilling the principles of sustainable development.
Mushtaq et al. provide an overview of various cross-linking techniques, properties, types of composites and their uses for gelatin-based hydrogels. The multifunctional nature of gelatin allows for simple and effective cross-linking while facilitating easy integration with other materials to form composites with desirable properties [35].
3.2.3. Silk fibroin
Silk fibroin are natural proteins extracted from silk, which have high mechanical strength, adjustable degradation rate and good biocompatibility. It is widely used in bone tissue engineering to control drug release and as a scaffold for ligament regeneration. However, its relatively complex processing is an unavoidable drawback [36] [37]. Zhang et al. studied the mechanical properties, biocompatibility and biodegradability of filipin proteins. They compared silk protein hydrogels prepared using two preparation strategies: physical and chemical cross-linking. Additionally, they conducted a systematic review of silk protein hydrogels for biomedical applications, including wearable sensors and drug delivery. However, research on filipin protein hydrogels is currently limited to small animal models, posing certain constraints [38].
Overall, the above candidates are compared and summarised in the table below.
|
Candidate Materials |
Source |
Functionality |
Water Solubility |
Application |
Citation |
|
Alginate |
Brown algae extraction |
Controllable gelation and cell loading |
Hydrosoluble |
Wound dressing, medicine delivery |
[18] |
|
Chitosan |
Crustacean extraction |
Antibacterial and film forming properties are strong |
Requires acidic medium to dissolve |
Wound repair, antibacterial material |
[22][23] |
|
Hyaluronic Acid (HA) |
Connective tissue, vitreous body |
Promote cell proliferation and tissue repair |
High hydrophilicity |
Cartilage repair, anti-inflammatory |
[26][27][28] |
|
Collagen |
Mammalian skin, bones |
Promote cell adhesion and ECM simulation |
Acidic medium required |
Tissue engineering, skin repair |
[30][31] |
|
Gelatin |
Partially hydrolyzed collagen |
Adjustable thermal response |
Hydrosoluble |
scaffold for tissue engineering |
[33][34] |
|
Silk Fibroin |
Fibroin |
High mechanical properties, controlled degradation |
Low water solubility, need to be treated |
Artificial ligament and bone repair |
[36][37] |
4. Advanced applications
Biologically-based hydrogels have evolved from basic biomedical uses to cutting-edge applications that address complex challenges in healthcare, biotechnology, and environmental science [39].
4.1. Advanced soft robotics
Hydrogels have attracted considerable attention in soft robotics due to their unique combination of flexibility, biocompatibility, and responsiveness to external stimuli such as temperature, pH, light, and electric fields. These properties enable hydrogels to mimic biological tissues and perform complex tasks, such as gripping, locomotion, and actuation [40].
4.1.1. Material selection
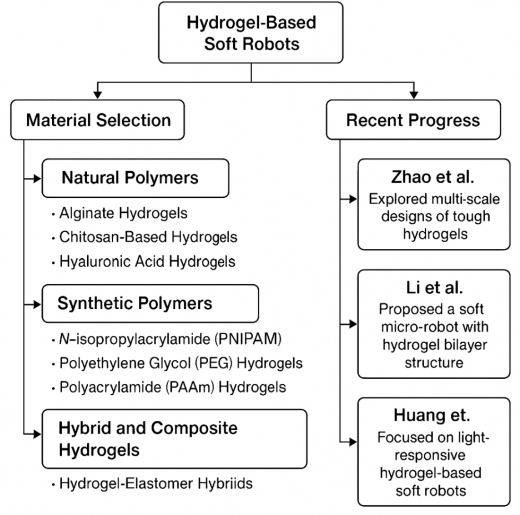
As for material selection aspects of hydrogel-based soft robots, they are typically divided into three categories: natural polymers, synthetic polymers, and hybrid and composite hydrogels. In terms of natural polymers, alginate hydrogels are used as soft robotic actuators for biomedical applications due to their gentle interaction with tissues. Chitosan-based hydrogels are ideal for soft robots designed for biomedical environments, such as surgical tools or drug delivery systems, due to their pH sensitivity and antimicrobial properties. Hyaluronic acid hydrogels, known for their viscoelastic properties, are used in robotic devices that require dynamic movement, such as artificial muscles [41].
In terms of synthetic polymers, N-isopropylacrylamide (PNIPAM) is a thermo-responsive hydrogel that undergoes reversible expansion and contraction near its lower critical solution temperature (~32°C). It is therefore widely used in actuators and temperature-sensitive robotic systems. Polyethylene Glycol (PEG) hydrogels are highly customisable and have been used in hybrid soft robotic systems where precise mechanical properties are required. Polyacrylamide (PAAm) hydrogels have excellent mechanical strength and elasticity for soft robotic grippers and artificial skin applications [42]. Regarding hybrid and composite hydrogels, Hydrogel-Elastomer Hybrids combine hydrogels with elastomers such as silicones or PDMS (polydimethylsiloxane), resulting in materials with high stretch and elasticity, ideal for bionic robotics applications [43].
4.1.2. Recent progress
Zhao et al. investigated multi-scale designs of tough hydrogels, incorporating energy dissipation mechanisms within stretchy hydrogel networks to enhance their mechanical properties, making them suitable for robotic applications [44]. However, the preparation process is relatively complex. Furthermore, Li et al. proposed a soft micro-robot composed of a hydrogel bilayer structure, capable of targeted movement via an electromagnetic actuation system that releases particles through a change in pH. This soft body robot is expected to be a tumour therapy tool in the future [45].
Huang et al. explored light-responsive hydrogel-based soft robots, improving actuation speed, precision, and durability. The study demonstrated the use of embedded photochromic nanoparticles for efficient light absorption, enabling soft robots to perform complex motions under light stimuli. This work highlighted the potential for optical control in biomedical devices and underwater robotics [46]. Future studies should focus on improving the mechanical robustness of hydrogels while maintaining their responsiveness.
