1. Artificial light collection system
With the increasing demand for energy supply and the continuous consumption of traditional fossil energy, the development of efficient renewable energy technology has become a top priority. Solar energy is expected to be clean, pollution-free, low operating costs and huge reserves.
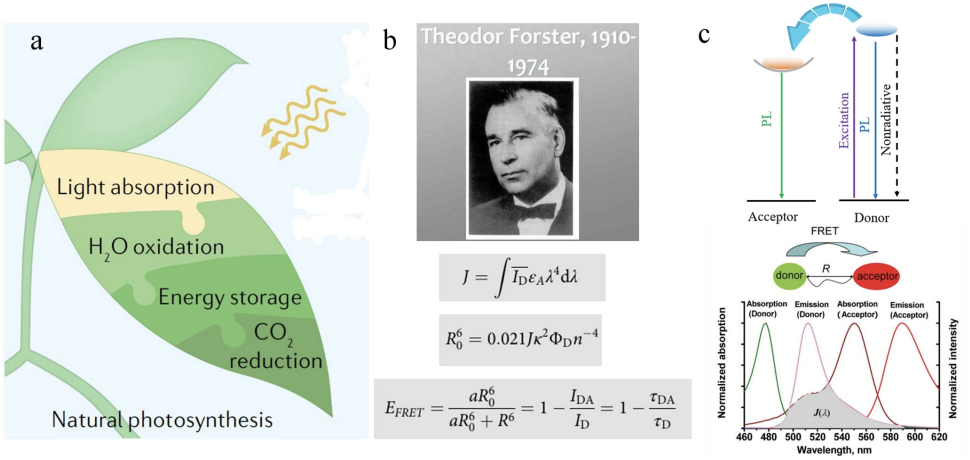
Photosynthesis in nature generally includes three processes: light capture, energy transfer and energy conversion, through which the efficient conversion of solar energy to chemical energy can be realized [1,2]. As the first stage of natural photosynthesis, the mechanism of light capture system (LHSS) has been deeply and carefully studied. In this process, chlorophyll and carotenoids and other pigment molecules are assembled into an ordered array through nanoscale supramolecular assembly, which can efficiently capture photons with a wide spectral band of 400-700 nm; After that, with the help of Förster resonance energy transfer (FRET) mechanism, the captured energy was accurately transferred to the reaction center, and finally more than 95% energy conversion efficiency was achieved [3-7].
Scientists have built an artificial light collection system based on the light capture process, so as to efficiently convert and utilize solar energy. Especially, the supramolecular artificial light capture system based on the Förster resonance energy transfer mechanism has shown great application potential in the field of photocatalysis [8-13].
2. Förster Resonance Energy Transfer (FRET)
FRET is a non radiative energy transfer process, which occurs between two fluorescent molecules (or chromophores). It was established by German physicist Theodor Förster through theoretical derivation in the 1940s [14], so it is named after it (Fig. 1a). It is an important tool for studying intermolecular interactions in biophysics, molecular biology and analytical chemistry.
The core of FRET is the energy transfer between the donor and the acceptor, and its formation needs to meet three conditions [15] (Fig. 1b, c):
(1) Donor recipient correspondence is close to each other (usually 10-100 μ m).
(2) The emission spectrum of the donor should overlap with the absorption spectrum of the receptor.
(3) The emission moment of the donor, the absorption moment of the receptor and their separation vector must be in a favorable mutual orientation.
The artificial light capture system constructed by researchers using covalent bond connection can not only obtain a very high donor/acceptor ratio, but also achieve a high antenna effect (AE), so that light energy can be concentrated and transmitted efficiently in the system. In addition, the system can also achieve efficient energy transfer efficiency (ΦET), ensuring that the captured light energy can be transferred to the reaction center quickly and accurately, creating favorable conditions for simulating natural photosynthesis.
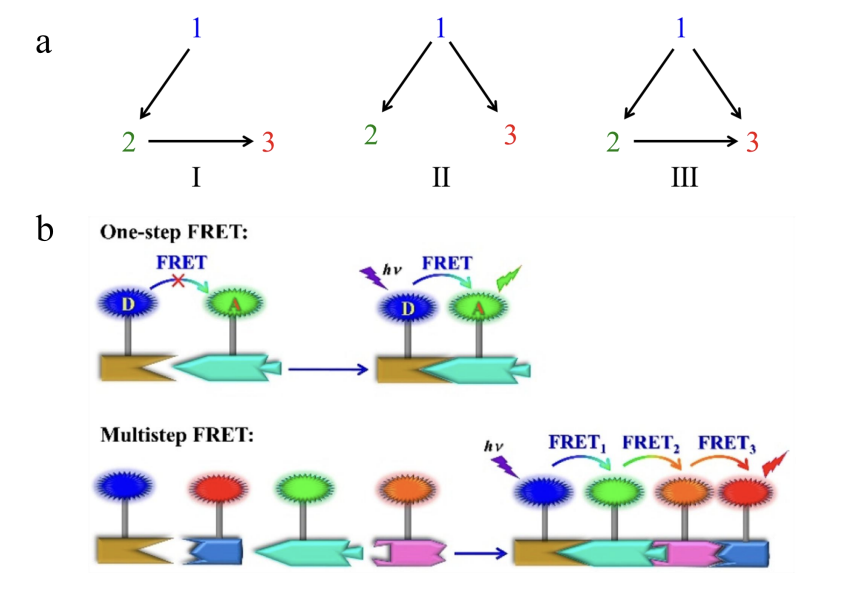
In recent years, FRET's energy transfer system has been expanded from the traditional single donor/acceptor system to a trichromatic system using two or more donor/acceptor pairs [16-22]. The trichromatic energy transfer system is composed of two donor/acceptor pairs that share the same chromophore, including three energy transfer paths (Fig. 2a):
(I) after chromophore 1 (donor) is excited, energy is transferred to chromophore 2 (receptor). After receiving the energy, chromophore 2 acts as a new donor to transfer the energy to chromophore 3, that is, multiple one-way single-step transfer.
