1.Introduction
The global energy structure is undergoing profound transformation, with renewable sources such as solar and wind power expanding rapidly but exhibiting inherent intermittency and vulnerability to climatic fluctuations, which creates instability in power output and challenges for grid reliability. To mitigate these issues, energy storage technologies play a critical role by balancing supply and demand, enabling peak shaving and valley filling, and enhancing system flexibility. Among the various storage approaches, electrochemical energy storage has attracted particular attention due to its high energy density, rapid response, and deployment flexibility, with lithium-ion batteries (LIBs) being the most mature and widely applied in electronics, electric vehicles, and power grids[1]. However, the uneven distribution and high cost of lithium resources, combined with environmental concerns from mining, constrain their long-term scalability [2,3]. This has driven research and industry to seek resource-abundant and cost-effective alternatives, among which sodium-ion batteries (SIBs) are emerging as a promising candidate due to the chemical similarity of sodium to lithium and its global abundance. Nevertheless, sodium’s larger ionic radius results in slower diffusion kinetics and incompatibility with many existing LIB electrode materials, creating both challenges and opportunities for material innovation. In particular, cathode materials largely determine the energy density, while anode materials strongly influence cycle life, making the development of high-capacity, long-life cathodes central to advancing practical SIB applications [4]. This paper therefore reviews the performance of three representative cathode materials, evaluates their respective advantages and limitations, and highlights future research directions, aiming to provide both a theoretical foundation and practical guidance for accelerating the commercialization of sodium-ion batteries in large-scale energy storage systems.
2. Methodology
This review adopts a structured research design to ensure the reliability and comprehensiveness of the analysis. Literature was retrieved primarily from the China National Knowledge Infrastructure (CNKI) database, with the keywords “cathode materials for sodium-ion batteries” and a time span from 2020 to 2025. To maintain academic rigor, only peer-reviewed journal articles and master’s theses were included, while patents were excluded. The collected literature was examined using a combination of qualitative, classification, and comparative analytical methods. Specifically, qualitative analysis was employed to describe the crystal structure characteristics, ion migration mechanisms, and electrochemical behaviors of different cathode materials. The materials were then classified by structural type and chemical composition into layered transition metal oxides, polyanionic compounds, and Prussian blue analogues, and the advantages and limitations of each category were systematically discussed. Finally, a comparative analysis was carried out to highlight the similarities and differences among these materials in terms of energy density, cycling stability, and ion transport rates, thereby providing a comprehensive and multi-perspective reference for the optimization and future design of high-performance sodium-ion battery cathodes. Ethical considerations were not applicable in this study, as the research is based entirely on previously published literature without the involvement of human participants or animal experiments.
3. Results
Sodium-ion battery cathode materials continue to encounter challenges related to cycle life, ionic/electronic conductivity, and rate performance. Given the diverse structural characteristics and degradation mechanisms across different material systems, targeted optimization strategies must be formulated to improve their overall electrochemical performance. Due to the differences in structural characteristics and failure mechanisms of different material systems, corresponding optimization strategies need to be formulated based on specific structural characteristics and failure mechanisms to improve their comprehensive electrochemical properties.
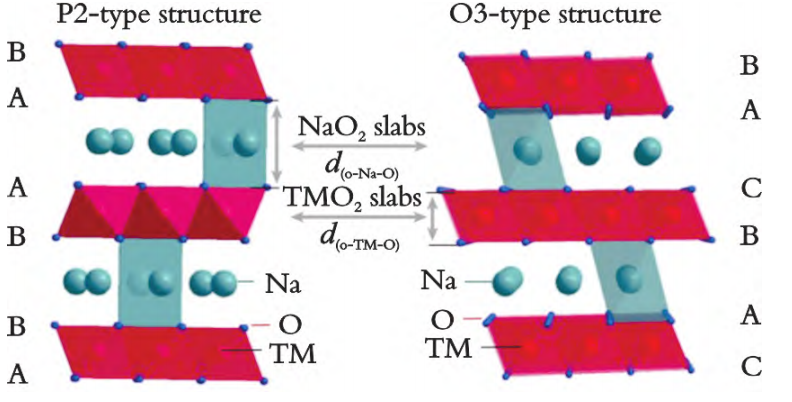
Layered oxides represent one of the most extensively studied cathode materials for sodium-ion batteries, with the general formula NaxMO2 (M = transition metals such as Ni, Mn, or Co). Depending on the Na+ arrangement and oxygen stacking sequence, the structures are typically classified into O3, P2, and P3 types. O3-type materials deliver high initial capacities but suffer from phase transitions during cycling, resulting in poor long-term stability. P2-type compounds provide wide Na+ diffusion channels and excellent rate performance, but their structural instability under high voltage limits practical application[5,6].P3-type structures share features of both O3 and P2 but have been less investigated. Overall, layered oxides offer high capacity and tunable structures, though they face challenges in cycling stability and air sensitivity, necessitating further optimization and surface modification. Figure 1 shows the structural diagram of P2 type (P63/mmc) and O3 type (R‐3m) materials.