Figure 2 below summarises the above information.
4.2. Cancer therapy
Hydrogels have emerged as a promising platform for cancer therapy due to their biocompatibility, adjustable mechanical properties, and ability to encapsulate and deliver therapeutic agents in a controlled manner. Their versatility enables them to serve as carriers for drug delivery, platforms for immunotherapy, and scaffolds for cancer research [47].
4.2.1. Material selection
Regarding material selection for cancer therapy, injectable hydrogels have been particularly highlighted. Injectable hydrogels, loaded with chemotherapeutic drugs or nanoparticles, can be delivered directly to tumour sites. For example, alginate or chitosan-based hydrogels have been employed to deliver doxorubicin or paclitaxel, ensuring sustained drug release over weeks [48]. Additionally, hydrogels sensitive to tumour-specific conditions, such as acidic pH or hypoxic environments, can release therapeutic agents in response to these stimuli. PNIPAM hydrogels with embedded nanoparticles have been used to target hypoxic tumours [49].
4.2.2. Recent progress
Hydrogels are increasingly being used to deliver combinations of therapies, such as chemotherapy and immunotherapy or photothermal therapy with checkpoint inhibitors, to enhance treatment outcomes [50]. Moreover, advances in smart hydrogels have enabled precision drug release. For instance, hydrogels that respond to tumour enzymes (e.g., matrix metalloproteinases) can degrade and release drugs specifically in the tumour microenvironment [51]. However, overcoming drug loading limitations and enhancing hydrogel penetration in solid tumours remain key challenges at this stage.
4.3. Biosensors and diagnostic platforms
Hydrogels have attracted significant attention in biosensors and diagnostic platforms due to their high water content, biocompatibility, and ability to mimic biological tissues. These materials are especially useful for integrating biological recognition elements and enabling real-time, sensitive detection of biomolecules, pathogens, and physiological signals [52]
4.3.1. Material selection
In the context of biosensors and diagnostic platforms, material selection is typically categorised into natural polymers and synthetic polymers. Regarding natural polymers, hyaluronic acid (HA) is used in biosensors for cancer biomarkers due to its biocompatibility and affinity for CD44, a surface marker overexpressed in tumours [53]. Chitosan is frequently employed in biosensors for DNA or protein detection, as its amino groups facilitate biomolecule binding [54]. Among synthetic polymers, poly(ethylene glycol) (PEG) is ideal for immobilising antibodies, enzymes and aptamers due to its low protein adsorption and non-fouling properties. It is widely used in optical biosensors for the detection of cardiac biomarkers [55]. Polyacrylamide (PAM) is known for its high mechanical stability and is commonly used in strain sensors and bioelectronic diagnostics [56].
4.3.2. Recent progress
Hydrogel-based sensors embedded in wearable devices can continuously monitor physiological parameters such as glucose, lactate and electrolytes. For example, sweat-based lactate sensors utilise alginate hydrogel patches loaded with enzymes, presenting new possibilities for personal healthcare [57]. Additionally, hydrogels serve as matrices for immobilising enzymes or other recognition elements in electrochemical sensors. For instance, the multifunctional sensing and actuation platform developed by Park et al., based on hydrogel, enables direct insertion into deep brain regions while minimising foreign body response limitations [58]. However, challenges remain, including limited mechanical stability and the need to balance sensitivity with scalability for commercial production [59]. Future developments should focus on exploring self-repairing hydrogels to improve the durability and longevity of wearable platforms.
5. Conclusion
Bio-based hydrogels, derived from natural materials such as alginate, chitosan, gelatin, and collagen, show tremendous potential for applications in fields such as soft robotics, cancer therapy, and biosensing due to their biocompatibility, biodegradability, and modifiability. Advances in preparation methods, including hybridisation and emerging cross-linking techniques, have expanded their versatility and utility.
However, challenges such as limited mechanical strength, instability, and complex fabrication processes still need to be addressed to realise a wider range of applications, especially in more complex systems such as soft robotics and cancer therapy. Future research should prioritise enhancing the strength, stability and efficiency of hydrogels to improve drug delivery and biosensing capabilities. With continued innovation, bio-based hydrogels hold great promise for advancing healthcare, biotechnology and environmental applications.