(II) when chromophore 1 (donor) is excited, it transfers energy to chromophore 2 (receptor) and chromophore 3 (receptor) at the same time, that is, parallel one-step multidirectional transfer.
(Ⅲ) chromophore 1 is excited to transfer energy to both chromophore 2 (1 → 2) and chromophore 3 (1 → 3). After receiving energy, chromophore 2 continues to transfer energy (2 → 3) to chromophore 3 as a donor. That is, the combination of parallel single step and double step.
R0 is the single donor recipient separation distance corresponding to 50% energy transfer efficiency. Unlike R, R0 depends on the donor emission and absorption spectral overlap integral (J), the orientation of the dipole moment (orientation factor κ2, usually assumed as 2/3), the donor quantum yield (ϕD) and the refractive index (n) of the medium. The spectral overlap between donor emission and acceptor absorption provides the necessary energy resonance for FRET. It is quantified by multiplying the normalized donor emission intensity (ID) by the molar extinction coefficient of the acceptor (εA, unit: M–1cm–1), and then multiplying by the quartic power of the wavelength (formula 1-2) within the spectral overlap range. Namely:
R0 can be expressed as:
ΦFRET is related to the FRET rate constant (kET), the radiative decay constant of the donor (kr), and the non-radiative decay constant (knr) as shown in the following equation:
The sum of kr and knr equals the reciprocal of the fluorescence lifetime (τD) of the donor in the absence of the acceptor. The average distance (R) between the donor and acceptor can be calculated from kET:
3. Covalently linked FRET system
The first type of FRET mainly includes covalent coupling of donors and acceptors to achieve long-wavelength emission, including near-infrared emission, and the preparation of highly sensitive fluorescent probes by opening/closing FRET through external stimulation. Due to their high molar extinction coefficient, strong emission characteristics, high quantum yield, and excellent photophysical stability, BODIPY derivatives have attracted much attention in FRET systems. Its core feature is the direct covalent labeling of the fluorescent donor (Donor) and acceptor (Acceptor). In recent years, scientists have innovated upon this system for applications in various fields, including material detection, cellular imaging, and fluorescent anti-counterfeiting.
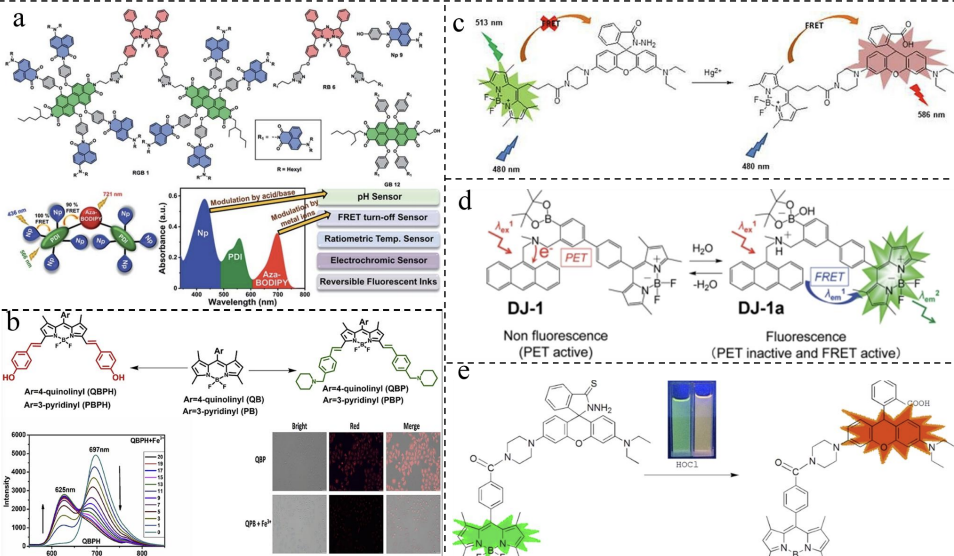
The Rani team used naphthyl imine as the donor, peryl imine as the intermediate acceptor, and Aza-BODIPY as the final acceptor to construct a two-step cascading SS-FRET system via covalent bonding [23] (Figure 3a). This system successfully achieved near-infrared emission at 721 nm and demonstrated excellent stimulus responsiveness. The Yu team developed a ratio-fluorescence probe with BODIPY-rhodamine for sensitive detection of Hg²⁺ ions [24] (Figure 3b). In the presence of Hg²⁺ ions, the hydrolysis of rhodamine's spirocaprolactam leads to SS-FRET between BODIPY and rhodamine, causing the system's fluorescence color to shift from green to orange. The Ooyama team developed a BODIPY-based fluorescent sensor using SS-FRET to measure trace amounts of water in organic samples [25] (Figure 3c). Using a similar strategy, the Li team developed two probes for detecting Fe³⁺, demonstrating high sensitivity and selectivity in live cells [26](Figure 3d). The Liu team developed a FRET fluorescent sensor based on the BODIPY and rhodamine thiocyanate system, which can effectively detect HOCl in live cells [27] (Figure 3e).