Polyanionic compounds, typically composed of stable three-dimensional frameworks formed by PO4, SO4, or SiO4 anions, are represented by materials such as Na3V2(PO4)3 and Na2Fe2(SO4)3. Their primary advantages lie in robust structural stability, high operating voltages, and excellent thermal safety, which contribute to long cycle life and reliable safety performance[4]. However, their specific capacities are generally lower than those of layered oxides, and poor electronic conductivity remains a major limitation, often requiring carbon coating or cation doping to enhance electrochemical performance. In summary, polyanionic compounds hold great promise for large-scale energy storage, though further improvements in energy density are essential. The crystal structure of polyanion-type compounds is shown in Figure 2 to illustrate their typical coordination framework [8].
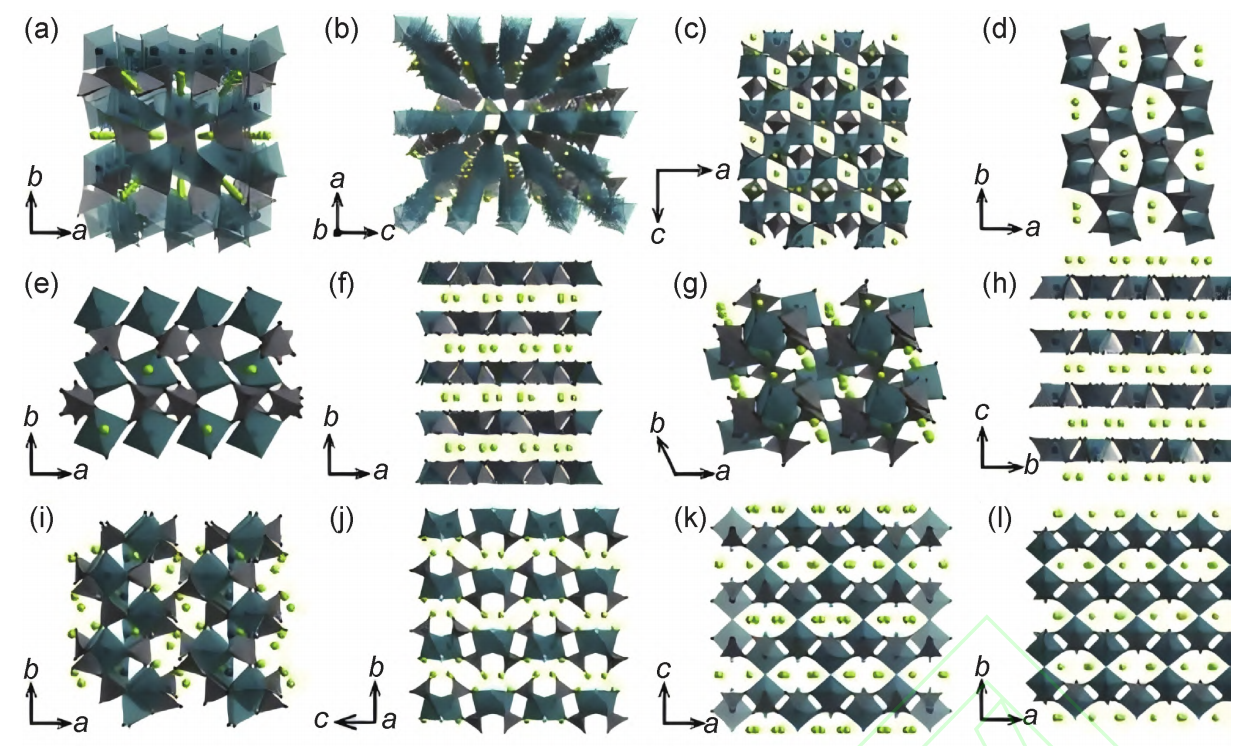
(a)NaFePO4-olivine;(b)NaFePO4-maricite;(c)NASICON-type Na3V2(PO4)3;(d)NaFe2PO7;(e)NaFeP2O7;(f)Na2CoP2O7;
(g)Na2CoP2O7;(h)Na2CoP2O7;(i)Na4Fe3(PO4)2P2O7;(j)Na2FePO4F;(k)Na3V2(PO4)2F3;(l)Na3V2(PO4)2O2F[8]
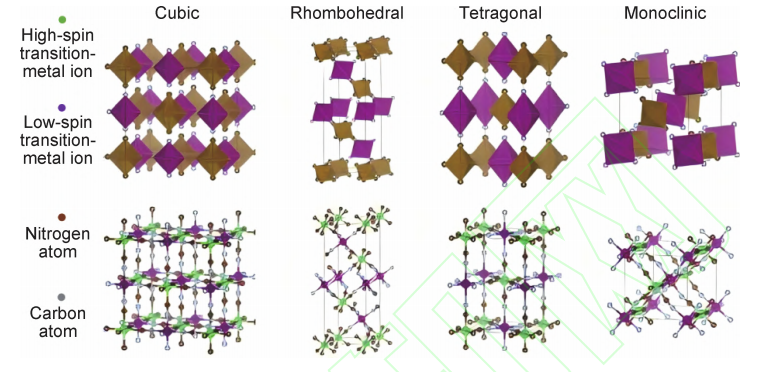
Prussian Blue Analogues (PBAs) possess an open cubic framework formed by M–C≡N–Fe linkages, which provide large Na+ diffusion channels, leading to excellent rate capability and cost advantages[9-11]. Moreover, PBAs can be synthesized under mild conditions, making them suitable for large-scale production. However, issues such as structural water and vacancy defects often impair electrochemical stability and capacity retention. Recent advances in synthesis techniques, defect