References
[1]. Pita-López, M. L. , Fletes-Vargas, G. , Espinosa-Andrews, H. , & Rodríguez-Rodríguez, R. (2021). Physically cross-linked chitosan-based hydrogels for tissue engineering applications: A state-of-the-art review. European Polymer Journal, 145, p. 110176, Feb. 2021, doi: https://doi. org/10. 1016/j. eurpolymj. 2020. 110176
[2]. Yang, J. , Chen, Y. , Zhao, L. , Zhang, J. , & Luo, H. (2022). Constructions and Properties of Physically Cross-Linked Hydrogels Based on Natural Polymers. Polymer Reviews, 63(3), pp. 574–612, Oct. 2022, doi: https://doi. org/10. 1080/15583724. 2022. 2137525
[3]. Jiang, P. , Lin, P. , Yang, C. , Qin, H. , Wang, X. , & Zhou, F. (2020). 3D Printing of Dual-Physical Cross-linking Hydrogel with Ultrahigh Strength and Toughness. Chemistry of Materials, 32(23), pp. 9983–9995, Nov. 2020, doi: https://doi. org/10. 1021/acs. chemmater. 0c02941
[4]. Tang, L. , et al. (2019) . Double-Network Physical Cross-Linking Strategy To Promote Bulk Mechanical and Surface Adhesive Properties of Hydrogels. Macromolecules, 52(24), pp. 9512–9525, Dec. 2019, doi: https://doi. org/10. 1021/acs. macromol. 9b01686
[5]. Muir, V. G. , & Burdick, J. A. (2020). Chemically Modified Biopolymers for the Formation of Biomedical Hydrogels. Chemical Reviews, 121(18), pp. 10908–10949, Dec. 2020, doi: https://doi. org/10. 1021/acs. chemrev. 0c00923
[6]. Echalier, C. , Valot, L. , Martinez, J. , Mehdi, A. , & Subra, G. (2019). Chemical cross-linking methods for cell encapsulation in hydrogels. Materials Today Communications, 20, p. 100536, Sep. 2019, doi: https://doi. org/10. 1016/j. mtcomm. 2019. 05. 012
[7]. Rebers, L. , et al. (2021). Differentiation of physical and chemical cross-linking in gelatin methacryloyl hydrogels. Scientific Reports, 11(1), p. 3256, Feb. 2021, doi: https://doi. org/10. 1038/s41598-021-82393-z
[8]. Annabi, N. , et al. (2013). 25th Anniversary Article: Rational Design and Applications of Hydrogels in Regenerative Medicine. Advanced Materials, 26(1), pp. 85–124, Nov. 2013, doi: https://doi. org/10. 1002/adma. 201303233
[9]. Zhao, D. , Huang, J. , Zhong, Y. , Li, K. , Zhang, L. , and Cai, J. (2016). High-Strength and High-Toughness Double-Cross-Linked Cellulose Hydrogels: A New Strategy Using Sequential Chemical and Physical Cross-Linking. Advanced Functional Materials, 26(34), pp. 6279–6287, Jul. 2016, doi: https://doi. org/10. 1002/adfm. 201601645
[10]. Xu, J. , Liu, X. , Ren, X. , & Gao, G. (2018). The role of chemical and physical crosslinking in different deformation stages of hybrid hydrogels. European Polymer Journal, 100, pp. 86–95, Mar. 2018, doi: https://doi. org/10. 1016/j. eurpolymj. 2018. 01. 020
[11]. Bartnikowski, M. , Wellard, R. , Woodruff, M. , and Klein, T. (2015). Tailoring Hydrogel Viscoelasticity with Physical and Chemical Crosslinking. Polymers, 7(12), pp. 2650–2669, Dec. 2015, doi: https://doi. org/10. 3390/polym7121539
[12]. Porwal, S. , Sridhar, S. B. , Talath, S. , Wali, A. F. , Warsi, M. H. , & Malviya, R. (2024). 3D printable sustainable hydrogel formulations for tissue engineering applications. Journal of Drug Delivery Science and Technology, 101, p. 106308, Oct. 2024, doi: https://doi. org/10. 1016/j. jddst. 2024. 106308.
[13]. Li, Z. , Zhou, Y. , Li, T. , Zhang, J. , & Tian, H. (2021). Stimuli‐responsive hydrogels: Fabrication and biomedical applications. VIEW, 3(2), p. 20200112, Aug. 2021, doi: https://doi. org/10. 1002/viw. 20200112
[14]. Zhang, A. , et al. (2021). 3D printing hydrogels for actuators: A review. Chinese Chemical Letters, 32(10), pp. 2923–2932, Oct. 2021, doi: https://doi. org/10. 1016/j. cclet. 2021. 03. 073
[15]. Appel, E. A. , Tibbitt, M. W. , Webber, M. J. , Mattix, B. A. , Veiseh, O. , & Langer, R. (2015). Self-assembled hydrogels utilizing polymer–nanoparticle interactions. Nature Communications, 6(1), Feb. 2015, doi: https://doi. org/10. 1038/ncomms7295
[16]. Catoira, M. C. , Fusaro, L. , Di Francesco, D. , Ramella, M. , & Boccafoschi, F. (2019). Overview of natural hydrogels for regenerative medicine applications. Journal of Materials Science: Materials in Medicine, 30(10), Oct. 2019, doi: https://doi. org/10. 1007/s10856-019-6318-7
[17]. Li, Z. , & Lin, Z. (2021). Recent advances in polysaccharide‐based hydrogels for synthesis and applications. Aggregate, Jan. 2021, doi: https://doi. org/10. 1002/agt2. 21
[18]. Lee, K. Y. , & Mooney, D. J. (2012). Alginate: Properties and biomedical applications. Progress in Polymer Science, 37(1), pp. 106–126, Jan. 2012, doi: https://doi. org/10. 1016/j. progpolymsci. 2011. 06. 003
[19]. Augst, A. D. , Kong, H. J. , & Mooney, D. J. (2006). Alginate Hydrogels as Biomaterials. Macromolecular Bioscience, 6(8), pp. 623–633, Aug. 2006, doi: https://doi. org/10. 1002/mabi. 200600069