4. Self-assembled FRET system
Self-assembly refers to the process by which basic structural units (such as molecules, nanoparticles, etc.) spontaneously organize or aggregate into ordered aggregates with specific structure and function through non-covalent interactions (such as hydrogen bonds, host-guest interactions, van der Waals forces, hydrophobic interactions, electrostatic interactions, etc.) without excessive external intervention (such as artificial deliberate arrangement, template-guided assembly, etc.). This process demonstrates the self-organizing capability at the molecular or nanoscale level, a phenomenon widely observed in nature, and serves as an important strategy for developing functional materials. The orderliness and functionality of self-assembly systems stem from the precise recognition and synergistic interactions between structural units, and this bottom-up assembly approach provides a significant avenue for designing and preparing novel functional materials [28] Lehn and Whitesides, among others, have classified self-assembly processes into two major categories from a thermodynamic perspective [29]: static self-assembly under thermodynamic equilibrium conditions, and dynamic self-assembly (also known as dissipative self-assembly) under non-equilibrium conditions. Static self-assembly refers to the spontaneous formation of ordered aggregates between molecules under thermodynamic equilibrium conditions. The aggregate structures formed in this process exhibit environmental independence and can remain stable under conditions without external interference. Dynamic self-assembly, on the other hand, refers to aggregate structures formed under non-equilibrium conditions, where the internal components are in a state of continuous dynamic equilibrium. The maintenance of such self-assembly systems relies on external energy input or internal energy dissipation. In recent years, significant progress has been made in the development of artificial light-harvesting systems based on supramolecular self-assembly principles. These systems exhibit outstanding performance characteristics, including extremely high donor-acceptor ratios, exceptional energy transfer efficiency, and significant antenna effects. Among these, systems based on host-guest complexation, biomolecules, metal coordination, and novel solvent-free SS-FRET systems have garnered significant attention due to their identifiable and controllable intermolecular interaction mechanisms and highly efficient energy transfer properties. The following sections will provide a detailed exploration of these systems.
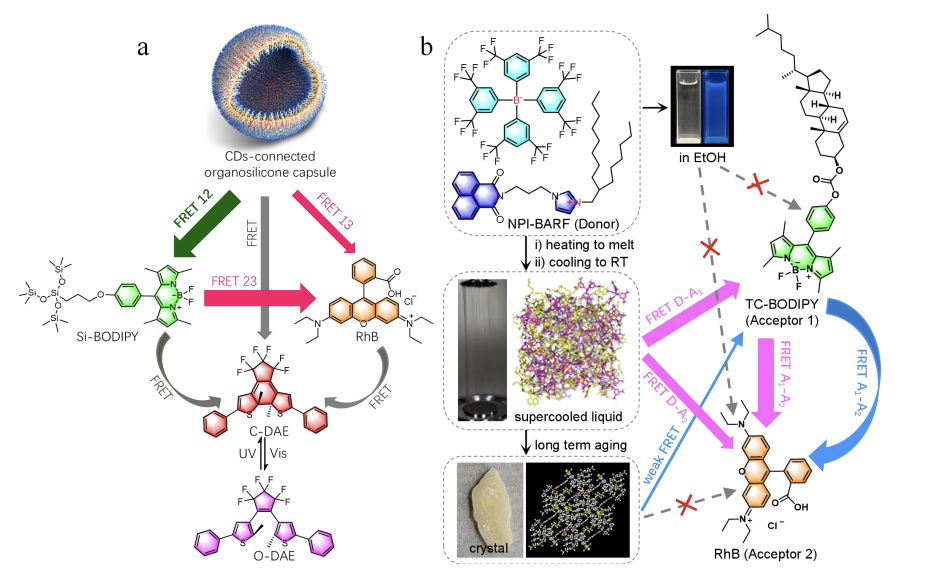
In nature, photosynthetic organisms achieve directed solar energy transfer and conversion through sophisticated light-harvesting complexes, a process closely related to Förster resonance energy transfer (FRET). If we focus solely on single photons capable of exciting chlorophyll molecules, the maximum energy transfer efficiency can reach ~95%. Inspired by this, a large number of artificial light harvesting systems (ALHSs) based on cascaded FRET have been developed. However, only a few examples have achieved an overall energy transfer efficiency exceeding 80%. Recently, Li group has made a breakthrough in this field. They prepared a novel organosilicon capsule (CDs-Si) using citric acid (CA) and amphiphilic polydimethylsiloxane as carbon sources via a solvent-free pyrolysis method [30]. The wide emission spectrum of the capsules covers the absorption spectra of green (siloxane-functionalized BODIPY, Si-BODIPY) and red (rhodamine B, RhB) dyes, promoting FRET in the CDs-Si/Si-BODIPY and CDs-Si/RhB binary systems. In the CDs-Si/Si-BODIPY/RhB ternary system, cascaded SS-FRET from CDs-Si to Si-BODIPY to RhB was achieved, accompanied by parallel FRET from CDs-Si directly to RhB, with an overall energy transfer efficiency of ~86%. By introducing photoswitching molecules (diaryl ethylene compounds, DAE), the system achieves switchable luminescence under alternating ultraviolet and visible light irradiation, forming intelligent AHLSs.