control, and water content regulation have significantly improved their performance. Nevertheless, long-term cycling stability and energy density remain critical challenges that need to be addressed for practical applications. The representative framework of Prussian-blue materials is depicted in figure3 [12].
4. Conclusion
4.1 Summary of Key Findings
Layered transition metal oxides, polyanionic compounds, and Prussian blue analogues each exhibit distinct advantages and limitations as sodium-ion battery cathode materials. Layered oxides provide relatively high capacity and voltage but suffer from phase transitions, Na⁺ loss, and structural collapse during cycling. Polyanionic compounds offer high structural stability and long cycle life but are limited by low ionic and electronic conductivity, resulting in poor rate performance. Prussian blue analogues feature simple synthesis, low cost, and fast ion diffusion but are prone to crystalline water incorporation and Fe–CN bond breakage, leading to severe capacity decay.
4.2 Comparison with Previous Studies
Sodium Layered Transition Metal Oxides.Layered oxides such as NaNixCoyMnzO₂ (x+y+z=1) have been widely investigated for their high capacity (160–200 mAh/g) and operating voltage (3.0–4.2 V vs. Na⁺/Na. For example, NaNi₀.₅Mn₀.₅O₂ delivers ~160 mAh/g with 80% retention after 500 cycles. However, phase transitions to rock-salt or spinel phases, particularly above 4.2 V, accelerate capacity fading (>30% within 100 cycles). Moreover, Na⁺ extraction induces interlayer shrinkage, triggering particle cracking and electrolyte corrosion, which further accelerates performance degradation.