[20]. Bidarra, S. J. , Barrias, C. C. , & Granja, P. L. (2014). Injectable alginate hydrogels for cell delivery in tissue engineering. Acta biomaterialia, 10(4), pp. 1646–62, 2014, doi: https://doi. org/10. 1016/j. actbio. 2013. 12. 006
[21]. Thakur, S. , Sharma, B. , Verma, A. , Chaudhary, J. , Tamulevicius, S. , & Thakur, V. K. (2018). Recent progress in sodium alginate based sustainable hydrogels for environmental applications. Journal of Cleaner Production, 198, pp. 143–159, Oct. 2018, doi: https://doi. org/10. 1016/j. jclepro. 2018. 06. 259
[22]. Islam, M. M. , Shahruzzaman, M. , Biswas, S. , Nurus Sakib, M. , & Rashid, T. U. (2020). Chitosan based bioactive materials in tissue engineering applications-A review. Bioactive Materials, 5(1), pp. 164–183, Mar. 2020, doi: https://doi. org/10. 1016/j. bioactmat. 2020. 01. 012
[23]. Fu, J. , Yang, F. , & Guo, Z. (2018). The chitosan hydrogels: from structure to function. New Journal of Chemistry, 42(21), pp. 17162–17180, 2018, doi: https://doi. org/10. 1039/c8nj03482f
[24]. Shariatinia, Z. , & Jalali, A. M. (2018). Chitosan-based hydrogels: Preparation, properties and applications. International Journal of Biological Macromolecules, 115, pp. 194–220, Aug. 2018, doi: https://doi. org/10. 1016/j. ijbiomac. 2018. 04. 034
[25]. Yang, J. , et al. (2021). Advanced applications of chitosan-based hydrogels: From biosensors to intelligent food packaging system. Trends in Food Science & Technology, 110, pp. 822–832, Apr. 2021, doi: https://doi. org/10. 1016/j. tifs. 2021. 02. 032
[26]. Saravanakumar, K. et al. (2022). Application of hyaluronic acid in tissue engineering, regenerative medicine, and nanomedicine: A review. International Journal of Biological Macromolecules, 222, pp. 2744–2760, Dec. 2022, doi: https://doi. org/10. 1016/j. ijbiomac. 2022. 10. 055
[27]. Luo, Z. , Wang, Y. , Li, J. , Wang, J. , Yu, Y. , & Zhao, Y. (2023). Tailoring Hyaluronic Acid Hydrogels for Biomedical Applications. Advanced Functional Materials, 33(49), Sep. 2023, doi: https://doi. org/10. 1002/adfm. 202306554
[28]. Pérez, L. A. , Hernández, R. , Alonso, J. M. , Pérez-González, R. , & Sáez-Martínez, V. (2021). Hyaluronic Acid Hydrogels Crosslinked in Physiological Conditions: Synthesis and Biomedical Applications. Biomedicines, 9(9), p. 1113, Aug. 2021, doi: https://doi. org/10. 3390/biomedicines9091113
[29]. Davari, N. , et al. (2022). Protein-Based Hydrogels: Promising Materials for Tissue Engineering. Polymers, 14(5), p. 986, Feb. 2022, doi: https://doi. org/10. 3390/polym14050986
[30]. She, J. , et al. (2025). Recent advances in collagen-based hydrogels: Materials, preparation and applications. Reactive and Functional Polymers, 207, p. 106136, Feb. 2025, doi: https://doi. org/10. 1016/j. reactfunctpolym. 2024. 106136
[31]. Yang, X. , et al. (2025). Collagen-based hydrogel sol-gel phase transition mechanism and their applications. Advances in Colloid and Interface Science, 340, p. 103456, Feb. 2025, doi: https://doi. org/10. 1016/j. cis. 2025. 103456
[32]. Benival, D. , et al. (2023). Collagen-Based Hydrogels for the Eye: A Comprehensive Review. Gels, 9(8), pp. 643–643, Aug. 2023, doi: https://doi. org/10. 3390/gels9080643
[33]. Wang, Y. , et al. (2024). A dual-crosslinking electroactive hydrogel based on gelatin methacrylate and dibenzaldehyde-terminated telechelic polyethylene glycol for 3D bio-printing. Scientific Reports, 14(1), Feb. 2024, doi: https://doi. org/10. 1038/s41598-024-54853-9
[34]. Andreazza, R. , Morales, A. , Pieniz, S. , & Labidi, J. (2023). Gelatin-Based Hydrogels: Potential Biomaterials for Remediation. Polymers, 15(4), p. 1026, Jan. 2023, doi: https://doi. org/10. 3390/polym15041026
[35]. Mushtaq, F. , et al. (2022). Preparation, properties, and applications of gelatin-based hydrogels (GHs) in the environmental, technological, and biomedical sectors. International Journal of Biological Macromolecules, 218, pp. 601–633, Oct. 2022, doi: https://doi. org/10. 1016/j. ijbiomac. 2022. 07. 168
[36]. Zheng, H. , & Zuo, B. (2021). Functional silk fibroin hydrogels: preparation, properties and applications. Journal of Materials Chemistry B, 2021, doi: https://doi. org/10. 1039/d0tb02099k
[37]. Kim, S. H. , et al. (2021). 3D bioprinted silk fibroin hydrogels for tissue engineering. Nature Protocols, 16(12), pp. 5484–5532, Dec. 2021, doi: https://doi. org/10. 1038/s41596-021-00622-1
[38]. Zhang, H. , Xu, D. , Zhang, Y. , Li, M. , & Chai, R. (2022). Silk fibroin hydrogels for biomedical applications. Smart Medicine, 1(1), Dec. 2022, doi: https://doi. org/10. 1002/smmd. 20220011
[39]. Cao, H. , Duan, L. , Zhang, Y. , Cao, J. , & Zhang, K. (2021). Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduction and Targeted Therapy, 6(1), pp. 1–31, Dec. 2021, doi: https://doi. org/10. 1038/s41392-021-00830-x.