Subsequently, they studied ionic compounds with naphthalimide (NPI) as the head group , and the energy transfer pathways and efficiencies of the same donor-acceptor combinations in solid, solution, and supercooled fluid states were compared [31]. Structurally, one end features a naphthalimide luminescent group, the other end a branched alkyl chain, and the middle a positively charged imidazole group, with tetrakis[3,5-bis(trifluoromethyl)phenyl]boronic acid (BARF-) serving as the counterion. Naphthalimide derivatives (NPI-BARF) heated above their melting point and then cooled to room temperature can form supercooled fluids lasting up to 16 days. In this system, the blue-emitting NPI-BARF serves as the donor, BODIPY derivatives (TC-BODIPY) as the intermediate acceptor, and RhB as the final acceptor. In the supercooled fluid state, the overall energy transfer efficiency reaches up to 97%, with the single-step energy transfer efficiency from TC-BODIPY to RhB as high as 99.9%, approaching the theoretical limit; in the solid state, due to poor spectral matching between the donor and acceptor, the overall energy transfer efficiency is approximately 40%; In the solution state, due to the donors and acceptors being in a monodisperse state in ethanol, the distance between them is insufficient to reach the FRET range, and thus FRET does not occur.
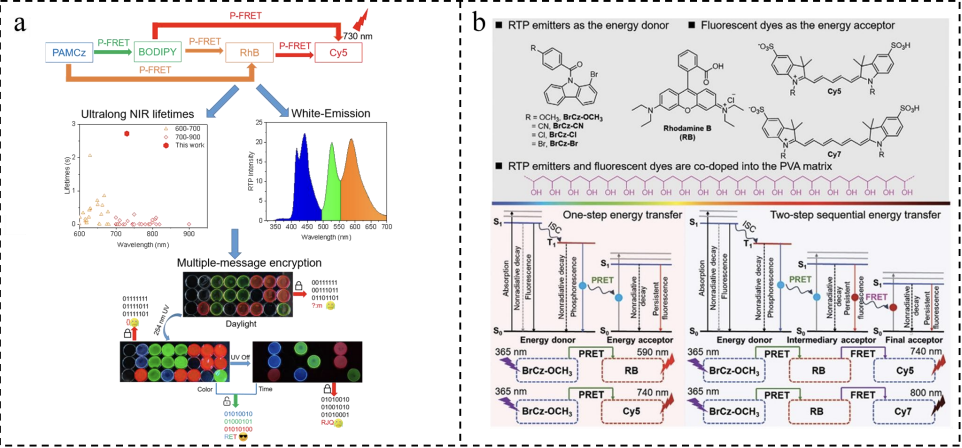
According to the bandgap law, the longer the emission wavelength, the lower the triplet energy level, and the easier it is for non-radiative transitions to occur, leading to phosphorescence quenching. Therefore, achieving RTP in the red or near-infrared (NIR) region remains an important challenge. The efficient ALHSs also contributes to long-lived near-infrared phosphorescence emission. Yu et al. used poly(acrylamide-co-N-vinylcarbazole) (PAMCz) with ultra-long room-temperature phosphorescence as the energy donor, and common green/red/NIR fluorescent dyes (BODIPY derivatives, RhB, and Cy5) as the energy acceptors [32]. In the quaternary system, three parallel cascading FRET pathways coexist: (I) PAMCz to BODIPY, then to RhB, and finally to Cy5; (II) PAMCz to Ala-BODIPY, then to Cy5; and (III) PAMCz to RhB, then to Cy5. White and NIR phosphorescence emission with a lifetime of 2.7 seconds was achieved through multi-path FRET. Multi-path cascaded TS-FRET demonstrates significant advantages over single-path cascaded TS-FRET, including higher antenna effect (AE), broader color-tunable spectra, and enhanced phosphorescence intensity and lifetime of the final acceptor. Additionally, these materials exhibit excellent stimulus responsiveness, making them highly promising for applications in information multi-encryption fields.