Polyanionic Compounds. Na₃V₂(PO₄)₃ (NVP) exemplifies polyanionic cathodes with a rigid three-dimensional framework, offering outstanding stability (>90% retention over 10,000 cycles) and an operating voltage of ~3.4 V. With a theoretical capacity of 117 mAh/g, NVP typically delivers ~100 mAh/g, making it suitable for grid-scale applications requiring long cycle life. Nonetheless, the insulating nature of polyanionic groups results in low electronic conductivity (~10⁻¹⁰ S/cm) and limited Na⁺ diffusivity (~10⁻¹⁴ cm²/s), yielding poor rate capability (1C discharge capacity ~60% of 0.1C).
Prussian Blue Analogues. Prussian blue analogues (PBAs) are attractive for low-cost and fast-charge applications due to facile aqueous co-precipitation synthesis, inexpensive precursors (ferrocyanide salts <10 USD/kg), and open three-dimensional frameworks (Na⁺ diffusivity up to 10⁻⁹ cm²/s). For instance, Na₂Fe[Fe(CN)₆] achieves ~150 mAh/g and supports rapid charging within 15 minutes (3C). However, crystalline water incorporated during synthesis occupies Na⁺ sites, causing an initial capacity loss exceeding 20%. Furthermore, Fe–CN bond breakage during cycling destabilizes the framework, leading to poor cycle life, with capacity retention below 60% after 100 cycles.
4.3 Implications of the Findings
This study systematically analyzes the performance bottlenecks and optimization strategies for sodium-ion battery cathode materials. The findings provide theoretical guidance and practical insights for improving electrochemical performance, offering valuable reference for advancing the large-scale application of sodium-ion batteries.
4.4 Limitations of the Study
This study focused on summarizing material categories and optimization strategies but did not propose specific candidate materials for immediate application.
4.5 Suggestions for Future Research
Future research should integrate structural regulation and performance balancing to develop more robust composite cathodes. Advanced characterization techniques are also needed to elucidate failure mechanisms, while efforts toward green, low-cost synthesis routes will be critical to accelerate commercialization of sodium-ion batteries.
References
[1]. Zhou, F.Y., Zeng, J.J. and Wang, X.B. (2024) Progress and Prospect of Electrochemical Energy Storage for Carbon Neutralization. Journal of Chinese Society of Power Engineering, 44(3), 396-405.
[2]. Zhou, J.Q., Yang, S.R., Ai, F.X., et al. (2022) Preparation of Co9S8@CNFs Composites and Their Application in Lithium-Ion Batteries. Journal of Liaoning Petrochemical University, 42(6), 21-27.
[3]. Zhou, Y., Shi, R.N., Lang, X.S., et al. (2022) Research Progress of V2O5 Cathode Material for Sodium-Ion Batteries. Journal of Petrochemical Universities, 35(6), 38-47.
[4]. Zhao, H., Liu, X.N., Wang, J.Y., et al. (2024) Research Progress of Sodium-Ion Battery Cathode Materials. Journal of Petrochemical Colleges and Universities, 37(6), 35-43.
[5]. Sun, Y.K. (2020) Direction for Commercialization of O3-Type Layered Cathodes for Sodium-Ion Batteries. ACS Energy Letters, 5(4), 1278-1280.
[6]. Gao, R.M., Zheng, Z.J., Wang, P.F., et al. (2020) Recent Advances and Prospects of Layered Transition Metal Oxide Cathodes for Sodium-Ion Batteries. Energy Storage Materials, 30, 9-26.
[7]. Wang Q D,Zhou D,Zhao C L,et al.Fast‐charge high ‐voltage layered cathodes for sodium ‐ion batteries[J].Nature
[8]. Zhao L,Zhang T,Zhao H, et al. Polyanion-type electrode materials for advanced sodium-ion batteries[J]. Materials Today Nano,2020,10:100072.
[9]. Qian J F, Wu C, Cao Y L, et al. Prussian blue cathode materials for sodium ‐ ion batteries and other ion batteries [J].Advanced Energy Materials, 2018, 8(17): 1702619.
[10]. Rehman R,Peng J,Yi H C,et al.Highly crystalline nickel hexacyanoferrate as a long‐life cathode material for sodium‐ ion batteries[J].RSC Advances,2020,10(45):27033‐27041.