[40]. Cianchetti, M. , Laschi, C. , Menciassi, A. , & Dario, P. (2018). Biomedical applications of soft robotics. Nature Reviews Materials, 3(6), pp. 143–153, May 2018, doi: https://doi. org/10. 1038/s41578-018-0022-y
[41]. Yuk, H. , Zhang, T. , Parada, G. A. , Liu, X. , & Zhao, X. (2016). Skin-inspired hydrogel–elastomer hybrids with robust interfaces and functional microstructures. Nature Communications, 7(1), Jun. 2016, doi: https://doi. org/10. 1038/ncomms12028
[42]. Shin, Y. , Lee, H. S. , Jeong, H. , & Kim, D. -H. (2024). Recent advances in conductive hydrogels for soft biointegrated electronics: Materials, properties, and device applications. Wearable Electronics, vol. 1, pp. 255–280, Dec. 2024, doi: https://doi. org/10. 1016/j. wees. 2024. 10. 004
[43]. Park, C. S. , Kang, Y. -W. , Na, H. , & Sun, J. -Y. (2024). Hydrogels for bioinspired soft robots. Progress in Polymer Science, pp. 101791–101791, Jan. 2024, doi: https://doi. org/10. 1016/j. progpolymsci. 2024. 101791
[44]. Zhao, X. (2014). Multi-scale multi-mechanism design of tough hydrogels: building dissipation into stretchy networks. Soft Matter, 10(5), pp. 672–687, 2014, doi: https://doi. org/10. 1039/c3sm52272e
[45]. Li, H. , Go, G. , Ko, S. Y. , Park, J. -O. , & Park, S. (2016). Magnetic actuated pH-responsive hydrogel-based soft micro-robot for targeted drug delivery. Smart Materials and Structures, 25(2), p. 027001, Jan. 2016, doi: https://doi. org/10. 1088/0964-1726/25/2/027001
[46]. Huang, Y. , Yu, Q. , Su, C. , Jiang, J. , Chen, N. , & Shao, H. (2021). Light-Responsive Soft Actuators: Mechanism, Materials, Fabrication, and Applications. Actuators, 10(11), p. 298, Nov. 2021, doi: https://doi. org/10. 3390/act10110298
[47]. Xie, Z. , Shen, J. , Sun, H. , Li, J. , & Wang, X. (2021). Polymer-based hydrogels with local drug release for cancer immunotherapy. Biomedicine & Pharmacotherapy, 137, p. 111333, Feb. 2021, doi: https://doi. org/10. 1016/j. biopha. 2021. 111333
[48]. Chen, C. -H. , et al. (2018). Thermosensitive Injectable Hydrogel for Simultaneous Intraperitoneal Delivery of Doxorubicin and Prevention of Peritoneal Adhesion. International Journal of Molecular Sciences, 19(5), p. 1373, May 2018, doi: https://doi. org/10. 3390/ijms19051373
[49]. Tang, L. , Wang, L. , Yang, X. , Feng, Y. , Li, Y. , & Feng, W. (2021). Poly(N-isopropylacrylamide)-based smart hydrogels: Design, properties and applications. Progress in Materials Science, 115, p. 100702, Jan. 2021, doi: https://doi. org/10. 1016/j. pmatsci. 2020. 100702
[50]. Cao, C. , et al. (2021). Biodegradable hydrogel with thermo-response and hemostatic effect for photothermal enhanced anti-infective therapy. Nano Today, 39, p. 101165, Aug. 2021, doi: https://doi. org/10. 1016/j. nantod. 2021. 101165
[51]. Chao, Y. , Chen, Q. , & Liu, Z. (2019). Smart Injectable Hydrogels for Cancer Immunotherapy. Advanced Functional Materials, 30(2), p. 1902785, Aug. 2019, doi: https://doi. org/10. 1002/adfm. 201902785
[52]. Bae, J. , et al. (2020). Tailored hydrogels for biosensor applications. Journal of Industrial and Engineering Chemistry, 89, pp. 1–12, Sep. 2020, doi: https://doi. org/10. 1016/j. jiec. 2020. 05. 001
[53]. Mattheolabakis, G. , Milane, L. , Singh, A. , & Amiji, M. M. (2015). Hyaluronic acid targeting of CD44 for cancer therapy: from receptor biology to nanomedicine. Journal of Drug Targeting, 23(7–8), pp. 605–618, Sep. 2015, doi: https://doi. org/10. 3109/1061186x. 2015. 1052072
[54]. Amirthalingam, S. , & Rangasamy, J. (2021). Chitosan-Based Biosensor Fabrication and Biosensing Applications. Advances in Polymer Science, pp. 233–255, Jan. 2021, doi: https://doi. org/10. 1007/12_2021_85
[55]. Qureshi, A. , Gurbuz, Y. , & Niazi, J. H. (2012). Biosensors for cardiac biomarkers detection: A review. Sensors and Actuators B: Chemical, 171–172, pp. 62–76, Aug. 2012, doi: https://doi. org/10. 1016/j. snb. 2012. 05. 077
[56]. Zhang, L. (2022). Strong and Tough PAm/SA Hydrogel with Highly Strain Sensitivity. Journal of Renewable Materials, 10(2), pp. 415–430, doi: https://doi. org/10. 32604/jrm. 2022. 016650
[57]. Nagamine, K. , et al. (2019). Noninvasive Sweat-Lactate Biosensor Emplsoying a Hydrogel-Based Touch Pad. Scientific Reports, 9(1), Jul. 2019, doi: https://doi. org/10. 1038/s41598-019-46611-z
[58]. Park, S. , et al. (2021). Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity. Nature Communications, 12(1), p. 3435, Jun. 2021, doi: https://doi. org/10. 1038/s41467-021-23802-9
[59]. Ma, J. , et al. (2023). Hydrogel sensors for biomedical electronics. Chemical Engineering Journal, 481, pp. 148317–148317, Dec. 2023, doi: https://doi. org/10. 1016/j. cej. 2023. 148317
Cite this article
Zhang,Y. (2025). Review on Biological-based Hydrogels for Advanced Applications. Applied and Computational Engineering,171,21-32.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of the 3rd International Conference on Functional Materials and Civil Engineering