Tang et al. constructed a multi-step TS-FRET system in a PVA matrix using 1-bromocarbazole derivatives with ultra-long blue RTP emission as the donor [33], RhB as the intermediate acceptor, and NIR dyes Cy5 or Cy7 as the final acceptor (Figure 1-18b). By adjusting the donor-acceptor ratio, the team successfully achieved long-lived phosphorescence emission spanning the visible to near-infrared regions. Compared to single-step TS-FRET, two-step sequential TS-FRET exhibits higher overall energy transfer efficiency, richer phosphorescence colors, and higher RTP quantum yields for the final acceptor. These water-soluble polymer afterglow materials with ultra-long lifetime, multicolor emission, and persistent near-infrared luminescence demonstrate significant potential applications in advanced anti-counterfeiting and information security technologies.
5. Conclusion
This paper provides a detailed discussion of two types of ALHSs: covalently linked and non-covalently linked. In terms of energy transfer pathways, the paper reviews single-step FRET, sequential FRET, and systems where single-step and sequential FRET occur in parallel. Regarding the construction of efficient ALHSs, the paper discusses efficient FRET based on organosilicon capsules and ionic liquids. In terms of material performance, the paper introduces polymer systems that achieve a 2.7 s near-infrared phosphorescence lifetime in polymers based on efficient FRET. Overall, the construction of efficient ALHSs has a profound impact on the preparation of high-performance materials. Further expanding the application of energy based on the construction of efficient ALHSs may be one of the key issues to be addressed in this field in the future.
References
[1]. Jiangquan L., Jiafang X., Aya G. A. M., Xiang Z., Yangyang F., Lei J., Enbo Z., Daqiang Y., and Yaobing W., Solar utilization beyond photosynthesis. Nat. Rev. Chem. 2023, 7, 91.
[2]. Blankenship, R.; Tiede, D.; Barber, J.; Brudvig, G.; Fleming, G.; Ghirardi, M.; Gunner, M.; Junge, W.; Kramer, D.; Melis, A.; et al. Comparing Photosynthetic and Photovoltaic Efficiencies and Recognizing the Potential for Improvement. Science 2011, 332 (6031), 805-809.
[3]. Yuan, Y.-X.; Jia, J.-H.; Song, Y.-P.; Ye, F.-Y.; Zheng, Y.-S.; Zang, S.-Q. Fluorescent TPE Macrocycle Relayed Light-Harvesting System for Bright Customized-Color Circularly Polarized Luminescence. J. Am. Chem. Soc. 2022, 144, 5389-5399.
[4]. Jiang, Y.; McNeill, J. Light-Harvesting and Amplified Energy Transfer in Conjugated Polymer Nanoparticles. Chem. Rev. 2017, 117, 838-859.
[5]. Frischmann, P.; Mahata, K.; Würthner, F. Powering the Future of Molecular Artificial Photosynthesis with Light-Harvesting Metallosupramolecular Dye Assemblies. Chem. Soc. Rev. 2013, 42, 1847-1870.
[6]. Croce, R.; van Amerongen, H. Light Harvesting in Oxygenic Photosynthesis: Structural Biology Meets Spectroscopy. Science 2020, 369, 933.
[7]. Dumele, O.; Chen, J.; Passarelli, J. V.; Stupp, S. I. Supramolecular Energy Materials. Adv. Mater. 2020, 32, 1907247.
[8]. Zhang, D.; Yu, W.; Li, S.; Xia, Y.; Li, X.; Li, Y.; Yi, T. Artificial Light-Harvesting Metallacycle System with Sequential Energy Transfer for Photochemical Catalysis. J. Am. Chem. Soc. 2021, 143, 1313-1317.
[9]. Peng, H.-Q.; Chen, Y.-Z.; Zhao, Y.; Yang, Q.-Z.; Wu, L.-Z.; Tung, C.-H.; Zhang, L.-P.; Tong, Q.-X. Artificial Light-Harvesting System Based on Multifunctional Surface-Cross-Linked Micelles. Angew. Chem. Int. Ed. 2012, 51, 2088-2092.
[10]. Yu, Z.; Bisoyi, H. K.; Chen, X.-M.; Nie, Z.-Z.; Wang, M.; Yang, H.; Li, Q. An Artificial Light-Harvesting System with Controllable Efficiency Enabled by an Annulene-Based Anisotropic Fluid. Angew. Chem. Int. Ed. 2022, 61, 2200466.
[11]. Xia, Y.; Chen, M.; Li, S.; Li, M.; Li, X.; Yi, T.; Zhang, D. An Artificial Light-Harvesting System with Sequential Energy Transfer for Information Dual Encryption and Anticounterfeiting. J. Mater. Chem. C 2022, 10, 12332-12337.
[12]. Zhang, G.; Yu, L.; Chen, J.; Dong, R.; Godbert, N.; Li, H.; Hao, J. Artificial Light-Harvesting System with White-Light Emission in a Bicontinuous Ionic Medium. J. Phys. Chem. Lett. 2022, 13, 8999−9006.
[13]. Yu, L.; Zhao, R.; Wang, N.; Feng, S.; Xu, X. Coordination Mode-Regulated Lanthanide Supramolecular Hydrogels with Tunable Luminescence and Stimuli-Responsive Properties. ACS Appl. Polym. Mater. 2021, 3, 3623-3630.