[11]. Simonov A,DE Baerdemaeker T,Boström H L B,et al.Hidden diversity of vacancy networks in Prussian blue analogues[J].Nature,2020,578(7794):256‐260.
[12]. Wang Q,Li J,Jin H, et al. Prussian-blue materials: revealing new opportunities for rechargeable batteries[J]. Infomat,2022,4(6): e12311.
Cite this article
Bi,L. (2025). Recent Advances in Cathode Materials for Sodium-Ion Batteries: Structure–Property Relationships and Performance Optimization Strategies. Applied and Computational Engineering,186,191-195.
Data availability
The datasets used and/or analyzed during the current study will be available from the authors upon reasonable request.
Disclaimer/Publisher's Note
The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of EWA Publishing and/or the editor(s). EWA Publishing and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
About volume
Volume title: Proceedings of CONF-FMCE 2025 Symposium: Semantic Communication for Media Compression and Transmission
© 2024 by the author(s). Licensee EWA Publishing, Oxford, UK. This article is an open access article distributed under the terms and
conditions of the Creative Commons Attribution (CC BY) license. Authors who
publish this series agree to the following terms:
1. Authors retain copyright and grant the series right of first publication with the work simultaneously licensed under a Creative Commons
Attribution License that allows others to share the work with an acknowledgment of the work's authorship and initial publication in this
series.
2. Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the series's published
version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgment of its initial
publication in this series.
3. Authors are permitted and encouraged to post their work online (e.g., in institutional repositories or on their website) prior to and
during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See
Open access policy for details).
References
[1]. Zhou, F.Y., Zeng, J.J. and Wang, X.B. (2024) Progress and Prospect of Electrochemical Energy Storage for Carbon Neutralization. Journal of Chinese Society of Power Engineering, 44(3), 396-405.
[2]. Zhou, J.Q., Yang, S.R., Ai, F.X., et al. (2022) Preparation of Co9S8@CNFs Composites and Their Application in Lithium-Ion Batteries. Journal of Liaoning Petrochemical University, 42(6), 21-27.
[3]. Zhou, Y., Shi, R.N., Lang, X.S., et al. (2022) Research Progress of V2O5 Cathode Material for Sodium-Ion Batteries. Journal of Petrochemical Universities, 35(6), 38-47.
[4]. Zhao, H., Liu, X.N., Wang, J.Y., et al. (2024) Research Progress of Sodium-Ion Battery Cathode Materials. Journal of Petrochemical Colleges and Universities, 37(6), 35-43.
[5]. Sun, Y.K. (2020) Direction for Commercialization of O3-Type Layered Cathodes for Sodium-Ion Batteries. ACS Energy Letters, 5(4), 1278-1280.
[6]. Gao, R.M., Zheng, Z.J., Wang, P.F., et al. (2020) Recent Advances and Prospects of Layered Transition Metal Oxide Cathodes for Sodium-Ion Batteries. Energy Storage Materials, 30, 9-26.
[7]. Wang Q D,Zhou D,Zhao C L,et al.Fast‐charge high ‐voltage layered cathodes for sodium ‐ion batteries[J].Nature
[8]. Zhao L,Zhang T,Zhao H, et al. Polyanion-type electrode materials for advanced sodium-ion batteries[J]. Materials Today Nano,2020,10:100072.
[9]. Qian J F, Wu C, Cao Y L, et al. Prussian blue cathode materials for sodium ‐ ion batteries and other ion batteries [J].Advanced Energy Materials, 2018, 8(17): 1702619.
[10]. Rehman R,Peng J,Yi H C,et al.Highly crystalline nickel hexacyanoferrate as a long‐life cathode material for sodium‐ ion batteries[J].RSC Advances,2020,10(45):27033‐27041.
[11]. Simonov A,DE Baerdemaeker T,Boström H L B,et al.Hidden diversity of vacancy networks in Prussian blue analogues[J].Nature,2020,578(7794):256‐260.
[12]. Wang Q,Li J,Jin H, et al. Prussian-blue materials: revealing new opportunities for rechargeable batteries[J]. Infomat,2022,4(6): e12311.