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Pita-López, M. L. , Fletes-Vargas, G. , Espinosa-Andrews, H. , & Rodríguez-Rodríguez, R. (2021). Physically cross-linked chitosan-based hydrogels for tissue engineering applications: A state-of-the-art review. European Polymer Journal, 145, p. 110176, Feb. 2021, doi: https://doi. org/10. 1016/j. eurpolymj. 2020. 110176
[2]. Yang, J. , Chen, Y. , Zhao, L. , Zhang, J. , & Luo, H. (2022). Constructions and Properties of Physically Cross-Linked Hydrogels Based on Natural Polymers. Polymer Reviews, 63(3), pp. 574–612, Oct. 2022, doi: https://doi. org/10. 1080/15583724. 2022. 2137525
[3]. Jiang, P. , Lin, P. , Yang, C. , Qin, H. , Wang, X. , & Zhou, F. (2020). 3D Printing of Dual-Physical Cross-linking Hydrogel with Ultrahigh Strength and Toughness. Chemistry of Materials, 32(23), pp. 9983–9995, Nov. 2020, doi: https://doi. org/10. 1021/acs. chemmater. 0c02941
[4]. Tang, L. , et al. (2019) . Double-Network Physical Cross-Linking Strategy To Promote Bulk Mechanical and Surface Adhesive Properties of Hydrogels. Macromolecules, 52(24), pp. 9512–9525, Dec. 2019, doi: https://doi. org/10. 1021/acs. macromol. 9b01686
[5]. Muir, V. G. , & Burdick, J. A. (2020). Chemically Modified Biopolymers for the Formation of Biomedical Hydrogels. Chemical Reviews, 121(18), pp. 10908–10949, Dec. 2020, doi: https://doi. org/10. 1021/acs. chemrev. 0c00923
[6]. Echalier, C. , Valot, L. , Martinez, J. , Mehdi, A. , & Subra, G. (2019). Chemical cross-linking methods for cell encapsulation in hydrogels. Materials Today Communications, 20, p. 100536, Sep. 2019, doi: https://doi. org/10. 1016/j. mtcomm. 2019. 05. 012
[7]. Rebers, L. , et al. (2021). Differentiation of physical and chemical cross-linking in gelatin methacryloyl hydrogels. Scientific Reports, 11(1), p. 3256, Feb. 2021, doi: https://doi. org/10. 1038/s41598-021-82393-z
[8]. Annabi, N. , et al. (2013). 25th Anniversary Article: Rational Design and Applications of Hydrogels in Regenerative Medicine. Advanced Materials, 26(1), pp. 85–124, Nov. 2013, doi: https://doi. org/10. 1002/adma. 201303233
[9]. Zhao, D. , Huang, J. , Zhong, Y. , Li, K. , Zhang, L. , and Cai, J. (2016). High-Strength and High-Toughness Double-Cross-Linked Cellulose Hydrogels: A New Strategy Using Sequential Chemical and Physical Cross-Linking. Advanced Functional Materials, 26(34), pp. 6279–6287, Jul. 2016, doi: https://doi. org/10. 1002/adfm. 201601645
[10]. Xu, J. , Liu, X. , Ren, X. , & Gao, G. (2018). The role of chemical and physical crosslinking in different deformation stages of hybrid hydrogels. European Polymer Journal, 100, pp. 86–95, Mar. 2018, doi: https://doi. org/10. 1016/j. eurpolymj. 2018. 01. 020
[11]. Bartnikowski, M. , Wellard, R. , Woodruff, M. , and Klein, T. (2015). Tailoring Hydrogel Viscoelasticity with Physical and Chemical Crosslinking. Polymers, 7(12), pp. 2650–2669, Dec. 2015, doi: https://doi. org/10. 3390/polym7121539
[12]. Porwal, S. , Sridhar, S. B. , Talath, S. , Wali, A. F. , Warsi, M. H. , & Malviya, R. (2024). 3D printable sustainable hydrogel formulations for tissue engineering applications. Journal of Drug Delivery Science and Technology, 101, p. 106308, Oct. 2024, doi: https://doi. org/10. 1016/j. jddst. 2024. 106308.
[13]. Li, Z. , Zhou, Y. , Li, T. , Zhang, J. , & Tian, H. (2021). Stimuli‐responsive hydrogels: Fabrication and biomedical applications. VIEW, 3(2), p. 20200112, Aug. 2021, doi: https://doi. org/10. 1002/viw. 20200112
[14]. Zhang, A. , et al. (2021). 3D printing hydrogels for actuators: A review. Chinese Chemical Letters, 32(10), pp. 2923–2932, Oct. 2021, doi: https://doi. org/10. 1016/j. cclet. 2021. 03. 073
[15]. Appel, E. A. , Tibbitt, M. W. , Webber, M. J. , Mattix, B. A. , Veiseh, O. , & Langer, R. (2015). Self-assembled hydrogels utilizing polymer–nanoparticle interactions. Nature Communications, 6(1), Feb. 2015, doi: https://doi. org/10. 1038/ncomms7295
[16]. Catoira, M. C. , Fusaro, L. , Di Francesco, D. , Ramella, M. , & Boccafoschi, F. (2019). Overview of natural hydrogels for regenerative medicine applications. Journal of Materials Science: Materials in Medicine, 30(10), Oct. 2019, doi: https://doi. org/10. 1007/s10856-019-6318-7
[17]. Li, Z. , & Lin, Z. (2021). Recent advances in polysaccharide‐based hydrogels for synthesis and applications. Aggregate, Jan. 2021, doi: https://doi. org/10. 1002/agt2. 21
[18]. Lee, K. Y. , & Mooney, D. J. (2012). Alginate: Properties and biomedical applications. Progress in Polymer Science, 37(1), pp. 106–126, Jan. 2012, doi: https://doi. org/10. 1016/j. progpolymsci. 2011. 06. 003
[19]. Augst, A. D. , Kong, H. J. , & Mooney, D. J. (2006). Alginate Hydrogels as Biomaterials. Macromolecular Bioscience, 6(8), pp. 623–633, Aug. 2006, doi: https://doi. org/10. 1002/mabi. 200600069