[14]. FORSTER, T. Zwischenmolekulare Energiewanderung und Fluoreszenz. Ann. Phys. 1948, 2, 55-75.
[15]. Wu, L.; Huang, C.; Emery, B.; Sedgwick, A.; Bull, S.; He, X.; Tian, H.; Yoon, J.; Sessler, J.; James, T. Forster Resonance Energy Transfer (FRET)-based Small-molecule Sensors and Imaging Agents. Chem. Soc. Rev. 2020, 49, 5110-5139.
[16]. Watrob, H.; Barkley, M. Two-step FRET as a Structural Tool. Biophys. J. 2003, 84, 475A-475A.
[17]. Jia, P.-P.; Xu, L.; Hu, Y.-X.; Li, W.-J.; Wang, X.-Q.; Ling, Q.-H.; Shi, X.; Yin, G.-Q.; Li, X.; Sun, H.; et al. Orthogonal Self-Assembly of a Two-Step Fluorescence-Resonance Energy Transfer System with Improved Photosensitization Efficiency and Photooxidation Activity. J. Am. Chem. Soc. 2021, 143, 399-408.
[18]. Algar, W. R.; Hildebrandt, N.; Vogel, S. S.; Medintz, I. L. FRET as a Biomolecular Research Tool-understanding its Potential While Avoiding Pitfalls. Nat. Methods 2019, 16, 815-829.
[19]. Lone, M. S.; Bhat, P. A.; Afzal, S.; Chat, O. A.; Dar, A. A. Energy Transduction Through FRET in Self-assembled Soft Nanostructures Based on Surfactants/Polymers: Current Scenario and Prospects. Soft Matter 2021, 17, 425-446.
[20]. Asadi-Zaki, N.; Mardani, H.; Roghani-Mamaqani, H.; Wang, F. Stimuli-Induced Adjustment of Spatial Distribution of Fluorescence Resonance Energy Transfer Dyads in Smart Polymers. Coord. Chem. Rev. 2024, 500.
[21]. Besford, Q.; Yong, H.; Merlitz, H.; Christofferson, A.; Sommer, J.; Uhlmann, P.; Fery, A. FRET-Integrated Polymer Brushes for Spatially Resolved Sensing of Changes in Polymer Conformation. Angew. Chem. Int. Ed. 2021, 60, 16600-16606.
[22]. Jia, D.; Luo, Q.; Liu, S.; Hou, C.; Liu, J. An Artificial Light-Harvesting System Based on Supramolecular AIEgen Assembly. Chem.-Eur. J. 2024, 30, 2402438.
[23]. Kavita R , Sanchita S . Multi-stimuli programmable FRET based RGB absorbing antennae towards ratiometric temperature, pH and multiple metal ion sensing. [J]. Chem. Sci. 2021, 12, 15533-15542.
[24]. Li C , Sun Q , Zhao Q , et al. Highly selective ratiometric fluorescent probes for the detection of Fe3+and its application in living cells [J]. Spectrochim. Acta, Part A, 2020, 228, 117720.
[25]. Liu Y , Zhao Z , Miao J , et al. A ratiometric fluorescent probe based on boron dipyrromethene and rhodamine Förster resonance energy transfer platform for hypochlorous acid and its application in living cells [J]. Anal. Chim. Acta, 2016, 921, 77-83.
[26]. Dan W, Xuankai D, Gongnv X, et al., A novel FRET fluorescent probe based on BODIPY- rhodamine system for Hg2+imaging in living cells [J]. J. Mol. Struct., 2021, 1236
[27]. Jinbo D , Imato K , Ooyama Y . Fluorescent sensor for water based on photo-induced electron transfer and Förster resonance energy transfer: anthracene-(aminomethyl)phenylboronic acid ester-BODIPY structure [J]. RSC Adv., 2019, 9, 15335-15340
[28]. Lehn, J. Perspectives in Chemistry-Steps towards Complex Matter. Angew. Chem. Int. Ed. 2013, 52, 2836-2850.
[29]. Nagarajan, R. Molecular Packing Parameter and Surfactant Self-assembly: The Neglected Role of the Surfactant Tail. Langmuir 2002, 18, 31-38.
[30]. Longyue Yu, Hailong Liu, Ning Feng, Gang Yi, Xia Xin, Jingcheng Hao, and Hongguang Li, Multi-Step and Switchable Energy Transfer in Photoluminescent Organosilicone Capsules. Adv. Sci. 2024, 11, 2402565
[31]. Longyue Yu, Xionghui Huang, Ning Feng, Wenwen Fu, Xia Xin, Jingcheng Hao, Hongguang Li, Solvent-Free Artificial Light-Harvesting System in a Fluid Donor with Highly Efficient Förster Resonance Energy Transfer. J. Phys. Chem. Lett. 2025, 16, 1305−1311.