[20]. Bidarra, S. J. , Barrias, C. C. , & Granja, P. L. (2014). Injectable alginate hydrogels for cell delivery in tissue engineering. Acta biomaterialia, 10(4), pp. 1646–62, 2014, doi: https://doi. org/10. 1016/j. actbio. 2013. 12. 006
[21]. Thakur, S. , Sharma, B. , Verma, A. , Chaudhary, J. , Tamulevicius, S. , & Thakur, V. K. (2018). Recent progress in sodium alginate based sustainable hydrogels for environmental applications. Journal of Cleaner Production, 198, pp. 143–159, Oct. 2018, doi: https://doi. org/10. 1016/j. jclepro. 2018. 06. 259
[22]. Islam, M. M. , Shahruzzaman, M. , Biswas, S. , Nurus Sakib, M. , & Rashid, T. U. (2020). Chitosan based bioactive materials in tissue engineering applications-A review. Bioactive Materials, 5(1), pp. 164–183, Mar. 2020, doi: https://doi. org/10. 1016/j. bioactmat. 2020. 01. 012
[23]. Fu, J. , Yang, F. , & Guo, Z. (2018). The chitosan hydrogels: from structure to function. New Journal of Chemistry, 42(21), pp. 17162–17180, 2018, doi: https://doi. org/10. 1039/c8nj03482f
[24]. Shariatinia, Z. , & Jalali, A. M. (2018). Chitosan-based hydrogels: Preparation, properties and applications. International Journal of Biological Macromolecules, 115, pp. 194–220, Aug. 2018, doi: https://doi. org/10. 1016/j. ijbiomac. 2018. 04. 034
[25]. Yang, J. , et al. (2021). Advanced applications of chitosan-based hydrogels: From biosensors to intelligent food packaging system. Trends in Food Science & Technology, 110, pp. 822–832, Apr. 2021, doi: https://doi. org/10. 1016/j. tifs. 2021. 02. 032
[26]. Saravanakumar, K. et al. (2022). Application of hyaluronic acid in tissue engineering, regenerative medicine, and nanomedicine: A review. International Journal of Biological Macromolecules, 222, pp. 2744–2760, Dec. 2022, doi: https://doi. org/10. 1016/j. ijbiomac. 2022. 10. 055
[27]. Luo, Z. , Wang, Y. , Li, J. , Wang, J. , Yu, Y. , & Zhao, Y. (2023). Tailoring Hyaluronic Acid Hydrogels for Biomedical Applications. Advanced Functional Materials, 33(49), Sep. 2023, doi: https://doi. org/10. 1002/adfm. 202306554
[28]. Pérez, L. A. , Hernández, R. , Alonso, J. M. , Pérez-González, R. , & Sáez-Martínez, V. (2021). Hyaluronic Acid Hydrogels Crosslinked in Physiological Conditions: Synthesis and Biomedical Applications. Biomedicines, 9(9), p. 1113, Aug. 2021, doi: https://doi. org/10. 3390/biomedicines9091113
[29]. Davari, N. , et al. (2022). Protein-Based Hydrogels: Promising Materials for Tissue Engineering. Polymers, 14(5), p. 986, Feb. 2022, doi: https://doi. org/10. 3390/polym14050986
[30]. She, J. , et al. (2025). Recent advances in collagen-based hydrogels: Materials, preparation and applications. Reactive and Functional Polymers, 207, p. 106136, Feb. 2025, doi: https://doi. org/10. 1016/j. reactfunctpolym. 2024. 106136
[31]. Yang, X. , et al. (2025). Collagen-based hydrogel sol-gel phase transition mechanism and their applications. Advances in Colloid and Interface Science, 340, p. 103456, Feb. 2025, doi: https://doi. org/10. 1016/j. cis. 2025. 103456
[32]. Benival, D. , et al. (2023). Collagen-Based Hydrogels for the Eye: A Comprehensive Review. Gels, 9(8), pp. 643–643, Aug. 2023, doi: https://doi. org/10. 3390/gels9080643
[33]. Wang, Y. , et al. (2024). A dual-crosslinking electroactive hydrogel based on gelatin methacrylate and dibenzaldehyde-terminated telechelic polyethylene glycol for 3D bio-printing. Scientific Reports, 14(1), Feb. 2024, doi: https://doi. org/10. 1038/s41598-024-54853-9
[34]. Andreazza, R. , Morales, A. , Pieniz, S. , & Labidi, J. (2023). Gelatin-Based Hydrogels: Potential Biomaterials for Remediation. Polymers, 15(4), p. 1026, Jan. 2023, doi: https://doi. org/10. 3390/polym15041026
[35]. Mushtaq, F. , et al. (2022). Preparation, properties, and applications of gelatin-based hydrogels (GHs) in the environmental, technological, and biomedical sectors. International Journal of Biological Macromolecules, 218, pp. 601–633, Oct. 2022, doi: https://doi. org/10. 1016/j. ijbiomac. 2022. 07. 168
[36]. Zheng, H. , & Zuo, B. (2021). Functional silk fibroin hydrogels: preparation, properties and applications. Journal of Materials Chemistry B, 2021, doi: https://doi. org/10. 1039/d0tb02099k
[37]. Kim, S. H. , et al. (2021). 3D bioprinted silk fibroin hydrogels for tissue engineering. Nature Protocols, 16(12), pp. 5484–5532, Dec. 2021, doi: https://doi. org/10. 1038/s41596-021-00622-1
[38]. Zhang, H. , Xu, D. , Zhang, Y. , Li, M. , & Chai, R. (2022). Silk fibroin hydrogels for biomedical applications. Smart Medicine, 1(1), Dec. 2022, doi: https://doi. org/10. 1002/smmd. 20220011
[39]. Cao, H. , Duan, L. , Zhang, Y. , Cao, J. , & Zhang, K. (2021). Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduction and Targeted Therapy, 6(1), pp. 1–31, Dec. 2021, doi: https://doi. org/10. 1038/s41392-021-00830-x.