[32]. Longyue Yu, Ning Feng, Wenwen Fu, Xionghui Huang, Xin Li, Xia Xin, Jingcheng Hao, Hongguang Li,Near-Infrared Organic Ultralong Room-Temperature Phosphorescence Materials Constructed via Multiple Phosphorescence Resonance Energy Transfer. Adv. Optical Mater. 2025, 2403003.
[33]. Chen, K.; Xiong, Y.; Wang, D.; Pan, Y.; Zhao, Z.; Wang, D.; Tang, B. A Facile Strategy for Achieving Polymeric Afterglow Materials with Wide Color-Tunability and Persistent Near-Infrared Luminescence. Adv. Funct. Mater. 2024, 34, 2312883.
Cite this article
Zhao,Y. (2025). Construction and Performance of Highly Efficient Artificial Light-Harvesting Systems. Applied and Computational Engineering,180,126-134.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of CONF-MCEE 2026 Symposium: Advances in Sustainable Aviation and Aerospace Vehicle Automation
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Jiangquan L., Jiafang X., Aya G. A. M., Xiang Z., Yangyang F., Lei J., Enbo Z., Daqiang Y., and Yaobing W., Solar utilization beyond photosynthesis. Nat. Rev. Chem. 2023, 7, 91.
[2]. Blankenship, R.; Tiede, D.; Barber, J.; Brudvig, G.; Fleming, G.; Ghirardi, M.; Gunner, M.; Junge, W.; Kramer, D.; Melis, A.; et al. Comparing Photosynthetic and Photovoltaic Efficiencies and Recognizing the Potential for Improvement. Science 2011, 332 (6031), 805-809.
[3]. Yuan, Y.-X.; Jia, J.-H.; Song, Y.-P.; Ye, F.-Y.; Zheng, Y.-S.; Zang, S.-Q. Fluorescent TPE Macrocycle Relayed Light-Harvesting System for Bright Customized-Color Circularly Polarized Luminescence. J. Am. Chem. Soc. 2022, 144, 5389-5399.
[4]. Jiang, Y.; McNeill, J. Light-Harvesting and Amplified Energy Transfer in Conjugated Polymer Nanoparticles. Chem. Rev. 2017, 117, 838-859.
[5]. Frischmann, P.; Mahata, K.; Würthner, F. Powering the Future of Molecular Artificial Photosynthesis with Light-Harvesting Metallosupramolecular Dye Assemblies. Chem. Soc. Rev. 2013, 42, 1847-1870.
[6]. Croce, R.; van Amerongen, H. Light Harvesting in Oxygenic Photosynthesis: Structural Biology Meets Spectroscopy. Science 2020, 369, 933.
[7]. Dumele, O.; Chen, J.; Passarelli, J. V.; Stupp, S. I. Supramolecular Energy Materials. Adv. Mater. 2020, 32, 1907247.
[8]. Zhang, D.; Yu, W.; Li, S.; Xia, Y.; Li, X.; Li, Y.; Yi, T. Artificial Light-Harvesting Metallacycle System with Sequential Energy Transfer for Photochemical Catalysis. J. Am. Chem. Soc. 2021, 143, 1313-1317.
[9]. Peng, H.-Q.; Chen, Y.-Z.; Zhao, Y.; Yang, Q.-Z.; Wu, L.-Z.; Tung, C.-H.; Zhang, L.-P.; Tong, Q.-X. Artificial Light-Harvesting System Based on Multifunctional Surface-Cross-Linked Micelles. Angew. Chem. Int. Ed. 2012, 51, 2088-2092.
[10]. Yu, Z.; Bisoyi, H. K.; Chen, X.-M.; Nie, Z.-Z.; Wang, M.; Yang, H.; Li, Q. An Artificial Light-Harvesting System with Controllable Efficiency Enabled by an Annulene-Based Anisotropic Fluid. Angew. Chem. Int. Ed. 2022, 61, 2200466.
[11]. Xia, Y.; Chen, M.; Li, S.; Li, M.; Li, X.; Yi, T.; Zhang, D. An Artificial Light-Harvesting System with Sequential Energy Transfer for Information Dual Encryption and Anticounterfeiting. J. Mater. Chem. C 2022, 10, 12332-12337.
[12]. Zhang, G.; Yu, L.; Chen, J.; Dong, R.; Godbert, N.; Li, H.; Hao, J. Artificial Light-Harvesting System with White-Light Emission in a Bicontinuous Ionic Medium. J. Phys. Chem. Lett. 2022, 13, 8999−9006.
[13]. Yu, L.; Zhao, R.; Wang, N.; Feng, S.; Xu, X. Coordination Mode-Regulated Lanthanide Supramolecular Hydrogels with Tunable Luminescence and Stimuli-Responsive Properties. ACS Appl. Polym. Mater. 2021, 3, 3623-3630.
[14]. FORSTER, T. Zwischenmolekulare Energiewanderung und Fluoreszenz. Ann. Phys. 1948, 2, 55-75.
[15]. Wu, L.; Huang, C.; Emery, B.; Sedgwick, A.; Bull, S.; He, X.; Tian, H.; Yoon, J.; Sessler, J.; James, T. Forster Resonance Energy Transfer (FRET)-based Small-molecule Sensors and Imaging Agents. Chem. Soc. Rev. 2020, 49, 5110-5139.