[40]. Cianchetti, M. , Laschi, C. , Menciassi, A. , & Dario, P. (2018). Biomedical applications of soft robotics. Nature Reviews Materials, 3(6), pp. 143–153, May 2018, doi: https://doi. org/10. 1038/s41578-018-0022-y
[41]. Yuk, H. , Zhang, T. , Parada, G. A. , Liu, X. , & Zhao, X. (2016). Skin-inspired hydrogel–elastomer hybrids with robust interfaces and functional microstructures. Nature Communications, 7(1), Jun. 2016, doi: https://doi. org/10. 1038/ncomms12028
[42]. Shin, Y. , Lee, H. S. , Jeong, H. , & Kim, D. -H. (2024). Recent advances in conductive hydrogels for soft biointegrated electronics: Materials, properties, and device applications. Wearable Electronics, vol. 1, pp. 255–280, Dec. 2024, doi: https://doi. org/10. 1016/j. wees. 2024. 10. 004
[43]. Park, C. S. , Kang, Y. -W. , Na, H. , & Sun, J. -Y. (2024). Hydrogels for bioinspired soft robots. Progress in Polymer Science, pp. 101791–101791, Jan. 2024, doi: https://doi. org/10. 1016/j. progpolymsci. 2024. 101791
[44]. Zhao, X. (2014). Multi-scale multi-mechanism design of tough hydrogels: building dissipation into stretchy networks. Soft Matter, 10(5), pp. 672–687, 2014, doi: https://doi. org/10. 1039/c3sm52272e
[45]. Li, H. , Go, G. , Ko, S. Y. , Park, J. -O. , & Park, S. (2016). Magnetic actuated pH-responsive hydrogel-based soft micro-robot for targeted drug delivery. Smart Materials and Structures, 25(2), p. 027001, Jan. 2016, doi: https://doi. org/10. 1088/0964-1726/25/2/027001
[46]. Huang, Y. , Yu, Q. , Su, C. , Jiang, J. , Chen, N. , & Shao, H. (2021). Light-Responsive Soft Actuators: Mechanism, Materials, Fabrication, and Applications. Actuators, 10(11), p. 298, Nov. 2021, doi: https://doi. org/10. 3390/act10110298
[47]. Xie, Z. , Shen, J. , Sun, H. , Li, J. , & Wang, X. (2021). Polymer-based hydrogels with local drug release for cancer immunotherapy. Biomedicine & Pharmacotherapy, 137, p. 111333, Feb. 2021, doi: https://doi. org/10. 1016/j. biopha. 2021. 111333
[48]. Chen, C. -H. , et al. (2018). Thermosensitive Injectable Hydrogel for Simultaneous Intraperitoneal Delivery of Doxorubicin and Prevention of Peritoneal Adhesion. International Journal of Molecular Sciences, 19(5), p. 1373, May 2018, doi: https://doi. org/10. 3390/ijms19051373
[49]. Tang, L. , Wang, L. , Yang, X. , Feng, Y. , Li, Y. , & Feng, W. (2021). Poly(N-isopropylacrylamide)-based smart hydrogels: Design, properties and applications. Progress in Materials Science, 115, p. 100702, Jan. 2021, doi: https://doi. org/10. 1016/j. pmatsci. 2020. 100702
[50]. Cao, C. , et al. (2021). Biodegradable hydrogel with thermo-response and hemostatic effect for photothermal enhanced anti-infective therapy. Nano Today, 39, p. 101165, Aug. 2021, doi: https://doi. org/10. 1016/j. nantod. 2021. 101165
[51]. Chao, Y. , Chen, Q. , & Liu, Z. (2019). Smart Injectable Hydrogels for Cancer Immunotherapy. Advanced Functional Materials, 30(2), p. 1902785, Aug. 2019, doi: https://doi. org/10. 1002/adfm. 201902785
[52]. Bae, J. , et al. (2020). Tailored hydrogels for biosensor applications. Journal of Industrial and Engineering Chemistry, 89, pp. 1–12, Sep. 2020, doi: https://doi. org/10. 1016/j. jiec. 2020. 05. 001
[53]. Mattheolabakis, G. , Milane, L. , Singh, A. , & Amiji, M. M. (2015). Hyaluronic acid targeting of CD44 for cancer therapy: from receptor biology to nanomedicine. Journal of Drug Targeting, 23(7–8), pp. 605–618, Sep. 2015, doi: https://doi. org/10. 3109/1061186x. 2015. 1052072
[54]. Amirthalingam, S. , & Rangasamy, J. (2021). Chitosan-Based Biosensor Fabrication and Biosensing Applications. Advances in Polymer Science, pp. 233–255, Jan. 2021, doi: https://doi. org/10. 1007/12_2021_85
[55]. Qureshi, A. , Gurbuz, Y. , & Niazi, J. H. (2012). Biosensors for cardiac biomarkers detection: A review. Sensors and Actuators B: Chemical, 171–172, pp. 62–76, Aug. 2012, doi: https://doi. org/10. 1016/j. snb. 2012. 05. 077
[56]. Zhang, L. (2022). Strong and Tough PAm/SA Hydrogel with Highly Strain Sensitivity. Journal of Renewable Materials, 10(2), pp. 415–430, doi: https://doi. org/10. 32604/jrm. 2022. 016650
[57]. Nagamine, K. , et al. (2019). Noninvasive Sweat-Lactate Biosensor Emplsoying a Hydrogel-Based Touch Pad. Scientific Reports, 9(1), Jul. 2019, doi: https://doi. org/10. 1038/s41598-019-46611-z
[58]. Park, S. , et al. (2021). Adaptive and multifunctional hydrogel hybrid probes for long-term sensing and modulation of neural activity. Nature Communications, 12(1), p. 3435, Jun. 2021, doi: https://doi. org/10. 1038/s41467-021-23802-9
[59]. Ma, J. , et al. (2023). Hydrogel sensors for biomedical electronics. Chemical Engineering Journal, 481, pp. 148317–148317, Dec. 2023, doi: https://doi. org/10. 1016/j. cej. 2023. 148317