[16]. Watrob, H.; Barkley, M. Two-step FRET as a Structural Tool. Biophys. J. 2003, 84, 475A-475A.
[17]. Jia, P.-P.; Xu, L.; Hu, Y.-X.; Li, W.-J.; Wang, X.-Q.; Ling, Q.-H.; Shi, X.; Yin, G.-Q.; Li, X.; Sun, H.; et al. Orthogonal Self-Assembly of a Two-Step Fluorescence-Resonance Energy Transfer System with Improved Photosensitization Efficiency and Photooxidation Activity. J. Am. Chem. Soc. 2021, 143, 399-408.
[18]. Algar, W. R.; Hildebrandt, N.; Vogel, S. S.; Medintz, I. L. FRET as a Biomolecular Research Tool-understanding its Potential While Avoiding Pitfalls. Nat. Methods 2019, 16, 815-829.
[19]. Lone, M. S.; Bhat, P. A.; Afzal, S.; Chat, O. A.; Dar, A. A. Energy Transduction Through FRET in Self-assembled Soft Nanostructures Based on Surfactants/Polymers: Current Scenario and Prospects. Soft Matter 2021, 17, 425-446.
[20]. Asadi-Zaki, N.; Mardani, H.; Roghani-Mamaqani, H.; Wang, F. Stimuli-Induced Adjustment of Spatial Distribution of Fluorescence Resonance Energy Transfer Dyads in Smart Polymers. Coord. Chem. Rev. 2024, 500.
[21]. Besford, Q.; Yong, H.; Merlitz, H.; Christofferson, A.; Sommer, J.; Uhlmann, P.; Fery, A. FRET-Integrated Polymer Brushes for Spatially Resolved Sensing of Changes in Polymer Conformation. Angew. Chem. Int. Ed. 2021, 60, 16600-16606.
[22]. Jia, D.; Luo, Q.; Liu, S.; Hou, C.; Liu, J. An Artificial Light-Harvesting System Based on Supramolecular AIEgen Assembly. Chem.-Eur. J. 2024, 30, 2402438.
[23]. Kavita R , Sanchita S . Multi-stimuli programmable FRET based RGB absorbing antennae towards ratiometric temperature, pH and multiple metal ion sensing. [J]. Chem. Sci. 2021, 12, 15533-15542.
[24]. Li C , Sun Q , Zhao Q , et al. Highly selective ratiometric fluorescent probes for the detection of Fe3+and its application in living cells [J]. Spectrochim. Acta, Part A, 2020, 228, 117720.
[25]. Liu Y , Zhao Z , Miao J , et al. A ratiometric fluorescent probe based on boron dipyrromethene and rhodamine Förster resonance energy transfer platform for hypochlorous acid and its application in living cells [J]. Anal. Chim. Acta, 2016, 921, 77-83.
[26]. Dan W, Xuankai D, Gongnv X, et al., A novel FRET fluorescent probe based on BODIPY- rhodamine system for Hg2+imaging in living cells [J]. J. Mol. Struct., 2021, 1236
[27]. Jinbo D , Imato K , Ooyama Y . Fluorescent sensor for water based on photo-induced electron transfer and Förster resonance energy transfer: anthracene-(aminomethyl)phenylboronic acid ester-BODIPY structure [J]. RSC Adv., 2019, 9, 15335-15340
[28]. Lehn, J. Perspectives in Chemistry-Steps towards Complex Matter. Angew. Chem. Int. Ed. 2013, 52, 2836-2850.
[29]. Nagarajan, R. Molecular Packing Parameter and Surfactant Self-assembly: The Neglected Role of the Surfactant Tail. Langmuir 2002, 18, 31-38.
[30]. Longyue Yu, Hailong Liu, Ning Feng, Gang Yi, Xia Xin, Jingcheng Hao, and Hongguang Li, Multi-Step and Switchable Energy Transfer in Photoluminescent Organosilicone Capsules. Adv. Sci. 2024, 11, 2402565
[31]. Longyue Yu, Xionghui Huang, Ning Feng, Wenwen Fu, Xia Xin, Jingcheng Hao, Hongguang Li, Solvent-Free Artificial Light-Harvesting System in a Fluid Donor with Highly Efficient Förster Resonance Energy Transfer. J. Phys. Chem. Lett. 2025, 16, 1305−1311.
[32]. Longyue Yu, Ning Feng, Wenwen Fu, Xionghui Huang, Xin Li, Xia Xin, Jingcheng Hao, Hongguang Li,Near-Infrared Organic Ultralong Room-Temperature Phosphorescence Materials Constructed via Multiple Phosphorescence Resonance Energy Transfer. Adv. Optical Mater. 2025, 2403003.
[33]. Chen, K.; Xiong, Y.; Wang, D.; Pan, Y.; Zhao, Z.; Wang, D.; Tang, B. A Facile Strategy for Achieving Polymeric Afterglow Materials with Wide Color-Tunability and Persistent Near-Infrared Luminescence. Adv. Funct. Mater. 2024, 34, 2312883.









